Exopolysaccharides Production by Cultivating a Bacterial Isolate from the Hypersaline Environment of Salar de Uyuni (Bolivia) in Pretreatment Liquids of Steam-Exploded Quinoa Stalks and Enzymatic Hydrolysates of Curupaú Sawdust
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism Isolation and Culture Conditions
2.2. Lignocellulosic Feedstocks
2.3. Pretreatment of Lignocellulosic Feedstocks
2.4. Enzymatic Hydrolysis
2.5. Cultivation of the Bacterial Isolate BU-4
2.6. EPS Extraction
2.7. Analyses
2.7.1. Monosaccharide Analysis in the EPS
2.7.2. Determination of Total Protein
2.7.3. FTIR and TGA Analysis of EPS
2.7.4. Scanning Electron Microscopy
3. Results
3.1. Effect of Salinity on EPS Production and Cell Growth
3.2. Effect of the Medium Components on Cell Growth and EPS Production
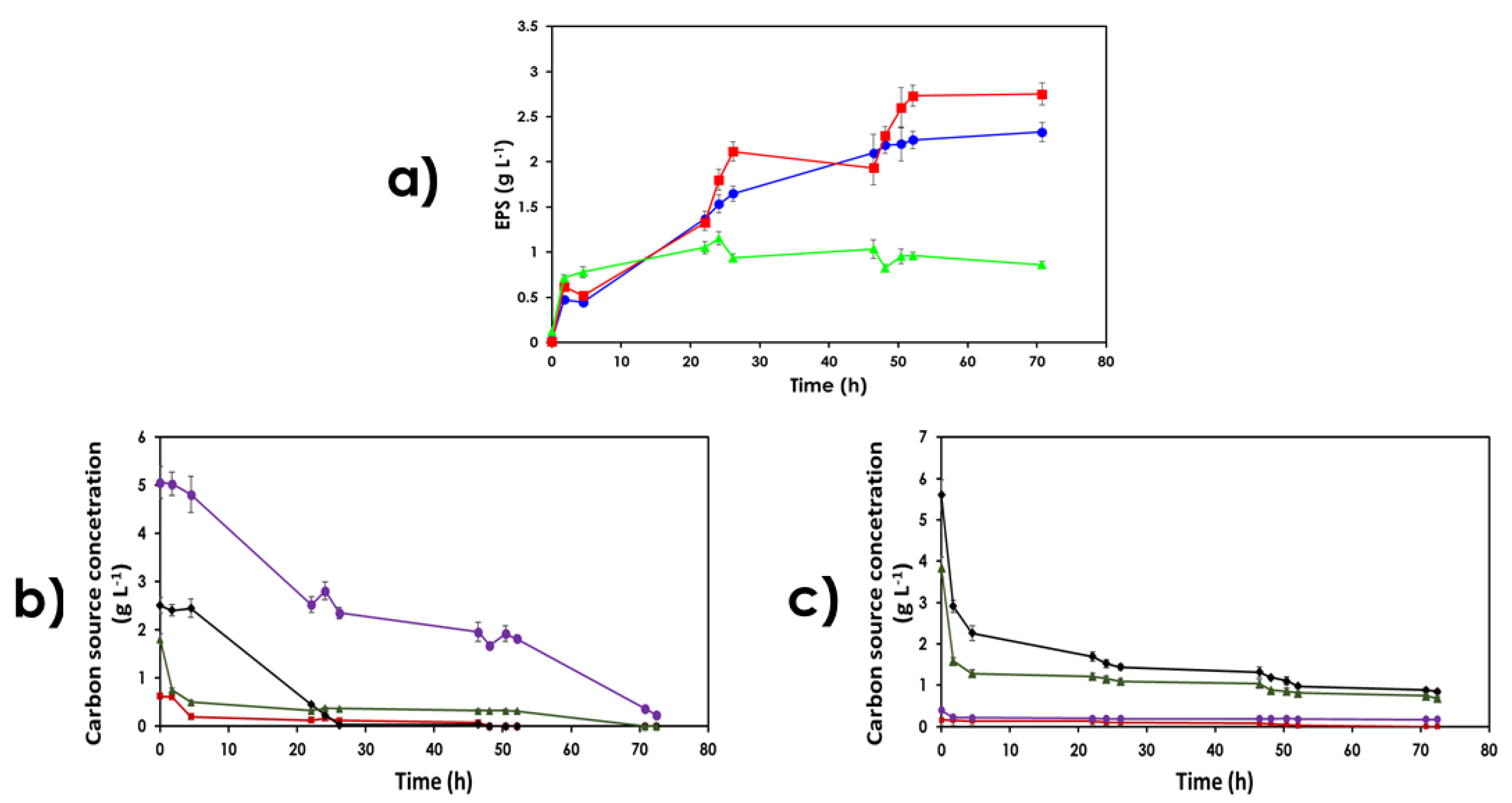
3.3. Use of Lignocellulose-Based Substrates for EPS Production
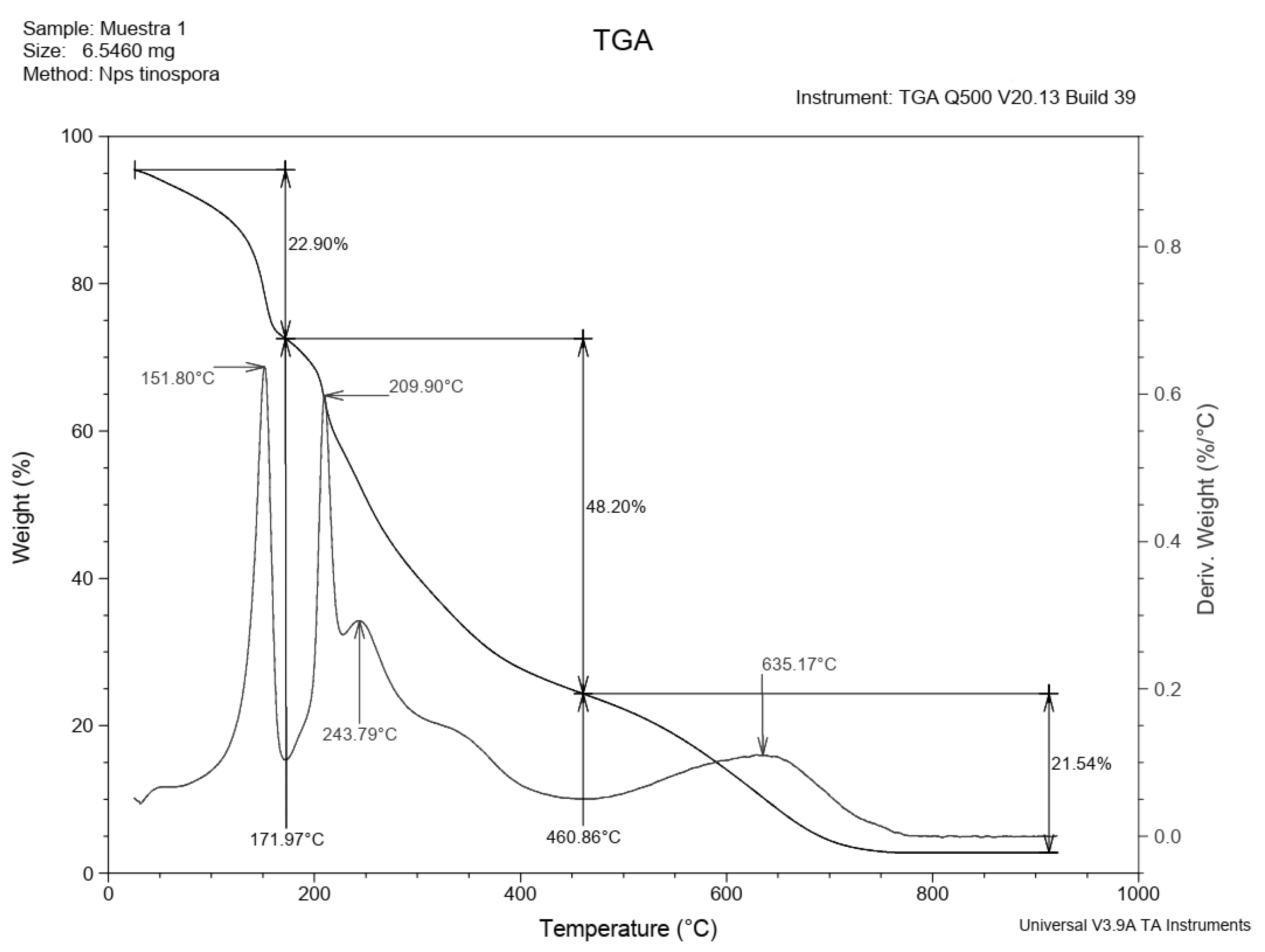
3.4. Characterization of the Produced EPS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A. Morphological Characterization
Appendix B. Physico-Chemical Characterization
Appendix C. Biochemical Tests
References
- Rampelotto, P.H. Extremophiles and extreme environments. Life (Basel) 2013, 3, 482–485. [Google Scholar] [CrossRef]
