Quinoa Flour, the Germinated Grain Flour, and Sourdough as Alternative Sources for Gluten-Free Bread Formulation: Impact on Chemical, Textural and Sensorial Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Quinoa Flour
2.2. Germinated Quinoa Flour
2.3. Quinoa Sourdough
2.4. Bread Making
2.5. Breads’ Proximal Composition Analysis
2.6. Breads’ Attributes
2.6.1. Determination of pH and Titrable Acidity
2.6.2. Bake Loss
2.6.3. Color
2.6.4. Texture
2.6.5. Shelf-Life and Microbial Quality
2.6.6. Amino Acids Composition
2.6.7. Sensory Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Flours’ Proximal Composition
3.2. Sourdough Development
3.3. Gluten-Free Breads Characterization
3.3.1. Color Analysis
3.3.2. Crumb Analysis
3.3.3. Chemical Composition
3.3.4. Amino Acid Profile
3.3.5. Shelf-Life
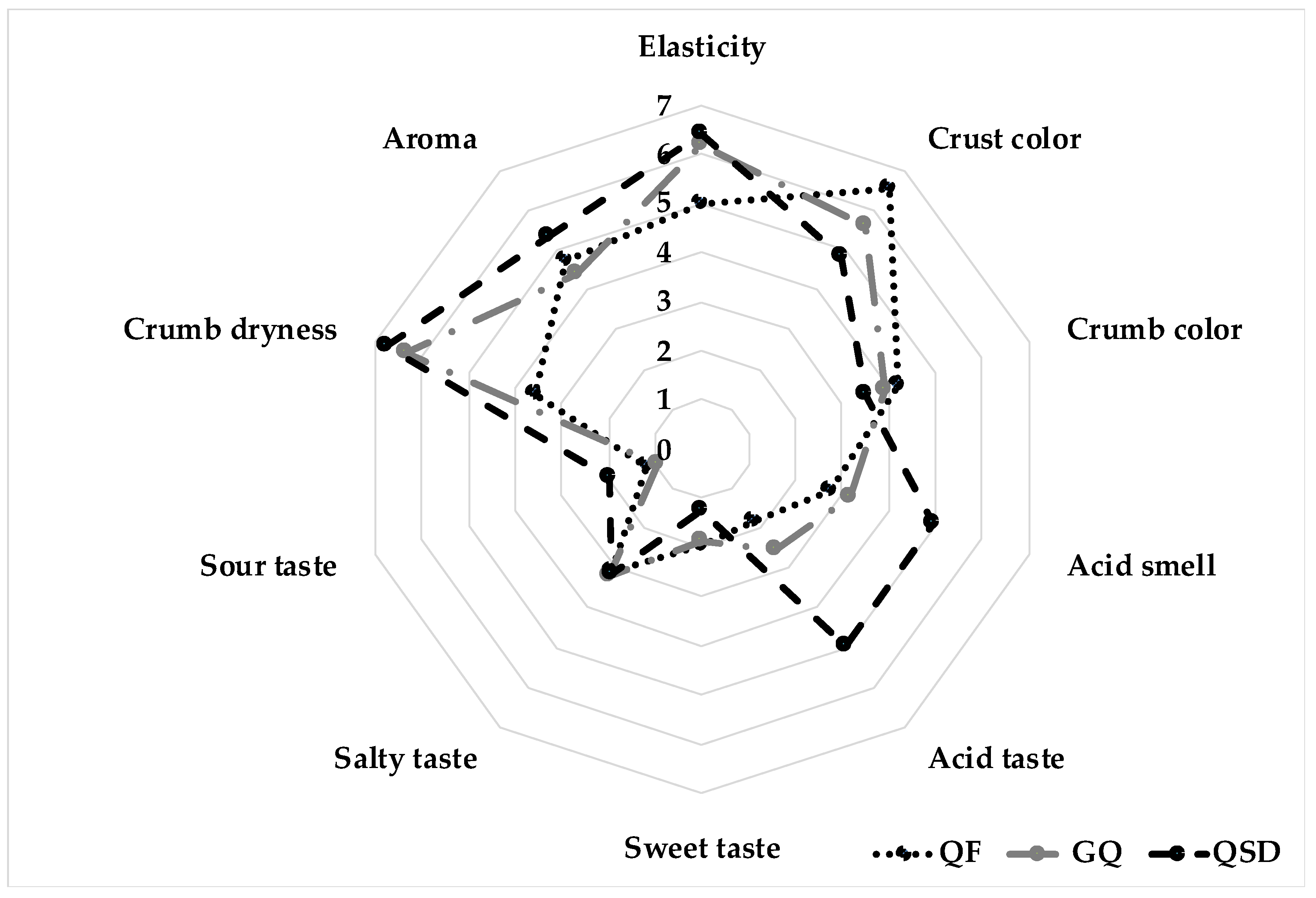
3.3.6. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez-Villaluenga, C.; Peñas, E.; Hernández-Ledesma, B. Pseudocereal grains: Nutritional value, health benefits and current applications for the development of gluten-free foods. In Food and Chemical Toxicology; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; Volume 137, p. 111178. [Google Scholar]
- Turkut, G.M.; Cakmak, H.; Kumcuoglu, S.; Tavman, S. Effect of quinoa flour on gluten-free bread batter rheology and bread quality. J. Cereal Sci. 2016, 69, 174–181. [Google Scholar] [CrossRef]
- Arendt, E.K.; Zannini, E. Quinoa. In Cereal Grains for the Food and Beverage Industries; Elsevier: Amsterdam, The Netherlands, 2013; pp. 409–438. [Google Scholar]
- Wood, L. Global Bread Market Growth, Trends & Forecast 2018–2023-ResearchAndMarkets.com—The Business Journals. 2018. Available online: https://www.businesswire.com/news/home/20180515005746/en/Global-Bread-Market-Growth-Trends-Forecast-2018–2023---ResearchAndMarkets.com (accessed on 3 December 2020).
- Graf, B.L.; Rojas-Silva, P.; Rojo, L.E.; Delatorre-Herrera, J.; Baldeón, M.E.; Raskin, I. Innovations in Health Value and Functional Food Development of Quinoa (Chenopodium quinoa Willd.). Compr. Rev. Food Sci. Food Saf. 2015, 14, 431–445. [Google Scholar] [CrossRef] [Green Version]
