Effect of Lactic Acid Fermentation on Legume Protein Properties, a Review
Abstract
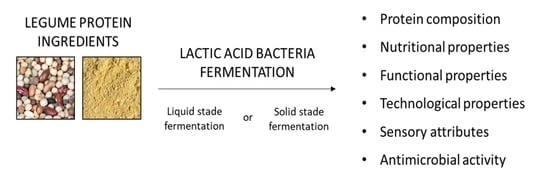
:1. Introduction
2. Lactic Acid Bacteria
3. Legume Proteins
4. The Effects of Applied Fermentation Techniques on Legume Products
5. Effect of Fermentation on Legume Protein Composition
6. Effect of Lactic Acid Fermentation on Nutritional Properties of Legume Protein
6.1. Protein Digestibility
6.2. Antinutritional Compounds
6.3. Antioxidant Activity
6.4. Other Vitamins and Bioactive Compounds
6.5. Allergenicity
7. Effect of Fermentation on the Functional Properties of Legume Protein
8. Effect of Fermentation on the Physicochemical and Technological Properties of Legume Protein
8.1. Protein Solubility
8.2. Charge of Proteins and Hydrophobicity
8.3. Water-Holding Capacity and Oil-Holding Capacity
8.4. Emulsifying Properties
8.5. Foaming Properties
8.6. Sourdough Preparation and Bread-Making Properties
8.7. Fermented Plant-Based Products
| Legume Type | Protein Ingredients Treated | LAB Strains | Techno-Functional Properties | References | |||
|---|---|---|---|---|---|---|---|
| Protein Solubility | Emulsifying Properties | Foaming Properties | Surface and Bulk Properties | ||||
| Chickpea | Flour | W. paramesenteroides Lb. plantarum | _ | EC increased | _ | WHC and OHC both increased Gel formation increased | [31] |
| Protein concentrate | P. pentosaceus P. acidilactici | _ | _ | FC decreased | _ | [10] | |
| Soybean | Flour | Lb. plantarum Lb. rhamnosus Lactobacillus nantensis Lb. fermentum Lb. reuteri P. acidilactici Lb. brevis | _ | EC increased | _ | WHC decreased OHC increased Gelation capacity decreased | [163] |
| Protein isolate | Lb. helveticus | Decreased at pH 7 Increased at pH 4 | EA decreased | FA increased FD decreased FS increased | WHC and OHC both increased | [53] | |
| Protein isolate | Lb. plantarum | Decreased at pH 7 | _ | _ | SH increased | [33] | |
| Pea | Pea protein-enriched | Lb. plantarum | _ | EA decreased Lower EA at pH 7 ES decreased at pH 4 | FC increased at pH 4 FC not changed at pH 7 | Surface charge decreased SH increased at pH 4 and decreased at pH 7 WHC and OHC changed with time but not pH | [150] |
| Protein isolate | Lb. plantarum Lb. fermentum Lb. casei Lc. mesenteroides P. pentosaceus Lb. perolens | Increased at pH 5 but decreased at pH 3, 7, and 8 | EC decreased The highest EC for Lb. plantarum The lowest EC for Lb. perolens EC increased for Lb. casei and Lc. cremoris after 48 h | Unable to form foam | _ | [147] | |
| Protein isolate | Lb. plantarum | Decreased | No differences | FS decreased No differences in FC | OHC increased WHC decreased | [154] | |
| Lupine | Protein concentrate | co-culture of Lc. Mesenteroides Lb. plantarum Lb. brevi | _ | Small effect on EA Decrease in the emulsifying properties | _ | Higher SH for samples with hulls SH decreased WHC increased | [59] |
| Protein isolate | P. pentosaceus | _ | EA increased with time and pH 8 ES increased with pH but was not affected by time | FC increased at pH 8 and 48 h FS increased at pH 8 compared to pH 6 | _ | [3] | |
| Protein isolate | Lb. Reuteri Lb. brevis Lb. amylolyticus Lb. parabuchneri Lb. sakei Lb. helveticus Lb. delbrueckii | Decreased at pH 7 No difference at pH 4 | Highest EC for Lb. parabuchneri and lowest for Lb. parabuchneri, EC decreased | FA increased FS increased in all strains except for Lb. parabuchneri and Lb. helveticus | _ | [60] | |
9. Effect of Lactic Acid Fermentation on the Sensory Attributes of Legumes
| Legume Type | Protein Ingredients Treated | LAB Strains (Addition of Sugar) | Sensorial Profile | References | |||
|---|---|---|---|---|---|---|---|
| Sensorial Attributes | Aromatic Related to Proteolysis Compounds | Aromatic Related to Glycolysis Compounds | Aromatic Related to Fatty Acid Compounds | ||||
| Soybean | Protein isolate | Lb. helveticus (No sugar) | Decrease in beany, bitter, mouthcoating, and astringent properties Increase in sour, tangy lactic acid taste and bitterness Better sensory results after 24 h rather than 48 h | Degradation of peptides rich in proline and leucine Degradation of bitter peptides | _ | Degradation of isopentanol, n-hexanal, and hexanol | [53] |
| Milk | Lb. pentosus Lb. plantarum (No sugar) | Slight sweet taste and good texture properties | _ | _ | _ | [182] | |
| Milk | Lb. acidophilus Lb. casei S. thermophilus Lb. delbrueckii (No sugar) | Reduction in beany flavor | _ | Decrease in methanol, acetaldehyde, and ethanol | Decrease in hexanal | [173] | |
| Juice | Leuconostoc Lactobacillus Lactococcous Streptococcous (No sugar) | Nuts, soy, fresh, caramel, and hay descriptors for S. thermophilus Acid, sour, floral, pineapple, spicy, cheesy, kefir, and sorrel descriptors for Lb. plantarum Lb. pentosus was described as “plastic” Soy sauce, black bread, cabbage, salty, and broth descriptors for Lc. lactis Lb. acidophilus had a “goat” odor Lb. lactis had a “cabbage” and/or a “broth” odor Lb. lactis, Lb. plantarum, had “floral” odors | _ | _ | Increase in aldehydes, carbonyl, and alcohol for S. thermophilus and L. delbrueckii Increase in pentane-2,3-dione, heptane-2,3-dione, methyl acetate, and ethyl acetate for S. thermophilus and Lb. delbrueckii Increase in 2,4-dimethylbenzaldehyde for S. thermophilus Lb. plantarum, Lb. pentosus, Lb. coryniformis, and Lb. lactis produced four acids (acetic, butanoic, pentanoic, and hexanoic acids), two carbonyl compounds (1-hydroxypropan-2-one and 3-hydroxybutan-2-one), and two alcohols (2-methylpropan-1-ol and ethanol) | [9] | |
| Faba bean | Flour | Lb. plantarum (No sugar) | Increase in pungent odor and flavor | _ | _ | _ | [39] |
| Flour | Lb. plantarum | Crumb flavor | _ | _ | _ | [55] | |
| Pea | Protein isolate | Lb. plantarum Lb. perolens Lb. fermentum, Lactobacillus Lb. casei L. mesenteroides Pediococcus P. pentosaceus (0.5% Glucose) | Better aroma after 48 h compared to 24 h Decrease in bitter and astringent attributes Lowest pea-like aroma after 24 h Lb. plantarum for 24 h also masked green and earthy notes Increase in buttery aroma for Lb. perolens for 24 h Increase in floury attribute for P. pentosaceus Fecal aroma for Lb. fermentum after 48 h Intense cheesy aroma for Lc. cremoris after 48 h Decrease in bitter intensity for Lb. plantarum and Lc. cremoris after 24 h Increase in bitter and acid tastes for Lb. perolens | Increase in undesirable compounds such as p-cresol, indole, and skatole for Lb. fermentum after 48 h | Increase in diacetyl for Lb. perolens | _ | [147] |