- Nicolaus, B.; Kambourova, M.; Oner, E.T. Exopolysaccharides from extremophiles: From fundamentals to Biotechnology. Environ. Technol. 2010, 31, 1145–1158. [Google Scholar] [CrossRef]
- Coker, J.A. Extremophiles and Biotechnology: Current Uses and Prospects. F1000Res 2016, 5. [Google Scholar] [CrossRef]
- Merino, N.; Aronson, H.S.; Bojanova, D.P.; Feyhl-Buska, J.; Wong, M.L.; Zhang, S.; Giovannelli, D. Living at the extremes: Extremophiles and the limits of life in a planetary context. Fron. Microbiol. 2019, 10, 780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galinski, E.; Truper, H. Microbial behavior in salt-stressed ecosystems. FEMS Microbiol. Rev. 1994, 15, 95–108. [Google Scholar] [CrossRef]
- Galinski, E. Osmoadaptation in bacteria. Adv. Microb. Physiol. 1995, 37, 273–328. [Google Scholar]
- Barghini, P.; Silvi, S.; Aquilanti, A.; Marcelli, M.; Fenice, M. Bacteria from marine salterns as a model of microorganisms adapted to high environmental variations. J. Environ. Prot. Ecol. 2014, 75, 897–906. [Google Scholar]
- Ollivier, B.; Caumette, P.; Garcia, J.L.; Mah, R.A. Anaerobic bacteria from hypersaline environments. Microbiol. Rev. 1994, 58, 27–38. [Google Scholar] [CrossRef]
- Margesin, R.; Schinner, F. Potential of halotolerant and halophilic microorganisms for Biotechnology. Extremophiles 2001, 5, 73–83. [Google Scholar] [CrossRef]