- Boukid, F.; Folloni, S.; Sforza, S.; Vittadini, E.; Prandi, B. Current Trends in Ancient Grains-Based Foodstuffs: Insights into Nutritional Aspects and Technological Applications. Compr. Rev. Food Sci. Food Saf. 2018, 17, 123–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Jubete, L.; Auty, M.; Arendt, E.K.; Gallagher, E. Baking properties and microstructure of pseudocereal flours in gluten-free bread formulations. Eur. Food Res. Technol. 2009, 230, 437–445. [Google Scholar] [CrossRef]
- Elgeti, D.; Nordlohne, S.D.; Föste, M.; Besl, M.; Linden, M.H.; Heinz, V.; Jekle, M.; Becker, T. Volume and texture improvement of gluten-free bread using quinoa white flour. J. Cereal Sci. 2014, 59, 41–47. [Google Scholar] [CrossRef]
- Gobbetti, M.; Gänzle, M. Handbook on Sourdough Biotechnology; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Franco, W.; Pérez-Díaz, I.M.; Connelly, L.; Diaz, J.T. Isolation of exopolysaccharide-producing yeast and lactic acid bacteria from quinoa (Chenopodium quinoa) sourdough fermentation. Foods 2020, 9, 337. [Google Scholar] [CrossRef] [Green Version]
- Galle, S.; Schwab, C.; Arendt, E.K.; Gänzle, M.G. Structural and rheological characterisation of heteropolysaccharides produced by lactic acid bacteria in wheat and sorghum sourdough. Food Microbiol. 2009, 78, 1–11. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Coffey, A.; Arendt, E.K. Lactic Acid Bacteria Exopolysaccharides in Foods and Beverages: Isolation, Properties, Characterization, and Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Boger, M.; van der Kaaij, R.M.; Woortman, A.J.J.; Pijning, T.; van Leeuwen, S.S.; Lammerts van Bueren, A.; Dijkhuizen, L. Lactobacillus reuteri Strains Convert Starch and Maltodextrins into Homoexopolysaccharides Using an Extracellular and Cell-Associated 4,6-α-Glucanotransferase. J. Agric. Food Chem. 2016, 64, 2941–2952. [Google Scholar] [CrossRef]
- Tieking, M.; Gänzle, M.G. Exopolisaccharides from cereal-associated lactobacilli. Trends Food Sci. Technol. 2005, 16, 79–84. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Lorusso, A.; Montemurro, M.; Marco, G. Use of sourdough made with quinoa (Chenopodium quinoa) flour and autochthonous selected lactic acid bacteria for enhancing the nutritional, textural and sensory features of white bread. Food Microbiol. 2016, 56, 1–13. [Google Scholar] [CrossRef]