| Protein isolate | Lb. plantarum | Lower color intensity, beany aroma, beany flavor, and lower amount of bitterness | _ | _ | Decrease in aldehydes and ketones Increase in alcohol | [154] | |
| Protein isolate | Co-culture Lb. acidophilus, S. thermophilus Lb. delbrueckii B. lactis (3% Sucrose) | Decrease “beer/yeast” notes | _ | _ | Presence of ester Increase in alcohol Decrease in aldehydes, ketones, and furans Presence of (E)-2-heptenal, 6-methyl-5-hepten-2-one, and trans-2-methyl-2-butenal Decrease in 2-pentyl-furan and 2-ethyl-furan | [167] | |
| Protein isolate | Lb. plantarum P. pentosaceus (No sugar) | Pleasant odor with weak milky attributes | 1-Pyrroline with a sperm-like odor produced from the degradation or oxidation of proline, spermine, spermidine, or putrescine | _ | No difference in the content of n-hexanal between strainsN-hexanal concentration reduced during fermentation and no negative effect on storage stability The pungent/cheese-like and floral/rose-like attributes in fermented samples were identified as butan-2-one and as β-damascenone, respectively During storage, slight increase in n-hexanal Presence of β-damascenone and butan-2-one, n-hexanal, n-botanal, and dimethyl trisulfid | [176] | |
| Lupine | Flour | P. pentosaceus (No sugar) | Intensive taste and acidity | _ | _ | _ | [3] |
| Protein isolate | Co-culture Lb. casei Lb. plantarum Lb. paracasei Lc. mesenteroides Lc. lactis S. thermophilus Lb. delbrueckii Streptococcus thermophilus Lb. delbruecki Lb. Acidophilus Bifidobacterium animalis Lc. lactis Leuconostoc pseudomesenteroides P. pentosaceus Lc. lactis Lb. plantarum (No sugar) | Increase in 3-methyl-1-butanol) Increase in alcohol compounds such as 3-methyl-1-butanol | Increase in 1-Nonen-2-ol | Increase in hexanal Increase in the content of acetic acid and hexanoic acid, responsible for sour and sweat odors Increase in 1-octen-3-ol | [183] | ||
| Protein isolate | Lb. reuteri Lb. brevis Lb. amylolyticus Lb. parabuchneri Lb. sakei Lb. helveticus Lb. delbrueckii (0.5% Glucose) | Increase in aroma perception of cheesy, roasty, and popcorn-like notes for Lb. brevis and Lb. amyloslyticus Lb. reuteri for cheesy, fatty, and oatmeal-like Lb. brevis, cheesy and oatmeal-like Lb. amylolyticus popcorn-like and roasty Lb. parabuchneri pea-like, green bell pepper, and cheesy Lb. sakei popcorn-like and roasty Lb. helvticus roasty and popcorn-like Lb. delbrukei oatmeal-like and fatty | _ | _ | Reduction of n-hexanal | [60] | |
| Protein isolate | Lb. Plantarum P. pentosaceus (No sugar) | Sweet, solvent, and fungal but also musty, earthy, burnt, dusty, or cereal-like odor | Presence of 1-pyrroline, which is known as the Strecker degradation product of proline | _ | Presence of hexanal Decrease in alcohol and aldehydes such as n-hexanal Decrease in lipid degradation compounds such as n-pentanal, n-heptanal, 1-octen-3-ol, and 2-pentylfuran Presence of 1-Octen-3-ol, which is the product of fatty acid degradation | [177] | |
| Protein isolate | Lb. sakei Lb. amylolyticus Lb. helveticus (0.5% Glucose) | Increase in intensity and aroma perception (cocoa-like and malty) Increase in bitter intensity Higher intensity of saltiness for Lb. helveticus | _ | _ | _ | [184] | |
| Mung beans | Seed | Lb. plantarum (No sugar) | More fragrant odor Stronger odor of grass and fat, related to the high content of aldehydes | Disappearance of nonanal, 5-methyl-2-formylthiophene, and phenylacetaldehyde | Increase in 2,3-butanediol Increase in ester Increase in isoamyl acetate and ethyl acetate | Decrease in alcohols (hexanol, 3-methyl-3-buten-1-ol, and (E)-2-hexen-1-ol) and aldehydes (nonanal, octanal, 2-furfural, and 3-methylbutanal) Decrease in hexanal, hexanol, and 1-octen-3-ol Increase in the content of acids Increase in the content of ketones Increase in 2-propanone and 3-hydroxy-2-butanone Majority of volatile flavors were ethyl hexanoate, heptanal, and butanal | [185] |
| Pea + cow protein | Protein isolate | Lb. delbrueckii S. thermophilous Lb. acidophilus Lb. helveticus Lb. casei Lb. rhamnosus Lb. fermentum (No sugar) | Lb. delbrueckii + Lb. fermentum, S. thermophilus + Lb. rhamnosus, Lb. rhamnosus have higher intensities for positive descriptors such as creamy, dairy, and sweet and lower intensities for negative descriptors such as vegetal, earth, and vinegar. Lb. delbrueckii + Lb. helveticus, Lb. delbrueckii + Lb. rhamnosus, S. thermophilus + Lb. helveticus: higher intensities for negative descriptors such as acid and astringent but rather low intensities for the negative descriptors pea and earth. | _ | _ | _ | [158] |
| Pea (P), Pea + milk (PM) | Protein isolate + milk protein | Lb. casei Lb. plantarum Lb. rhamnosus Lactococcus lactis, Leuconostoc lactis W. cibaria (No sugar) | Increase in fruity and flowery notes related to the presence of Lb. plantarum Increase in sweety and creamy descriptors | Proteolysis of pea vicilin by LAB strains, leading to roasted/grilled notes | Decrease in hexanal and heptanal Production of 3-methyl-1-butanol in the mixed emulsion and 2-methylpropanal and 2-butanone in the pea protein isolate emulsion | [168] | |
| Protein isolate | Microbial communities including some bacteria, yeast, and Lc. lactis Leuconostoc lactis Lb. rhamnosus (No sugar) | _ | Formation of 3-methylbutanal and benzaldehyde, which are responsible for chocolate and roasted coffee notes | Formation of butane-2,3-dione (=diacetyl) (for PM), pentan-2-one, 2-butan-2one, and 3-methylbutan-2-one (for P), which are responsible for buttery and creamy flavors | Elimination of aldehydes responsible for green notes, i.e., hexanal, heptanal, nonanal, octanal, and (E)-2-ethylbut-2-enal Increase in aldehydes responsible for grilled and roasted note, i.e., (2E,4E)-hepta-2,4-dienal, 3-methybutanal, and 2-methylpropanal Formation of other aromatic hydrocarbons, including toluene, benzene, and 2pentylfuran | [171] | |
10. Antimicrobial Activity of Lactic Acid Bacteria on Legume Protein
11. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Steinkraus, K.H. Handbook of Indigenous Fermented Foods, Revised and Expanded, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Steinkraus, K.H. Fermentations in world food processing. Compr. Rev. Food Sci. Food Saf. 2002, 1, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Klupsaite, D.; Juodeikiene, G.; Zadeike, D.; Bartkiene, E.; Maknickiene, Z.; Liutkute, G. The influence of lactic acid fermentation on functional properties of narrow-leaved lupine protein as functional additive for higher value wheat bread. LWT 2017, 75, 180–186. [Google Scholar] [CrossRef]
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Diversity of microorganisms in global fermented foods and beverages. Front. Microbiol. 2016, 7, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rui, X.; Wang, M.; Zhang, Y.; Chen, X.; Li, L.; Liu, Y.; Dong, M. Optimization of soy solid-state fermentation with selected lactic acid bacteria and the effect on the anti-nutritional components. J. Food Process. Preserv. 2017, 41, e13290. [Google Scholar] [CrossRef]
- Bartkiene, E.; Krungleviciute, V.; Juodeikiene, G.; Vidmantiene, D.; Maknickiene, Z. Solid state fermentation with lactic acid bacteria to improve the nutritional quality of lupin and soya bean. J. Sci. Food Agric. 2015, 95, 1336–1342. [Google Scholar] [CrossRef] [Green Version]
- Ayivi, R.; Gyawali, R.; Krastanov, A.; Aljaloud, S.; Worku, M.; Tahergorabi, R.; Da Silva, R.; Ibrahim, S. Lactic acid bacteria: Food safety and human health applications. Dairy 2020, 1, 202–232. [Google Scholar] [CrossRef]
- Pereira, G.V.M.; De Carvalho Neto, D.P.; Junqueira, A.C.D.O.; Karp, S.G.; Letti, L.A.; Magalhães Júnior, A.I.; Soccol, C.R. A review of selection criteria for starter culture development in the food fermentation industry. Food Rev. Int. 2020, 36, 135–167. [Google Scholar] [CrossRef]
- Harlé, O.; Falentin, H.; Niay, J.; Valence, F.; Courselaud, C.; Chuat, V.; Maillard, M.-B.; Guédon, É.; Deutsch, S.-M.; Thierry, A. Diversity of the metabolic profiles of a broad range of lactic acid bacteria in soy juice fermentation. Food Microbiol. 2020, 89, 103410. [Google Scholar] [CrossRef]
- Xing, Q.; Dekker, S.; Kyriakopoulou, K.; Boom, R.M.; Smid, E.J.; Schutyser, M.A. Enhanced nutritional value of chickpea protein concentrate by dry separation and solid state fermentation. Innov. Food Sci. Emerg. Technol. 2020, 59, 102269. [Google Scholar] [CrossRef]
- Reuben, R.; Roy, P.; Sarkar, S.; Alam, A.R.U.; Jahid, I. Characterization and evaluation of lactic acid bacteria from indigenous raw milk for potential probiotic properties. J. Dairy Sci. 2020, 103, 1223–1237. [Google Scholar] [CrossRef]
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS microbiol. 2018, 4, 665. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Tsai, S.-P.; Bonsignore, P.; Moon, S.-H.; Frank, J.R. Technological and economic potential of poly (lactic acid) and lactic acid derivatives. FEMS Microbiol. Rev. 1995, 16, 221–231. [Google Scholar] [CrossRef]
- Trontel, A.; Batušić, A.; Gusić, I.; Slavica, A.; Šantek, B.; Novak, S. Production of D-and L-lactic acid by mono-and mixed cultures of Lactobacillus sp. Food Technol. Biotechnol. 2011, 49, 75–82. [Google Scholar]
- Müller, V. Bacterial fermentation. In eLS; John Wiley & Sons Ltd.: Chichester, UK, 2001. [Google Scholar]
- Gänzle, M.G.; Follador, R. Metabolism of oligosaccharides and starch in lactobacilli: A review. Front. Microbiol. 2012, 3, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semba, R.D.; Ramsing, R.; Rahman, N.; Kraemer, K.; Bloem, M.W. Legumes as a sustainable source of protein in human diets. Global Food Security 2021, 28, 100520. [Google Scholar] [CrossRef]
- Lam, A.C.Y.; Can Karaca, A.; Tyler, R.T.; Nickerson, M.T. Pea protein isolates: Structure, extraction, and functionality. Food Rev. Int. 2018, 34, 126–147. [Google Scholar] [CrossRef]
- Crévieu, I.; Berot, S.; Guéguen, J. Large scale procedure for fractionation of albumins and globulins from pea seeds. Food/Nahrung 1996, 40, 237–244. [Google Scholar] [CrossRef]
- Boye, J.; Aksay, S.; Roufik, S.; Ribéreau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res. Int. 2010, 43, 537–546. [Google Scholar] [CrossRef]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef]
- Díaz-Batalla, L.; Widholm, J.M.; Fahey, G.C.; Castaño-Tostado, E.; Paredes-López, O. Chemical components with health implications in wild and cultivated Mexican common bean seeds (Phaseolus vulgaris L.). J. Agric. Food Chem. 2006, 54, 2045–2052. [Google Scholar] [CrossRef]
- Garrido-Galand, S.; Asensio-Grau, A.; Calvo-Lerma, J.; Heredia, A.; Andrés, A. The potential of fermentation on nutritional and technological improvement of cereal and legume flours: A review. Food Res. Int. 2021, 145, 110398. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Liu, X.; Kokare, C. Microbial Enzymes of Use in Industry. In Biotechnology of Microbial Enzymes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 267–298. [Google Scholar]
- Limón, R.I.; Peñas, E.; Torino, M.I.; Martínez-Villaluenga, C.; Dueñas, M.; Frias, J. Fermentation enhances the content of bioactive compounds in kidney bean extracts. Food Chem. 2015, 172, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Orozco, R.; Frias, J.; Muñoz, R.; Zielinski, H.; Piskula, M.K.; Kozlowska, H.; Vidal-Valverde, C. Fermentation as a bio-process to obtain functional soybean flours. J. Agric. Food Chem. 2007, 55, 8972–8979. [Google Scholar] [CrossRef]
- Torino, M.I.; Limón, R.I.; Martínez-Villaluenga, C.; Mäkinen, S.; Pihlanto, A.; Vidal-Valverde, C.; Frias, J. Antioxidant and antihypertensive properties of liquid and solid state fermented lentils. Food Chem. 2013, 136, 1030–1037. [Google Scholar] [CrossRef] [Green Version]
- Skrzypczak, K.; Jabłońska-Ryś, E.; Gustaw, K.; Sławińska, A.; Waśko, A.; Radzki, W.; Michalak-Majewska, M.; Gustaw, W. Reinforcement of the Antioxidative Properties of Chickpea Beverages Through Fermentation Carried Out by Probiotic Strain Lactobacillus plantarum 299v. J. Pure. Appl. Microbiol. 2019, 13, 01–12. [Google Scholar] [CrossRef] [Green Version]
- Adeyemo, S.; Onilude, A. Enzymatic reduction of anti-nutritional factors in fermenting soybeans by Lactobacillus plantarum isolates from fermenting cereals. Niger. Food J. 2013, 31, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Sáez, G.D.; Sabater, C.; Fara, A.; Zárate, G. Fermentation of chickpea flour with selected lactic acid bacteria for improving its nutritional and functional properties. J. Appl. Microbiol. 2021. [Google Scholar] [CrossRef]