- Quillaguamán, J.; Hatti-Kaul, R.; Mattiason, B.; Alvarez, M.; Delgado, O. Halomonas boliviensis sp. nov., an alkalitolerant, moderate halophile isolated from soil around a Bolivian hypersaline lake. Int. J. Syst. Evol. Microbiol. 2004, 54, 721–725. [Google Scholar] [CrossRef]
- Mata, J. Caracterización de los exopolisacáridos producidos por microorganismos halófilos pertenecientes a los géneros Halomonas, Alteromonas, Idiomarina, Palleronia y Salipiger. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 2006. [Google Scholar]
- Guzmán, H.; Van-Thuoc, D.; Martín, J.; Hatti-Kaul, R.; Quillaguamán, J. A process for the production of ectoine and poly(3-hydroxybutyrate) by Halomonas boliviensis. Appl. Microbiol. Biotechnol. 2009, 84, 1069–1077. [Google Scholar] [CrossRef]
- Czaczyk, K.; Myszka, K. Biosynthesis of extracellular polymeric substances (EPS) and its role in microbial biofilm formation. Pol. J. Environ. Stud. 2007, 16, 799–806. [Google Scholar]
- Orgad, O.; Oren, Y.; Walker, S.L.; Herzberg, M. The role of alginate in Pseudomonas aeruginosa EPS adherence, viscoelastic properties and cell attachment. Biofouling 2011, 27, 787–798. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; de Vin, F. Exopolysaccharides from lactic acid bacteria. In Comprehensive Glycoscience; Kamerling, J.P., Ed.; Elsevier: Amsterdam, The Netherlands; volume 2, pp. 477–519.