- Jagelaviciute, J.; Cizeikiene, D. The influence of non-traditional sourdough made with quinoa, hemp and chia flour on the characteristics of gluten-free maize/rice bread. LWT 2020, 137, 110457. [Google Scholar] [CrossRef]
- Nionelli, L.; Rizzello, C. Sourdough-Based Biotechnologies for the Production of Gluten-Free Foods. Foods 2016, 5, 65. [Google Scholar] [CrossRef]
- Arendt, E.K.; O’Brien, C.M.; Schober, T.; Gormley, T.R.; Gallagher, E. Development of gluten-free cereal products. Farm Food 2002, 12, 21–27. [Google Scholar]
- Axel, C.; Röcker, B.; Brosnan, B.; Zannini, E.; Furey, A.; Coffey, A.; Arendt, E.K. Application of Lactobacillus amylovorus DSM19280 in gluten-free sourdough bread to improve the microbial shelf life. Food Microbiol. 2015, 47, 36–44. [Google Scholar] [CrossRef]
- Moore, M.M.; Bello, F.D.; Arendt, E.K. Sourdough fermented by Lactobacillus plantarum FST 1.7 improves the quality and shelf life of gluten-free bread. Eur. Food Res. Technol. 2008, 226, 1309–1316. [Google Scholar] [CrossRef]
- Phiarais, B.P.N.; Arendt, E.K. Malting and brewing with gluten-free cereals. In Gluten-Free Cereal Products and Beverages; Elsevier Inc.: Amsterdam, The Netherlands, 2008; pp. 348–371. [Google Scholar]
- Kaur, I.; Tanwar, B. Quinoa beverages: Formulation, processing and potential health benefits. Rom. J. Diabetes, Nutr. Metab. Dis. 2016, 23, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Miranda-Villa, P.P.; Mufari, J.R.; Bergesse, A.E.; Calandri, E.L. Effects of Whole and Malted Quinoa Flour Addition on Gluten-Free Muffins Quality. J. Food Sci. 2019, 84, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFeeters, R.F.; Barish, A.O. Sulfite analysis of fruits and vegetables by high-performance liquid chromatography (HPLC) with ultraviolet spectrophotometric detection. J. Agric. Food Chem. 2003, 51, 1513–1517. [Google Scholar] [CrossRef]
- Lattanzi, A.; Minervini, F.; Di Cagno, R.; Diviccaro, A.; Antonielli, L.; Cardinali, G.; Cappelle, S.; De Angelis, M.; Gobbetti, M. The lactic acid bacteria and yeast microbiota of eighteen sourdoughs used for the manufacture of traditional Italian sweet leavened baked goods. Int. J. Food Microbiol. 2013, 163, 71–79. [Google Scholar] [CrossRef] [PubMed]
- America Association of Cereals and Grains. AACC Approved Methods of Analysis-11 Edition. Available online: https://methods.aaccnet.org/default.aspx (accessed on 19 November 2017).