- Niyibituronasa, M.; Onyango, A.; Gaidashova, S.; Imathiu, S.; De Boevre, M.; Leenknecht, D.; Neyrinck, E.; De Saeger, S.; Vermeir, P.; Raes, K. The growth of different probiotic microorganisms in soymilk from different soybean varieties and their effects on antioxidant activity and oligosaccharide content. J. Food Res. 2019, 8, 41–51. [Google Scholar] [CrossRef]
- Rui, X.; Huang, J.; Xing, G.; Zhang, Q.; Li, W.; Dong, M. Changes in soy protein immunoglobulin E reactivity, protein degradation, and conformation through fermentation with Lactobacillus plantarum strains. LWT 2019, 99, 156–165. [Google Scholar] [CrossRef]
- Aguirre, L.; Garro, M.S.; De Giori, G.S. Enzymatic hydrolysis of soybean protein using lactic acid bacteria. Food Chem. 2008, 111, 976–982. [Google Scholar] [CrossRef]
- Coda, R.; Melama, L.; Rizzello, C.G.; Curiel, J.A.; Sibakov, J.; Holopainen, U.; Pulkkinen, M.; Sozer, N. Effect of air classification and fermentation by Lactobacillus plantarum VTT E-133328 on faba bean (Vicia faba L.) flour nutritional properties. Int. J. Food Microbiol. 2015, 193, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Verni, M.; Wang, C.; Montemurro, M.; De Angelis, M.; Katina, K.; Rizzello, C.G.; Coda, R. Exploring the Microbiota of Faba Bean: Functional Characterization of Lactic Acid Bacteria. Front. Microbiol. 2017, 8, 2461. [Google Scholar] [CrossRef] [PubMed]
- Verni, M.; De Mastro, G.; De Cillis, F.; Gobbetti, M.; Rizzello, C.G. Lactic acid bacteria fermentation to exploit the nutritional potential of Mediterranean faba bean local biotypes. Food Res. Int. 2019, 125, 108571. [Google Scholar] [CrossRef]
- Rosa-Sibakov, N.; Re, M.; Karsma, A.; Laitila, A.; Nordlund, E. Phytic Acid Reduction by Bioprocessing as a Tool To Improve the In Vitro Digestibility of Faba Bean Protein. J. Agric. Food Chem. 2018, 66, 10394–10399. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Verni, M.; Koivula, H.; Montemurro, M.; Seppa, L.; Kemell, M.; Katina, K.; Coda, R.; Gobbetti, M. Influence of fermented faba bean flour on the nutritional, technological and sensory quality of fortified pasta. Food Funct. 2017, 8, 860–871. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Losito, I.; Facchini, L.; Katina, K.; Palmisano, F.; Gobbetti, M.; Coda, R. Degradation of vicine, convicine and their aglycones during fermentation of faba bean flour. Sci. Rep. 2016, 6, 32452. [Google Scholar] [CrossRef] [Green Version]
- Pulkkinen, M.; Coda, R.; Lampi, A.-M.; Varis, J.; Katina, K.; Piironen, V. Possibilities of reducing amounts of vicine and convicine in faba bean suspensions and sourdoughs. Eur. Food Res. Technol. 2019, 245, 1507–1518. [Google Scholar] [CrossRef] [Green Version]
- Starzyńska-Janiszewska, A.; Stodolak, B. Effect of inoculated lactic acid fermentation on antinutritional and antiradical properties of grass pea (Lathyrus sativus ‘Krab’) flour. Pol. J. Food Nutr. Sci. 2011, 61, 245–249. [Google Scholar] [CrossRef] [Green Version]
- Cabuk, B.; Nosworthy, M.G.; Stone, A.K.; Korber, D.R.; Tanaka, T.; House, J.D.; Nickerson, M.T. Effect of Fermentation on the Protein Digestibility and Levels of Non-Nutritive Compounds of Pea Protein Concentrate. Food Technol. Biotechnol. 2018, 56, 257–264. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I.; Hu, X. Nutritional quality and techno-functional changes in raw, germinated and fermented yellow field pea (Pisum sativum L.) upon pasteurization. LWT 2018, 92, 147–154. [Google Scholar] [CrossRef]
- Stanisavljevic, N.; Vukotic, G.; Pastor, F.; Suznjevic, D.; Jovanovic, Z.; Strahinic, I.; Fira, D.; Radovic, S. Antioxidant activity of pea protein hydrolysates produced by batch fermentation with lactic acid bacteria. Arch. Biol. Sci. 2015, 67, 1033–1042. [Google Scholar] [CrossRef]
- Czarnecka, M.; Czarnecki, Z.; Nowak, J.; Roszyk, H. Effect of lactic fermentation and extrusion of bean and pea seeds on nutritional and functional properties. Food Nahr. 1998, 42, 7–11. [Google Scholar] [CrossRef]
- Vermeirssen, V.; Van Camp, J.; Decroos, K.; Van Wijmelbeke, L.; Verstraete, W. The impact of fermentation and in vitro digestion on the formation of angiotensin-I-converting enzyme inhibitory activity from pea and whey protein. J. Dairy Sci. 2003, 86, 429–438. [Google Scholar] [CrossRef]
- De Pasquale, I.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Nutritional and functional effects of the lactic acid bacteria fermentation on gelatinized legume flours. Int. J. Food Microbiol. 2020, 316, 108426. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, M.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Investigation of the nutritional, functional and technological effects of the sourdough fermentation of sprouted flours. Int. J. Food Microbiol. 2019, 302, 47–58. [Google Scholar] [CrossRef]
- Fritsch, C.; Vogel, R.F.; Toelstede, S. Fermentation performance of lactic acid bacteria in different lupin substrates—Influence and degradation ability of antinutritives and secondary plant metabolites. J. Appl. Microbiol. 2015, 119, 1075–1088. [Google Scholar] [CrossRef]
- García-Cano, I.; Rocha-Mendoza, D.; Ortega-Anaya, J.; Wang, K.; Kosmerl, E.; Jiménez-Flores, R. Lactic acid bacteria isolated from dairy products as potential producers of lipolytic, proteolytic and antibacterial proteins. Appl. Microbiol. Biotechnol. 2019, 103, 5243–5257. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Li, L. Effects of extracellular proteases and its inhibitors on the gel characteristics of soy protein induced by lactic acid bacteria. Int. J. Food Sci. Tech. 2022, 57, 1587–1597. [Google Scholar] [CrossRef]
- Meinlschmidt, P.; Ueberham, E.; Lehmann, J.; Schweiggert-Weisz, U.; Eisner, P. Immunoreactivity, sensory and physicochemical properties of fermented soy protein isolate. Food Chem. 2016, 205, 229–238. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I.; Hu, X. In vitro digestibility, protein composition and techno-functional properties of Saskatchewan grown yellow field peas (Pisum sativum L.) as affected by processing. Food Res. Int. 2017, 92, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Sozer, N.; Melama, L.; Silbir, S.; Rizzello, C.G.; Flander, L.; Poutanen, K. Lactic acid fermentation as a pre-treatment process for faba bean flour and its effect on textural, structural and nutritional properties of protein-enriched gluten-free faba bean breads. Foods 2019, 8, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raveschot, C.; Cudennec, B.; Coutte, F.; Flahaut, C.; Fremont, M.; Drider, D.; Dhulster, P. Production of Bioactive Peptides by Lactobacillus Species: From Gene to Application. Front. Microbiol. 2018, 9, 2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rui, X.; Zhang, Q.; Huang, J.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Does lactic fermentation influence soy yogurt protein digestibility: A comparative study between soymilk and soy yogurt at different pH. J. Sci. Food Agric. 2019, 99, 861–867. [Google Scholar] [CrossRef]