- Prasanna, P.H.P.; Grandison, A.S.; Charalampopoulos, D. Bifidobacteria in milk products: An overview of physiological and biochemical properties, exopolysaccharide production, selection criteria of milk products and health benefits. Food Res. Int. 2014, 55, 247–262. [Google Scholar] [CrossRef]
- Raposo, M.F.D.J.; de Morais, R.M.S.C.; de Morais, B.; Miranda, A.M. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Czajka, D.; Lion, L.; Shuler, M.; Ghiorse, W. Trace metal mobilization in soil by bacterial polymers. Environ. Health Perspec. 1995, 103, 53–58. [Google Scholar]
- Sheng, G.P.; Yu, H.Q.; Li, X.Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Bourven, I.; Guibaud, G.; van Hullebusch, E.D.; Panico, A.; Pirozzi, F.; Esposito, G. Role of extracellular polymeric substances (EPS) Production in bioaggregation: Application to wastewater treatment. Appl. Microbiol. Biotechnol. 2015, 99, 9883–9905. [Google Scholar] [CrossRef]
- Quillaguamán, J.; Delgado, O.; Mattiasson, B.; Hatti-Kaul, R. Chromohalobacter sarecensis sp. nov., a psychrotolerant moderate halophile bacterium isolated from the saline Andean region of Bolivia. Int. J. Syst. Evol. Microbiol 2004, 54, 1921–1926. [Google Scholar]
- Guzmán, D.; Quillaguamán, J.; Munoz, M.; Hatti-Kaul, R. Halomonas andesensis sp. nov., a moderate halophile isolated from the saline lake Laguna Colorada in Bolivia. Int. J. Syst. Evol. Microbiol. 2010, 60, 749–753. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Contreras, A.; Koller, M.; de Sousa Dias, M.M.; Calafell, M.; Braunegg, G.; Marqués-Calvo, M.S. Novel poly[(R)-3-hydroxybutyrate]-producing bacterium isolated from a Bolivian hypersaline lake. Food Technol. Biotechnol. 2013, 51, 123–130. [Google Scholar]
- Haferburg, G.; Gröning, J.A.; Schmidt, N.; Kummer, N.A.; Erquicia, J.C.; Schlömann, M. Microbial diversity of the hypersaline and lithium-rich Salar de Uyuni, Bolivia. Microbiol. Res. 2017, 199, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Ehling-Schulz, M.; Bilger, W.; Scherer, S. UV-B-induced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium Nostoc commune. J. Bacteriol. 1997, 1940–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alandia, G.; Rodriguez, J.P.; Jacobsen, S.E.; Bazile, D.; Condori, B. Global expansion of quinoa and challenges for the Andean region. Glob. Food Sec. 2020, 26, 100429. [Google Scholar] [CrossRef]
- Canales, N.; Gomez, J.; Fielding, M.; Dugarte, M. The potential of quinoa in Bolivia’s bioeconomy. In SEI Report; Swedish Environment Institute: Stockholm, Sweden, 2020; p. 14. [Google Scholar]
- Carrasco, C. Lignocellulosic Production Studies on Sugarcane Bagasse, Paja Brava, Wheat Straw, Quinoa Stalks and Curupaú. Ph.D. Thesis, Department of Chemical Engineering, Lund University, Lund, Sweden, 2013. [Google Scholar]
- Salas-Veizaga, D.M.; Villagomez, R.; Linares-Pastén, J.A.; Carrasco, C.; Álvarez, M.T.; Adlercreutz, P.; Nordberg Karlsson, E. Extraction of glucuronoarabinoxylan from quinoa stalks (Chenopodium Quinoa Willd.) and evaluation of xylooligosaccharides produced by GH10 and GH11 xylanases. J. Agric. Food Chem. 2017, 65, 8663–8673. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, C.; Quispe, L.; Lidén, G. Study of sulphuric acid-catalyzed steam pretreatment of the hardwood Anadenanthera colubrina. ENERLAC 2018, 2, 1, 54–68. [Google Scholar]