- McCleary, B.V.; De Vries, J.W.; Rader, J.I.; Cohen, G.; Prosky, L.; Mugford, D.C.; Champ, M.; Okuma, K. Determination of Total Dietary Fiber (CODEX Definition) by Enzymatic-Gravimetric Method and Liquid Chromatography: Collaborative Study. J. AOAC Int. 2010, 93, 221–233. [Google Scholar] [CrossRef] [Green Version]
- Bello, F.D.; Clarke, C.I.; Ryan, L.A.M.; Ulmer, H.; Schober, T.J.; Ström, K.; Sjögren, J.; Van Sinderen, D.; Schnürer, J.; Arendt, E.K. Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. J. Cereal Sci. 2007, 45, 309–318. [Google Scholar] [CrossRef]
- El-Sohaimy, S.A.; Shehata, M.G.; Mehany, T.; Zeitoun, M.A. Nutritional, Physicochemical, and Sensorial Evaluation of Flat Bread Supplemented with Quinoa Flour. Int. J. Food Sci. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Jiménez, B.; Torres-Vargas, O.L.; Rodríguez-García, M.E. Physicochemical characterization of quinoa (Chenopodium quinoa) flour and isolated starch. Food Chem. 2019, 298, 124982. [Google Scholar] [CrossRef]
- Cunniff, P.A.; AOAC. Official Methods of Analysis of AOAC International. Assoc. Off. Anal. Chem. Int. 1998, 2, 2–66. [Google Scholar]
- Poutanen, K.; Flander, L.; Katina, K. Sourdough and cereal fermentation in a nutritional perspective. Food Microbiol. 2009, 26, 693–699. [Google Scholar] [CrossRef]
- Azizi, S.; Azizi, M.H.; Moogouei, R.; Rajaei, P. The effect of Quinoa flour and enzymes on the quality of gluten-free bread. Food Sci. Nutr. 2020, 8, 2373–2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, G.S.; Zillman, R.R.; Eskin, N.A.M. Dough mixing and breadmaking properties of quinoa-wheat flour blends. Int. J. Food Sci. Technol. 1992, 27, 701–705. [Google Scholar] [CrossRef]
- Welman, A.D.; Maddox, I.S. Exopolysaccharides from lactic acid bacteria: Perspectives and challenges. In Trends in Biotechnology; Elsevier Ltd.: Amsterdam, The Netherlands, 2003; Volume 21, pp. 269–274. [Google Scholar]
- Schawb, C.; Mastrangelo, M.; Cordsdetti, A.; Gänzle, M.G. Formation of oligosaccharides and polysaccharides by Lactobacillus reuteri LTH5448 and Weisella cibardia 10 M in sorghum sourdough. Cereal Chem. 2008, 85, 679–684. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Rizzello, C.G. Design of a ‘Clean-Label’ Gluten-Free Bread to Meet Consumers Demand. Foods 2021, 10, 462. [Google Scholar] [CrossRef]
- Coda, R.; di Cagno, R.; Gobbetti, M.; Rizzello, C.G. Sourdough lactic acid bacteria: Exploration of non-wheat cereal-based fermentation. Food Microbiol. 2014, 37, 51–58. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Zannini, E.; Arendt, E.K. Germination of Oat and Quinoa and Evaluation of the Malts as Gluten Free Baking Ingredients. Plant Foods Hum. Nutr. 2013, 68, 90–95. [Google Scholar] [CrossRef]