- Emkani, M.; Oliete, B.; Saurel, R. Pea Protein Extraction Assisted by Lactic Fermentation: Impact on Protein Profile and Thermal Properties. Foods 2021, 10, 549. [Google Scholar] [CrossRef]
- Lampart-Szczapa, E.; Konieczny, P.; Nogala-Kałucka, M.; Walczak, S.; Kossowska, I.; Malinowska, M. Some functional properties of lupin proteins modified by lactic fermentation and extrusion. Food Chem. 2006, 96, 290–296. [Google Scholar] [CrossRef]
- Schlegel, K.; Leidigkeit, A.; Eisner, P.; Schweiggert-Weisz, U. Technofunctional and Sensory Properties of Fermented Lupin Protein Isolates. Foods 2019, 8, 678. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Mung bean (Vigna radiata) as probiotic food through fermentation with Lactobacillus plantarum B1-6. LWT 2015, 63, 445–451. [Google Scholar] [CrossRef]
- Xu, Y.; Coda, R.; Holopainen-Mantila, U.; Laitila, A.; Katina, K.; Tenkanen, M. Impact of in situ produced exopolysaccharides on rheology and texture of fava bean protein concentrate. Food Res. Int. 2019, 115, 191–199. [Google Scholar] [CrossRef]
- Chandra-Hioe, M.V.; Wong, C.H.; Arcot, J. The potential use of fermented chickpea and faba bean flour as food ingredients. Plant Foods Hum. Nutr. 2016, 71, 90–95. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Starkute, V.; Zadeike, D.; Juodeikiene, G. The Nutritional and Safety Challenges Associated with Lupin Lacto-Fermentation. Front Plant Sci. 2016, 7, 951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oomah, B.D.; Caspar, F.; Malcolmson, L.J.; Bellido, A.-S. Phenolics and antioxidant activity of lentil and pea hulls. Int. Food Res. J. 2011, 44, 436–441. [Google Scholar] [CrossRef]
- Gupta, Y. Anti-nutritional and toxic factors in food legumes: A review. Plant Foods Hum. Nutr. 1987, 37, 201–228. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D.; Luc, G.; Leprelle, C.; Drover, J.C.; Harrison, J.E.; Olson, M. Phenolics, phytic acid, and phytase in Canadian-grown low-tannin faba bean (Vicia faba L.) genotypes. J. Agric. Food Chem. 2011, 59, 3763–3771. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.S.; McNeill, V.; Gänzle, M.G. Levansucrase and sucrose phoshorylase contribute to raffinose, stachyose, and verbascose metabolism by lactobacilli. Food Microbiol. 2012, 31, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, W.H. Appropriate starter culture technologies for small-scale fermentation in developing countries. Int. J. Food Microbiol. 2002, 75, 197–212. [Google Scholar] [CrossRef]
- Egounlety, M.; Aworh, O. Effect of soaking, dehulling, cooking and fermentation with Rhizopus oligosporus on the oligosaccharides, trypsin inhibitor, phytic acid and tannins of soybean (Glycine max Merr.), cowpea (Vigna unguiculata L. Walp) and groundbean (Macrotyloma geocarpa Harms). J. Food Eng. 2003, 56, 249–254. [Google Scholar] [CrossRef]
- Coda, R.; Varis, J.; Verni, M.; Rizzello, C.G.; Katina, K. Improvement of the protein quality of wheat bread through faba bean sourdough addition. LWT 2017, 82, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Curiel, J.A.; Coda, R.; Centomani, I.; Summo, C.; Gobbetti, M.; Rizzello, C.G. Exploitation of the nutritional and functional characteristics of traditional Italian legumes: The potential of sourdough fermentation. Int. J. Food Microbiol. 2015, 196, 51–61. [Google Scholar] [CrossRef]
- Robinson, G.; Balk, J.; Domoney, C. Improving pulse crops as a source of protein, starch and micronutrients. Nutr. Bull. 2019, 44, 202–215. [Google Scholar] [CrossRef] [Green Version]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Lai, L.-R.; Hsieh, S.-C.; Huang, H.-Y.; Chou, C.-C. Effect of lactic fermentation on the total phenolic, saponin and phytic acid contents as well as anti-colon cancer cell proliferation activity of soymilk. J. Biosci. Bioeng. 2013, 115, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Fernández, D.; Hernández, T.; Estrella, I.; Muñoz, R. Bioactive phenolic compounds of cowpeas (Vigna sinensis L). Modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC 14917. J. Sci. Food Agric. 2005, 85, 297–304. [Google Scholar] [CrossRef]
- McCue, P.P.; Shetty, K. A model for the involvement of lignin degradation enzymes in phenolic antioxidant mobilization from whole soybean during solid-state bioprocessing by Lentinus edodes. Process Biochem. 2005, 40, 1143–1150. [Google Scholar] [CrossRef]
- Osawa, R.; Kuroiso, K.; Goto, S.; Shimizu, A. Isolation of tannin-degrading lactobacilli from humans and fermented foods. Appl. Environ. Microbiol. 2000, 66, 3093–3097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; De las Rivas, B.; De Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Granito, M.; Frias, J.; Doblado, R.; Guerra, M.; Champ, M.; Vidal-Valverde, C. Nutritional improvement of beans (Phaseolus vulgaris) by natural fermentation. Eur. Food Res. Technol. 2002, 214, 226–231. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Singh, N. Pulse Chemistry and Technology; Royal Society of Chemistry: Cambridge, UK, 2012. [Google Scholar]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Angural, S.; Rana, M.; Puri, N.; Kondepudi, K.K.; Gupta, N. Phytase producing lactic acid bacteria: Cell factories for enhancing micronutrient bioavailability of phytate rich foods. Trends Food Sci. Technol. 2020, 96, 1–12. [Google Scholar] [CrossRef]