- Gandla, M.L.; Martín, C.; Jönsson, L.J. Analytical enzymatic saccharification of lignocellulosic biomass for conversion to biofuels and bio-based chemicals. Energies 2018, 11, 2936. [Google Scholar] [CrossRef] [Green Version]
- Sella, S.R.; Vandenberghe, L.P.; Soccol, C.R. Bacillus atrophaeus: Main characteristics and biotechnological applications-a review. Crit. Rev. Biotechnol. 2015, 35, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, Y.Z.; Yan, F.; Song, R.T.; Li, Z.J.; Li, Y.Q.; Song, B. Physical and chemical properties, percutaneous absorption-promoting effects of exopolysaccharide produced by Bacillus atrophaeus WYZ strain. Carbohydr. Polym. 2018, 192, 52–60. [Google Scholar] [CrossRef]
- Carrasco, C.; Cuno, D.; Carlqvist, K.; Galbe, M.; Lidén, G. SO2-catalysed steam pretreatment of quinoa stalks. J. Chem. Technol. Biotechnol. 2015, 90, 64–71. [Google Scholar] [CrossRef]
- Romero-Soto, A.; Thabet, H.; Maghembe, R.; Gameiro, D.; Van-Thuoc, D.; Dishisha, T.; Hatti-Kaul, R. Metabolic potential of the moderate halophile Yangia sp. ND199 for co-production of polyhydroxybutyrate and exopolysaccharides. MicrobiologyOpen 2021, 10, e1160. [Google Scholar] [CrossRef]
- Kanamarlapudi, S.L.R.K.; Muddada, S. Characterization of exopolysaccharide produced by Streptococcus thermophilus CC30. Biomed. Res. Int. 2017, 4201809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mota, R.; Guimarães, R.; Buttel, Z.; Rossi, F.; Colica, G.; Silva, C.; Santos, C.; Gales, L.; Zille, A.; Philippis, R.; et al. Production and characterization of extracellular carbohydrate polymer from Cyanothece sp. CCY 0110. Carbohydr. Polym. 2013, 92, 1408–1415. [Google Scholar] [CrossRef]
- Shrivastaw, K.P.; Jhamb, S.S.; Kumar, A. Quantitation of the protein content of diphtheria and tetanus toxoids by the Biuret method during production of combined vaccines. Biologicals 1995, 23, 61–63. [Google Scholar] [CrossRef]
- Liu, Z.L.; Pan, J.H. A practical method for extending the biuret assay to protein determination of corn-based products. Food Chem. 2017, 224, 289–293. [Google Scholar] [CrossRef]
- Mora-Murillo, L.D.; Orozco-Gutiérrez, F.; Vega-Baudrit, J.; González-Paz, R.J. Thermal-mechanical characterization of polyurethane rigid foams: Effect of modifying bio-polyol content in isocyanate prepolymers. J. Renew. Mater. 2017, 5, 220–230. [Google Scholar] [CrossRef]
- Rani, R.P.; Anandharaj, M.; Sabhapathy, P.; Ravindran, A.D. Physiochemical and biological characterization of novel exopolysaccharide produced by Bacillus tequilensis FR9 isolated from chicken. Int. J. Biol. Macromol. 2017, 96, 1–10. [Google Scholar] [CrossRef]
- Sutherland, I.W. Structure-function relationships in microbial exopolysaccharide. Biotechnol. Adv. 1994, 12, 393–448. [Google Scholar] [CrossRef]
- Rasulov, B.A.; Rozi, P.; Pattaeva, M.A.; Yili, A.; Aisa, H.A. Exopolysaccharide-based bioflocculant matrix of Azotobacter chroococcum XU1 for synthesis of AgCl nanoparticles and its application as a novel biocidal nanobiomaterial. Materials 2016, 9, 528. [Google Scholar] [CrossRef] [Green Version]