- de Meo, B.; Freeman, G.; Marconi, O.; Booer, C.; Perretti, G.; Fantozzi, P. Behaviour of malted cereals and pseudo-cereals for gluten-free beer production. J. Inst. Brew. 2011, 117, 541–546. [Google Scholar] [CrossRef]
- Cappelli, A.; Oliva, N.; Cini, E. A systematic review of gluten-free dough and bread: Dough rheology, bread characteristics, and improvement strategies. Appl. Sci. 2020, 10, 6559. [Google Scholar] [CrossRef]
- Kozioł, M.J. Chemical composition and nutritional evaluation of quinoa (Chenopodium quinoa Willd.). J. Food Compos. Anal. 1992, 5, 35–68. [Google Scholar] [CrossRef]
- Zannini, E.; Jones, J.M.; Renzetti, S.; Arendt, E.K. Functional Replacements for Gluten. Annu. Rev. Food Sci. Technol. 2012, 3, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Rehal, J.; Kaur, A.; Jyot, G. Enhancement of Attributes of Cereals by Germination and Fermentation: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1575–1589. [Google Scholar] [CrossRef]
- Adebiyi, J.A.; Obadina, A.O.; Adebo, O.A.; Kayitesi, E. Comparison of nutritional quality and sensory acceptability of biscuits obtained from native, fermented, and malted pearl millet (Pennisetum glaucum) flour. Food Chem. 2017, 232, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Pontonio, E.; Dingeo, C.; Gobbetti, M.; Rizzello, C.G. Maize milling by-products: From food wastes to functional ingredients through lactic acid bacteria fermentation. Front. Microbiol. 2019, 10, 561. [Google Scholar] [CrossRef] [Green Version]
- Thiele, C.; Gänzle, M.G.; Vogel, R.F. Contribution of sourdough lactobacilli, yeast, and cereal enzymes to the generation of amino acids in dough relevant for bread flavor. Cereal Chem. 2002, 79, 45–51. [Google Scholar] [CrossRef]
- Katina, K.; Poutanen, K.; Autio, K. Influence and interactions of processing conditions ans starter culture on formation of acids, volatile compounds, and amino acids in wheat sourdoughs. Cereal Chem. 2002, 81, 598–610. [Google Scholar]
- Celus, I.; Brijs, K.; Delcour, J.A. The effects of malting and mashing on barley protein extractability. J. Cereal Sci. 2006, 44, 203–211. [Google Scholar] [CrossRef]
- Motta, C.; Castanheira, I.; Gonzales, G.B.; Delgado, I.; Torres, D.; Santos, M.; Matos, A.S. Impact of cooking methods and malting on amino acids content in amaranth, buckwheat and quinoa. J. Food Compos. Anal. 2019, 76, 58–65. [Google Scholar] [CrossRef]
- Dallagnol, A.; Pescuma, M.; Rollán, G.; Torino, M.; Valdez, G. Optimization of lactic ferment with quinoa flour as bio-preservative alternative for packed bread. Appl. Microbiol. Biotechnol. 2015, 99, 3839–3849. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Gobbetti, M.; Giuseppe, C. Investigation of the nutritional, functional and technological e ff ects of the sourdough fermentation of sprouted flours. Int. J. Food Microbiol. 2019, 302, 47–58. [Google Scholar] [CrossRef]