- Rekha, C.; Vijayalakshmi, G. Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and vitamin B complex in fermented soymilk by probiotic bacteria and yeast. J. Appl. Microbiol. 2010, 109, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, M.; Sparvoli, F.; Bollini, R.; Lubrano, V.; Longo, V.; Pucci, L. The impact of sourdough fermentation on non-nutritive compounds and antioxidant activities of flours from different Phaseolus vulgaris L. genotypes. J. Food Sci. 2019, 84, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Van Vo, B.; Bui, D.P.; Nguyen, H.Q.; Fotedar, R. Optimized fermented lupin (Lupinus angustifolius) inclusion in juvenile barramundi (Lates calcarifer) diets. Aquaculture 2015, 444, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Olukomaiya, O.O.; Adiamo, O.Q.; Fernando, W.C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Effect of solid-state fermentation on proximate composition, anti-nutritional factor, microbiological and functional properties of lupin flour. Food Chem. 2020, 315, 126238. [Google Scholar] [CrossRef]
- CHU, G.M.; Ohmori, H.; Kawashima, T.; Funaba, M.; Matsui, T. Brewer’s yeast efficiently degrades phytate phosphorus in a corn-soybean meal diet during soaking treatment. Anim. Sci. J. 2009, 80, 433–437. [Google Scholar] [CrossRef]
- Kasprowicz-Potocka, M.; Borowczyk, P.; Zaworska, A.; Nowak, W.; Frankiewicz, A.; Gulewicz, P. The Effect of Dry Yeast Fermentation on Chemical Composition and Protein Characteristics of Blue Lupin Seeds. Food Technol. Biotechnol. 2016, 54, 360–366. [Google Scholar] [CrossRef]
- De Angelis, M.; Gallo, G.; Corbo, M.R.; McSweeney, P.L.; Faccia, M.; Giovine, M.; Gobbetti, M. Phytase activity in sourdough lactic acid bacteria: Purification and characterization of a phytase from Lactobacillus sanfranciscensis CB1. Int. J. Food Microbiol. 2003, 87, 259–270. [Google Scholar] [CrossRef]
- Zhao, H.-M.; Guo, X.-N.; Zhu, K.-X. Impact of solid state fermentation on nutritional, physical and flavor properties of wheat bran. Food Chem. 2017, 217, 28–36. [Google Scholar] [CrossRef]
- Vats, P.; Banerjee, U.C. Production studies and catalytic properties of phytases (myo-inositolhexakisphosphate phosphohydrolases): An overview. Enzyme Microb. Technol. 2004, 35, 3–14. [Google Scholar] [CrossRef]
- Khokhar, S.; Apenten, R.K.O. Antinutritional factors in food legumes and effects of processing. Role Food Agric. For. Fish. Hum. Nutr. 2003, 4, 82–116. [Google Scholar]
- Lin, C.-Y.; Tsai, C.-Y.; Lin, S.-H. Effects of soy components on blood and liver lipids in rats fed high-cholesterol diets. World J. Gastroenterol. WJG 2005, 11, 5549. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.A.; Berhow, M.A.; Singletary, K.W. Inhibition of Akt signaling and enhanced ERK1/2 activity are involved in induction of macroautophagy by triterpenoid B-group soyasaponins in colon cancer cells. Carcinogenesis 2006, 27, 298–306. [Google Scholar] [CrossRef]
- Ishii, Y.; Tanizawa, H. Effects of soyasaponins on lipid peroxidation through the secretion of thyroid hormones. Biol. Pharm. Bull 2006, 29, 1759–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubert, J.; Berger, M.; Nepveu, F.; Paul, F.; Daydé, J. Effects of fermentation on the phytochemical composition and antioxidant properties of soy germ. Food Chem. 2008, 109, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Gacek, M. Soy and legume seeds as sources of isoflavones: Selected individual determinants of their consumption in a group of perimenopausal women. Menopausal Rev. 2014, 13, 27. [Google Scholar] [CrossRef]
- Dowling, S.; Regan, F.; Hughes, H. The characterisation of structural and antioxidant properties of isoflavone metal chelates. J. Inorg. Biochem. 2010, 104, 1091–1098. [Google Scholar] [CrossRef]
- Csaba, G. Effect of endocrine disruptor phytoestrogens on the immune system: Present and future. Acta Microbiol. Immunol. Hung. 2018, 65, 1–14. [Google Scholar] [CrossRef] [Green Version]
- De Camargo, A.C.; Favero, B.T.; Morzelle, M.C.; Franchin, M.; Alvarez-Parrilla, E.; de la Rosa, L.A.; Geraldi, M.V.; Maróstica Júnior, M.R.; Shahidi, F.; Schwember, A.R. Is chickpea a potential substitute for soybean? Phenolic bioactives and potential health benefits. Int. J. Mol. Sci. 2019, 20, 2644. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-J.; Li, D.; Zou, L.; Dong Chen, X.; Cheng, Y.-Q.; Yamaki, K.; Li, L.-T. Antioxidative activity of douchi (a Chinese traditional salt-fermented soybean food) extracts during its processing. Int. J. Food Prop. 2007, 10, 385–396. [Google Scholar] [CrossRef] [Green Version]
- Sung, J.H.; Choi, S.J.; Lee, S.W.; Park, K.H.; Moon, T.W. Isoflavones found in Korean soybean paste as 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Biosci., Biotechnol., Biochem. 2004, 68, 1051–1058. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Kim, J.-D.; Zheng, J.; Row, K.H. Comparisons of isoflavones from Korean and Chinese soybean and processed products. Biochem. Eng. J. 2007, 36, 49–53. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, W.; Shan, Y.; Zhi-Qiang, E.; Wang, L.-Q. Study on the inhibition of fermented soybean to cancer cells. J. Northeast Agric. Univ. (Engl. Ed.) 2009, 16, 25–28. [Google Scholar]
- De Olmos, A.R.; Garro, M.S. Metabolic profile of Lactobacillus paracasei subsp. paracasei CRL 207 in solid state fermentation using commercial soybean meal. Food Biosci. 2020, 35, 100584. [Google Scholar] [CrossRef]
- Delgado, S.; Guadamuro, L.; Flórez, A.B.; Vázquez, L.; Mayo, B. Fermentation of commercial soy beverages with lactobacilli and bifidobacteria strains featuring high β-glucosidase activity. Innov. Food Sci. Emerg. Technol. 2019, 51, 148–155. [Google Scholar] [CrossRef]
- Budryn, G.; Klewicka, E.; Grzelczyk, J.; Gałązka-Czarnecka, I.; Mostowski, R. Lactic acid fermentation of legume seed sprouts as a method of increasing the content of isoflavones and reducing microbial contamination. Food Chem. 2019, 285, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Spano, G.; Russo, P.; Lonvaud-Funel, A.; Lucas, P.; Alexandre, H.; Grandvalet, C.; Coton, E.; Coton, M.; Barnavon, L.; Bach, B. Biogenic amines in fermented foods. Eur. J. Clin. Nutr. 2010, 64, S95–S100. [Google Scholar] [CrossRef] [Green Version]
- Suzzi, G.; Torriani, S. Biogenic amines in foods. Front. Microbiol. 2015, 6, 472. [Google Scholar] [CrossRef] [Green Version]
- Stojanović, Z.; Kos, J. Detection of metabolites of microbial origin in beverages with harmful effect on human health—Biogenic amines and mycotoxins. In Safety Issues in Beverage Production; Grumezescu, A., Holban, A.M., Eds.; Elsevier: Cambridge, MA, USA, 2020; pp. 39–77. [Google Scholar]
- Anal, A.K.; Perpetuini, G.; Petchkongkaew, A.; Tan, R.; Avallone, S.; Tofalo, R.; Van Nguyen, H.; Chu-Ky, S.; Ho, P.H.; Phan, T.T. Food safety risks in traditional fermented food from South-East Asia. Food Control 2020, 109, 106922. [Google Scholar] [CrossRef]