- Kielak, A.; Castellane, T.; Campanharo, J.; Colnago, L.A.; Costa, O.Y.A.; Corradi da Silva, M.; van Veen, J.A.; Lemos, E.G.M.; Kuramae, E.E. Characterization of novel Acidobacteria exopolysaccharides with potential industrial and ecological applications. Sci. Rep. 2017, 7, 41193. [Google Scholar] [CrossRef]
- Copikova, J.; Barros, A.S.; Šmídová, I.; Černá, M.; Teixeira, D.H.; Delgadillo, I.; Synytsya, A.; Coimbra, M.A. Influence of hydration of food additive polysaccharides on FT-IR spectra distinction. Carbohydr. Polym. 2006, 63, 355–359. [Google Scholar] [CrossRef]
- Barros, F.C.; Silva, D.C.; Sombra, V.G.; Maciel, J.S.; Feitosa, J.P.; Freitas, A.L.; Paula, R.C. Structural characterization of polysaccharide obtained from red seaweed Gracilaria caudata (J Agardh). Carbohydr. Polym. 2013, 92, 598–603. [Google Scholar] [CrossRef] [Green Version]
- Mata, J.A.; Béjar, V.; Llamas, I.; Arias, S.; Bressollier, P.; Tallon, R.; Urdaci, M.C.; Quesada, E. Exopolysaccharides produced by the recently described halophilic bacteria Halomonas ventosae and Halomonas anticariensis. Res. Microbiol. 2006, 157, 827–835. [Google Scholar] [CrossRef]
- Daoub, R.; Elmurabak, A.; Misran, M.; Hassan, E.; Osman, M. Characterization and functional properties of some natural acacia gums. J. Saudi Soc. Agric. Sci. 2016, 17, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Dinić, M.; Pecikoza, U.; Djokić, J.; Stepanović-Petrović, R.; Milenković, M.; Stevanović, M.; Filipović, N.; Begović, J.; Golić, N.; Lukić, J. Exopolysaccharide produced by probiotic strain Lactobacillus paraplantarum BGCG11 reduces inflammatory hyperalgesia in rats. Front Pharmacol. 2018, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.; Petkowicz, C.; Morais, S.; Terrones, M.; Resende, M.; França, F.; Cardoso, V. Characterization of xanthan gum produced from sugar cane broth. Carbohydr. Polym. 2011, 86, 469–476. [Google Scholar] [CrossRef] [Green Version]
- Suja, L.D.; Summers, S.; Gutierrez, T. Role of EPS, dispersant and nutrients on the microbial response and MOS formation in the Subarctic Northeast Atlantic. Fron. Microbiol. 2017, 8, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The microbial “protective clothing” in extreme environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef] [Green Version]
- Bouchotroch, S.; Quesada, E.; Izquierdo, I.; Rodriguez, M.; Béjar, V. Bacterial exopolysaccharides produced by newly discovered bacteria belonging to the genus Halomonas, isolated from hypersaline habitats in Morocco. J. Ind. Microbiol. Biotechnol. 2000, 24, 374–378. [Google Scholar] [CrossRef]
- Martínez-Cánovas, M.J.; Quesada, E.; Martínez-Checa, F.; del Moral, A.; Bejar, V. Salipiger mucescens gen. nov., sp. nov., a moderately halophilic, exopolysaccharide-producing bacterium isolated from hypersaline soil, belonging to the α-proteobacteria. Int. J. Syst. Evol. Microbiol 2004, 54, 1735–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, J.; Paul, A.K. Diversity and production of extracellular polysaccharide by halophilic microorganisms. Biodiversity Int. J. 2017, 1, 32–39. [Google Scholar]