- Guarniz-Benites, J.; Valdez-Arana, J.D.C. Morphological identification of mycotoxigenic fungi in accessions of quinoa (Chenopodium quinoa Wild.) of the peruvian coast and sierra. Sci. Agropecu. 2019, 10, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Alencar, N.M.M.; de Morais, E.C.; Steel, C.J.; Bolini, H.M.A. Sensory characterisation of gluten-free bread with addition of quinoa, amaranth flour and sweeteners as an alternative for coeliac patients. Int. J. Food Sci. Technol. 2017, 52, 872–879. [Google Scholar] [CrossRef]



| % Dried Weight | ||||
|---|---|---|---|---|
| Ingredients | Control | QF | GQ | QSD |
| Flours | ||||
| Refined rice flour | 50 | 30 | 30 | 30 |
| Whole quinoa flour | - | 20 | - | - |
| Germinated quinoa flour | - | - | 20 | - |
| Starches | ||||
| Corn Starch | 25 | 25 | 25 | 25 |
| Potato Starch | 25 | 25 | 25 | 25 |
| Other | ||||
| Xhantan gum | 2 | 2 | 2 | 2 |
| Salt | 2 | 2 | 2 | 2 |
| Ferment | ||||
| Dried baker’s yeast | 0.5 | 0.5 | 0.5 | 0.5 |
| Sourdough | - | - | - | 30 |
| Water | 100 | 100 | 100 | 80 |
| Parameter (%) | Control | QF | GQ |
|---|---|---|---|
| Moisture | 14.3 ± 0.31 a | 13.71 ± 0.87 b | 13.76 ± 0.45 b |
| Protein | 9.75 ± 0.24 b | 14.8 ± 0.57 a | 15.3 ± 0.18 a |
| Lipid | 0.61 ± 0.03 b | 5.22 ± 1.01 a | 5.55 ± 0.41 a |
| Ash | 0.14 ± 0.01 c | 2.00 ± 0.29 a | 2.55 ± 0.28 b |
| Carbohydrate | 89.5 ± 2.31 a | 77.9 ± 1.52 b | 76.4 ± 2.45 b |
| 4.03 ± 1.05 c | 10.5 ± 0.11 a | 9.15 ± 0.21 b |
| 2.17 ± 0.02 b | 3.15 ± 0.05 a | 0.85 ± 0.01 c |
| 1.86 ± 1.06 c | 7.31 ± 0.13 b | 8.30 ± 0.06 a |
| Time (h) | LAB Count (log CFU/g) | pH |
|---|---|---|
| 0 | 6.01 ± 0.01 | 6.01 ± 0.01 |
| 2 | 6.11 ± 0.01 | 6.19 ± 0.03 |
| 4 | 6.43 ± 0.08 | 6.01 ± 0.02 |
| 6 | 6.94 ± 0.10 | 5.63 ± 0.07 |
| 8 | 7.29 ± 0.14 | 4.94 ± 0.13 |
| 10 | 7.62 ± 0.09 | 4.33 ± 0.22 |
| 12 | 7.93 ± 0.10 | 3.93 ± 0.11 |
| 14 | 8.22 ± 0.17 | 3.91 ± 0.10 |
| 16 | 8.24 ± 0.16 | 3.88 ± 0.09 |
| Characteristic | Value |
|---|---|
| LAB count (log CFU/g) | 8.40 ± 0.01 |
| pH | 3.88 ± 0.09 |
| TTA (mL of NaOH) | 17.5 ± 0.89 |
| Lactic acid (mM) | 18.4 ± 2.35 |
| Acetic acid (mM) | 21.6 ± 1.17 |
| FQ | 8.52 ± 0.75 |
| Moisture (% d.m) | 11.2 ± 0.03 |
| Protein (% d.m) | 14.5 ± 0.04 |
| Lipid (% d.m) | 5.47 ± 0.03 |
| Carbohydrate (% d.m) | 70.1 ± 1.13 |
| Soluble fiber (% d.m) | 4.50 ± 0.20 |
| Insoluble fiber (% d.m) | 5.50 ± 0.13 |
| Ash (%) | 2.19 ± 0.02 |
| Characteristic | Bread Type | |||
|---|---|---|---|---|
| Control | QF | GQ | QSD | |
| Color crust | ||||
| L * | 60.7 ± 1.13 c | 61.0 ± 3.62 c | 70.0 ± 3.23 b | 71.6 ± 3.94 a |
| a * | 12.7 ± 2.34 a | 13.2 ± 1.76 a | 8.33 ± 1.10 b | 7.29 ± 1.47 b |
| b * | 31.89 ± 0.35 b | 33.65 ± 3.43 a | 32.54 ± 3.50 a | 32.42 ± 2.10 a |
| Color crumb | ||||
| L * | 69.9 ± 1.54 c | 72.2 ± 3.07 b | 73.10± 3.86 a | 72.8 ± 2.64 a |