- Leuschner, R.G.; Heidel, M.; Hammes, W.P. Histamine and tyramine degradation by food fermenting microorganisms. Int. J. Food Microbiol. 1998, 39, 1–10. [Google Scholar] [CrossRef]
- Rahate, K.A.; Madhumita, M.; Prabhakar, P.K. Nutritional composition, anti-nutritional factors, pretreatments-cum-processing impact and food formulation potential of faba bean (Vicia faba L.): A comprehensive review. LWT 2021, 138, 110796. [Google Scholar] [CrossRef]
- Khazaei, H.; Purves, R.W.; Hughes, J.; Link, W.; O’Sullivan, D.M.; Schulman, A.H.; Björnsdotter, E.; Geu-Flores, F.; Nadzieja, M.; Andersen, S.U. Eliminating vicine and convicine, the main anti-nutritional factors restricting faba bean usage. Trends Food Sci. Technol. 2019, 91, 549–556. [Google Scholar] [CrossRef]
- Doblado, R.; Zielinski, H.; Piskula, M.; Kozlowska, H.; Muñoz, R.; Frías, J.; Vidal-Valverde, C. Effect of processing on the antioxidant vitamins and antioxidant capacity of Vigna sinensis Var. Carilla. J. Agric. Food Chem. 2005, 53, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Yu, R.-C.; Chou, C.-C. Antioxidative activities of soymilk fermented with lactic acid bacteria and bifidobacteria. Food Microbiol. 2006, 23, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lee, S.Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Li, W.; Wang, T. Effect of solid-state fermentation with Bacillus subtilis lwo on the proteolysis and the antioxidative properties of chickpeas. Int. J. Food Microbiol. 2021, 338, 108988. [Google Scholar] [CrossRef]
- Frias, J.; Miranda, M.L.; Doblado, R.; Vidal-Valverde, C. Effect of germination and fermentation on the antioxidant vitamin content and antioxidant capacity of Lupinus albus L. var. Multolupa. Food Chem. 2005, 92, 211–220. [Google Scholar] [CrossRef]
- Bendich, A.; Machlin, L.; Scandurra, O.; Burton, G.; Wayner, D. The antioxidant role of vitamin C. Adv. Free Radic. Biol. Med. 1986, 2, 419–444. [Google Scholar] [CrossRef]
- Villacrés, E.; Quelal, M.B.; Jácome, X.; Cueva, G.; Rosell, C.M. Effect of debittering and solid-state fermentation processes on the nutritional content of lupine (Lupinus mutabilis Sweet). Int. J. Food Sci. Tech. 2020, 55, 2589–2598. [Google Scholar] [CrossRef]
- Torres, A.; Frías, J.; Granito, M.; Vidal-Valverde, C. Fermented pigeon pea (Cajanus cajan) ingredients in pasta products. J. Agric. Food Chem. 2006, 54, 6685–6691. [Google Scholar] [CrossRef]
- Niki, E. Evidence for beneficial effects of vitamin E. KJM Korean J. Intern. Med. 2015, 30, 571. [Google Scholar] [CrossRef]
- Schowen, R.L. Principles of biochemistry 2nd ed. (Lehninger, Albert L.; Nelson, David L.; Cox, Michael M.). J. Chem. Educ. 1993, 70, A223. [Google Scholar] [CrossRef] [Green Version]
- James, S.; Nwabueze, T.U.; Ndife, J.; Onwuka, G.I.; Usman, M.A.A. Influence of fermentation and germination on some bioactive components of selected lesser legumes indigenous to Nigeria. J. Agric. Food Res. 2020, 2, 100086. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.; Suberviola, J.; Bartolomé, B.; Colomo, B.; Suárez, J. Adsorption of anthocyanins by yeast cell walls during the fermentation of red wines. J. Agric. Food Chem. 2003, 51, 4084–4088. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Coda, R.; Chamlagain, B.; Edelmann, M.; Varmanen, P.; Piironen, V.; Katina, K. Fermentation of cereal, pseudo-cereal and legume materials with Propionibacterium freudenreichii and Levilactobacillus brevis for vitamin B12 fortification. LWT 2021, 137, 110431. [Google Scholar] [CrossRef]
- Watanabe, F. Vitamin B12 sources and bioavailability. Exp. Biol. Med. 2007, 232, 1266–1274. [Google Scholar] [CrossRef]
- Anzani, C.; Boukid, F.; Drummond, L.; Mullen, A.M.; Álvarez, C. Optimising the use of proteins from rich meat co-products and non-meat alternatives: Nutritional, technological and allergenicity challenges. Food Res. Int. 2020, 137, 109575. [Google Scholar] [CrossRef]
- Moore, L.E.; Stewart, P.H.; deShazo, R.D. Food allergy: What we know now. Am. J. Med. Sci. 2017, 353, 353–366. [Google Scholar] [CrossRef]
- Licandro, H.; Ho, P.H.; Nguyen, T.K.C.; Petchkongkaew, A.; Van Nguyen, H.; Chu-Ky, S.; Nguyen, T.V.A.; Lorn, D.; Waché, Y. How fermentation by lactic acid bacteria can address safety issues in legumes food products? Food Control 2020, 110, 106957. [Google Scholar] [CrossRef]
- Untersmayr, E.; Jensen-Jarolim, E. The role of protein digestibility and antacids on food allergy outcomes. J. Allergy Clin. Immunol. 2008, 121, 1301–1308. [Google Scholar] [CrossRef] [Green Version]
- Yang, A.; Zuo, L.; Cheng, Y.; Wu, Z.; Li, X.; Tong, P.; Chen, H. Degradation of major allergens and allergenicity reduction of soybean meal through solid-state fermentation with microorganisms. Food Funct. 2018, 9, 1899–1909. [Google Scholar] [CrossRef]
- Song, Y.-S.; Frías, J.; Martinez-Villaluenga, C.; Vidal-Valdeverde, C.; de Mejia, E.G. Immunoreactivity reduction of soybean meal by fermentation, effect on amino acid composition and antigenicity of commercial soy products. Food Chem. 2008, 108, 571–581. [Google Scholar] [CrossRef] [PubMed]
- AT, D.; Action, E.C. Scientific concepts of functional foods in Europe: Consensus document. Br. J. Nutr. 1999, 81, S1–S27. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamang, J.P.; Shin, D.-H.; Jung, S.-J.; Chae, S.-W. Functional properties of microorganisms in fermented foods. Front. Microbiol. 2016, 7, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, T.; Tamang, J. Health benefits of Natto. In Health Benefits of Fermented Foods; Tamang, J., Ed.; CRC Press: New York, NY, USA, 2015; pp. 433–453. [Google Scholar]
- Tamang, J.P. Naturally fermented ethnic soybean foods of India. J. Ethn. Foods 2015, 2, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.-R.; Jo, Y.N.; Jeong, J.H.; Jin, D.E.; Song, B.G.; Choi, S.J.; Shin, D.-H.; Heo, H.J. Antiamnesic effects of ethyl acetate fraction from chestnut (Castanea crenata var. dulcis) inner skin on Aβ25–35-induced cognitive deficits in mice. J. Med. Food 2012, 15, 1051–1056. [Google Scholar] [CrossRef] [Green Version]
- Omizu, Y.; Tsukaoto, C.; Chettri, R.; Tamang, J.P. Determination of saponin contents in raw soybean and fermented soybean foods of India. J. Sci. Ind. Res. 2011, 70, 533–538. [Google Scholar]