- Pérez-Llano, Y.; Rodríguez-Pupo, E.C.; Druzhinina, I.S.; Chenthamara, K.; Cai, F.; Gunde-Cimerman, N.; Batista-García, R.A. Stress reshapes the physiological response of halophile fungi to salinity. Cells 2020, 9, 525. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, N. Hydrogeological and hydrochemical investigations at the Salar de Uyuni (Bolivia) with regard to the extraction of lithium. FOG 2010, 26, 1–131. [Google Scholar]
- Pérez-Fernández, C.A.; Iriarte, M.; Rivera-Pérez, J.; Tremblay, R.L.; Toranzos, G.A. Microbiota dispersion in the Uyuni salt flat (Bolivia) as determined by community structure analyses. Int. Microbiol. 2019, 22, 325–336. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Degeest, B.; De Vuyst, L. Indication that the nitrogen source influences both amount and size of exopolysaccharides produced by Streptococcus thermophilus LY03 and modelling of the bacterial growth and exopolysaccharide production in a complex medium. Appl. Environ. Microbiol. 1999, 65, 2863–2870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zisu, B.; Shah, N.P. Effects of pH, temperature, supplementation with whey protein concentrate, and adjunct cultures on the production of exopolysaccharides by Streptococcus thermophiles 1275. J. Dairy Sci. 2003, 86, 3405–3415. [Google Scholar] [CrossRef] [Green Version]
- Ewert, M.; Deming, J.W. Sea ice microorganisms: Environmental constraints and extracellular responses. Biology (Basel) 2013, 2, 603–628. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Li, J.; Liu, Y.; Xu, Z.; Wu, J.Y.; Ding, Z.; Shi, G. Effects of mixed carbon sources on galactose and mannose content of exopolysaccharides and related enzyme activities in Ganoderma lucidum. RSC Adv. 2016, 6, 39284–39291. [Google Scholar] [CrossRef]
- Savi, A.; Calegari, G.; Queiroz, V.; Pereira, E.; Teixeira, S. Chemical characterization and antioxidant of polysaccharide extracted from Dioscorea bulbifera. J. King Saud Univ. Sci. 2020, 32, 636–642. [Google Scholar] [CrossRef]
- Georgieva, V.; Zvezdova, D.; Vlaev, L. Non-isothermal kinetics of thermal degradation of chitosan. Chem. Cent. J. 2012, 6, 81. [Google Scholar] [CrossRef] [Green Version]
- Kumar, C.G.; Joo, H.S.; Choi, J.W.; Koo, Y.M.; Chang, C.S. Purification and characterization of an extracellular polysaccharide from haloalkalophilic Bacillus sp. I-450. Enzyme Microb. Technol. 2004, 34, 673–681. [Google Scholar] [CrossRef]
- Joulak, I.; Azabou, S.; Finore, I.; Poli, A.; Nicolaus, B.; Donato, P.D. and Attia, H. Structural characterization and functional properties of novel exopolysaccharide from the extremely halotolerant Halomonas elongata S6. Int. J. Biol. Macromol. 2020, 164, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Nicolaus, B.; Lama, L.; Panico, A.; Schiano Moriello, V.; Romano, I.; Gambacorta, A. Production and characterization of exopolysaccharides excreted by thermophilic bacteria from shallow, marine hydrothermal vents of Flegrean areas (Italy). Syst. Appl. Microbiol. 