| a * | 0.22 ± 0.21 a | 0.25 ± 0.19 a | 0.01 ± 0.24 b | 0.01 ± 0.46 b |
| b * | 12.8 ± 1.65 d | 15.1 ± 1.14 c | 17.1 ± 1.85 a | 16.6 ± 0.67 b |
| Texture | ||||
| Hardness (N) | 22.3 ± 3.24 d | 59.9 ± 4.06 a | 35.6 ± 4.67 b | 21.1 ± 4.89 c |
| Bake loss (%) | 9.04 ± 1.32 c | 9.05 ± 1.57 c | 9.2 ± 1.28 b | 10.5 ± 1.63 a |
| Chemical composition | ||||
| pH | 6.14 ± 0.01 a | 6.09 ± 0.04 a | 5.62 ± 0.09 b | 4.47 ± 0.03 c |
| TTA (mL NaOH) | 2.01 ± 0.04 c | 2.13 ± 0.06 c | 3.32 ± 0.06 b | 8.51 ± 0.05 a |
| Moisture (%) | 41.5 ± 2.31 a | 41.6 ± 1.74 a | 40.6 ± 1.28 a | 41.6 ± 2.89 a |
| Protein (%) | 2.70 ± 0.12 c | 9.61 ± 0.51 b | 9.77 ± 0.50 a | 9.82 ± 0.23 a |
| Lipid (%) | 0.34 ± 0.34 c | 0.58 ± 0.19 b | 0.78 ± 0.13 a | 0.62 ± 0.12 b |
| Ash (%) | 2.76 ± 0.12 b | 2.97 ± 0.24 a | 2.66 ± 0.36 b | 2.87 ± 0.29 a |
| Carbohydrate (%) | 57.9 ± 0.45 b | 58.8 ± 1.15 a | 59.1 ± 1.34 a | 58.2 ± 2.77 b |
| Sensory Attribute | RF | GQF | QSD | F | p-Value |
|---|---|---|---|---|---|
| Elasticity | 4.85 ± 0.11 | 6.02 ± 0.32 | 6.46 ± 0.09 | 5.82 | 3.00 × 10−3 |
| Crust color | 6.62 ± 1.01 | 4.71 ± 0.24 | 4.83 ± 0.18 | 16.1 | 2.62 × 10−7 |
| Crumb color | 4.35 ± 0.55 | 4.00 ± 0.33 | 3.61 ± 0.08 | 2.39 | 9.30 × 10−2 |
| Acid smell | 2.98 ± 1.12 | 3.27 ± 0.11 | 4.86 ± 0.10 | 12.9 | 4.62 × 10−6 |
| Acid taste | 2.01 ± 0.93 | 2.50 ± 0.05 | 2.81 ± 0.07 | 33.1 | 1.54 × 10−13 |
| Sweet taste | 1.93 ± 0.12 | 1.88 ± 0.32 | 1.21 ± 0.32 | 4.04 | 1.90 × 10−2 |
| Salty taste | 3.11 ± 0.10 | 3.15 ± 0.09 | 3.24 ± 0.38 | 0.08 | 9.30 × 10−1 |
| Sour taste | 1.29 ± 0.05 | 1.07 ± 0.02 | 2.17 ± 0.07 | 7.07 | 1.00 × 10−3 |
| Crumb dryness | 3.67 ± 0.23 | 6.68 ± 0.11 | 6.36 ± 0.09 | 34.7 | 3.94 × 10−14 |
| Aroma | 4.89 ± 0.59 | 4.45 ± 0.10 | 5.48 ± 0.05 | 3.99 | 1.90 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, W.; Evert, K.; Van Nieuwenhove, C. Quinoa Flour, the Germinated Grain Flour, and Sourdough as Alternative Sources for Gluten-Free Bread Formulation: Impact on Chemical, Textural and Sensorial Characteristics. Fermentation 2021, 7, 115. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation7030115
Franco W, Evert K, Van Nieuwenhove C. Quinoa Flour, the Germinated Grain Flour, and Sourdough as Alternative Sources for Gluten-Free Bread Formulation: Impact on Chemical, Textural and Sensorial Characteristics. Fermentation. 2021; 7(3):115. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation7030115
Chicago/Turabian StyleFranco, Wendy, Katherine Evert, and Carina Van Nieuwenhove. 2021. "Quinoa Flour, the Germinated Grain Flour, and Sourdough as Alternative Sources for Gluten-Free Bread Formulation: Impact on Chemical, Textural and Sensorial Characteristics" Fermentation 7, no. 3: 115. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation7030115