2002, 25, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Arena, A.; Maugeri, T.L.; Pavone, B.; Iannello, D.; Gugliandolo, C.; Bisignano, G. Antiviral and Immunoregulatory Effect of a novel Exopolysaccharide from a Marine Thermotolerant Bacillus licheniformis. Int. Immunopharmacol. 2006, 6, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Kianpour, S.; Ebrahiminezhad, A.; Mohkam, M.; Tamaddon, A.M.; Dehshahri, A.; Heidari, R.; Ghasemi, Y. Physicochemical and biological characteristics of the nanostructured polysaccharide-iron hydrogel produced by microorganism Klebsiella oxytoca. J. Basic Microbiol. 2017, 57, 132–140. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles–a review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef]
- Saroia, J.; Yanen, W.; Wei, Q.; Zhang, K.; Lu, T.; Zhang, B. A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio-Des. Manuf. 2018, 1, 265–279. [Google Scholar] [CrossRef]




| No. | Peptone | Yeast Extract | Glucose | EPS Production |
|---|---|---|---|---|
| 1 | 1 | 1 | 10 | 0.508 |
| 2 | 1 | 1 | 30 | 0.586 |
| 3 | 1 | 5 | 10 | 1.090 |
| 4 | 1 | 5 | 30 | 0.361 |
| 5 | 5 | 1 | 10 | 2.008 |
| 6 | 5 | 1 | 30 | 1.684 |
| 7 | 5 | 5 | 10 | 2.358 |
| 8 | 5 | 5 | 30 | 2.242 |
| Lignocellulose-Based Substrates | Concentration (g L−1) | ||||
|---|---|---|---|---|---|
| Cellobiose | Glucose | Xylose | Galactose | Arabinose | |
| PL-QS | 1.13 | 4.51 | 9.03 | 3.28 | 3.24 |
| EH-CS | 0.16 | 4.44 | 0.39 | - | 3.84 |
| Glucose | Xylose | Arabinose | |
|---|---|---|---|
| EPS from glucose-based medium | 90.5 | - | - |
| EPS from quinoa stalks | 30.2 | 48.7 | 16.3 |
| Feedstock | Cellulose | Hemicellulose | Lignin | Ash |
|---|---|---|---|---|
| Quinoa stalks | 35.7 | 15.4 | 21.9 | 4.2 |
| Curupaú sawdust | 43.3 | 15.6 | 20.0 | 14.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chambi, D.; Romero-Soto, L.; Villca, R.; Orozco-Gutiérrez, F.; Vega-Baudrit, J.; Quillaguamán, J.; Hatti-Kaul, R.; Martín, C.; Carrasco, C. Exopolysaccharides Production by Cultivating a Bacterial Isolate from the Hypersaline Environment of Salar de Uyuni (Bolivia) in Pretreatment Liquids of Steam-Exploded Quinoa Stalks and Enzymatic Hydrolysates of Curupaú Sawdust. Fermentation 2021, 7, 33. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation7010033
Chambi D, Romero-Soto L, Villca R, Orozco-Gutiérrez F, Vega-Baudrit J, Quillaguamán J, Hatti-Kaul R, Martín C, Carrasco C. Exopolysaccharides Production by Cultivating a Bacterial Isolate from the Hypersaline Environment of Salar de Uyuni (Bolivia) in Pretreatment Liquids of Steam-Exploded Quinoa Stalks and Enzymatic Hydrolysates of Curupaú Sawdust. Fermentation. 2021; 7(1):33. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation7010033
Chicago/Turabian StyleChambi, Diego, Luis Romero-Soto, Roxana Villca, Felipe Orozco-Gutiérrez, José Vega-Baudrit, Jorge Quillaguamán, Rajni Hatti-Kaul, Carlos Martín, and Cristhian Carrasco. 2021. "Exopolysaccharides Production by Cultivating a Bacterial Isolate from the Hypersaline Environment of Salar de Uyuni (Bolivia) in Pretreatment Liquids of Steam-Exploded Quinoa Stalks and Enzymatic Hydrolysates of Curupaú Sawdust" Fermentation 7, no. 1: 33. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation7010033