- Sanjukta, S.; Rai, A.K. Production of bioactive peptides during soybean fermentation and their potential health benefits. Trends Food Sci. Technol. 2016, 50, 1–10. [Google Scholar] [CrossRef]
- Park, K.-B.; Oh, S.-H. Enhancement of γ-aminobutyric acid production in Chungkukjang by applying a Bacillus subtilis strain expressing glutamate decarboxylase from Lactobacillus brevis. Biotechnol. Lett. 2006, 28, 1459–1463. [Google Scholar] [CrossRef]
- Gänzle, M.G. Food fermentations for improved digestibility of plant foods—An essential ex situ digestion step in agricultural societies? Curr. Opin. Food Sci. 2020, 32, 124–132. [Google Scholar] [CrossRef]
- Arteaga, V.G.; Leffler, S.; Muranyi, I.; Eisner, P.; Schweiggert-Weisz, U. Sensory profile, functional properties and molecular weight distribution of fermented pea protein isolate. CRFS Curr. Res. Food Sci. 2021, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Jakobsone, I.; Juodeikiene, G.; Vidmantiene, D.; Pugajeva, I.; Bartkevics, V. Effect of lactic acid fermentation of lupine wholemeal on acrylamide content and quality characteristics of wheat-lupine bread. Int. J. Food Sci. Nutr. 2013, 64, 890–896. [Google Scholar] [CrossRef]
- Parca, F.; Koca, Y.O.; Aydın, U. Nutritional and Antinutritional factors of some pulses seed and their effects on human health. Int. J. Second. 2018, 5, 331–342. [Google Scholar] [CrossRef]
- Cabuk, B.; Stone, A.K.; Korber, D.R.; Tanaka, T.; Nickerson, M.T. Effect of Lactobacillus plantarum Fermentation on the Surface and Functional Properties of Pea Protein-Enriched Flour. Food Technol. Biotechnol. 2018, 56, 411–420. [Google Scholar] [CrossRef]
- Bureau, S.; Ruiz, D.; Reich, M.; Gouble, B.; Bertrand, D.; Audergon, J.-M.; Renard, C.M. Rapid and non-destructive analysis of apricot fruit quality using FT-near-infrared spectroscopy. Food Chem. 2009, 113, 1323–1328. [Google Scholar] [CrossRef]
- Konieczny, P.; Uchman, W. Comparative characterization of surface hydrophobicity and other physico-chemical properties of selected protein prepartions. Electron. J. Pol. Agric. Univ. Series Food Sci. Technol. 2002, 5. [Google Scholar]
- Sharif, H.R.; Williams, P.A.; Sharif, M.K.; Abbas, S.; Majeed, H.; Masamba, K.G.; Safdar, W.; Zhong, F. Current progress in the utilization of native and modified legume proteins as emulsifiers and encapsulants—A review. Food Hydrocoll. 2018, 76, 2–16. [Google Scholar] [CrossRef]
- Shi, Y.; Singh, A.; Kitts, D.; Pratap-Singh, A. Lactic acid fermentation: A novel approach to eliminate unpleasant aroma in pea protein isolates. LWT 2021, 150, 111927. [Google Scholar] [CrossRef]
- Bartkiene, E.; Juodeikiene, G.; Vidmantiene, D.; Viskelis, P.; Urbonaviciene, D. Nutritional and quality aspects of wheat sourdough bread using L. luteus and L. angustifolius flours fermented by Pedioccocus acidilactici. Int. J. Food Sci. Tech. 2011, 46, 1724–1733. [Google Scholar] [CrossRef]
- Perri, G.; Coda, R.; Rizzello, C.G.; Celano, G.; Ampollini, M.; Gobbetti, M.; De Angelis, M.; Calasso, M. Sourdough fermentation of whole and sprouted lentil flours: In situ formation of dextran and effects on the nutritional, texture and sensory characteristics of white bread. Food Chem. 2021, 355, 129638. [Google Scholar] [CrossRef]
- Boeck, T.; Sahin, A.W.; Zannini, E.; Arendt, E.K. Nutritional properties and health aspects of pulses and their use in plant-based yogurt alternatives. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3858–3880. [Google Scholar] [CrossRef] [PubMed]
- Yousseef, M.; Lafarge, C.; Valentin, D.; Lubbers, S.; Husson, F. Fermentation of cow milk and/or pea milk mixtures by different starter cultures: Physico-chemical and sensorial properties. LWT 2016, 69, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Zare, F.; Boye, J.; Champagne, C.; Orsat, V.; Simpson, B. Probiotic milk supplementation with pea flour: Microbial and physical properties. Food Bioprocess Technol. 2013, 6, 1321–1331. [Google Scholar] [CrossRef]
- Klost, M.; Giménez-Ribes, G.; Drusch, S. Enzymatic hydrolysis of pea protein: Interactions and protein fractions involved in fermentation induced gels and their influence on rheological properties. Food Hydrocoll. 2020, 105, 105793. [Google Scholar] [CrossRef]
- Vasilean, I.; Aprodu, I.; Garnai, M.; Munteanu, V.; Patrașcu, L. Preliminary Investigations into the Use of Amylases and Lactic Acid Bacteria to Obtain Fermented Vegetable Products. Foods 2021, 10, 1530. [Google Scholar] [CrossRef]
- Cheng, Y.; Thompson, L.; Brittin, H. Sogurt, a yogurt-like soybean product: Development and properties. J. Food Sci. 1990, 55, 1178–1179. [Google Scholar] [CrossRef]
- Ogodo, A.; Ugbogu, O.; Onyeagba, R. Variations in the Functional Properties of Soybean Flour Fermented with Lactic Acid Bacteria (LAB)-Consortium. Appli. Microbiol. Open Access 2018, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Roland, W.S.; Pouvreau, L.; Curran, J.; van de Velde, F.; de Kok, P.M. Flavor aspects of pulse ingredients. Cereal Chem. 2017, 94, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Trindler, C.; Kopf-Bolanz, K.A.; Denkel, C. Aroma of peas, its constituents and reduction strategies–effects from breeding to processing. Food Chem. 2021, 376, 131892. [Google Scholar] [CrossRef]
- Fischer, E.; Cayot, N.; Cachon, R. Potential of Microorganisms to Decrease the “Beany” Off-Flavor: A Review. J. Agric. Food Chem. 2022, 70, 4493–4508. [Google Scholar] [CrossRef]
- El Youssef, C.; Bonnarme, P.; Fraud, S.; Péron, A.-C.; Helinck, S.; Landaud, S. Sensory Improvement of a Pea Protein-Based Product Using Microbial Co-Cultures of Lactic Acid Bacteria and Yeasts. Foods 2020, 9, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Harb, S.; Saint-Eve, A.; Panouillé, M.; Souchon, I.; Bonnarme, P.; Dugat-Bony, E.; Irlinger, F. Design of microbial consortia for the fermentation of pea-protein-enriched emulsions. Int. J. Food Microbiol. 2019, 293, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Grujović, M.Ž.; Mladenović, K.G.; Semedo-Lemsaddek, T.; Laranjo, M.; Stefanović, O.D.; Kocić-Tanackov, S.D. Advantages and disadvantages of non-starter lactic acid bacteria from traditional fermented foods: Potential use as starters or probiotics. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1537–1567. [Google Scholar] [CrossRef] [PubMed]
- Cogan, T.M.; Hill, C. Cheese starter cultures. In Cheese: Chemistry, Physics and Microbiology; Fox, P.F., Ed.; Springer: Boston, MA, USA, 1993; pp. 193–255. [Google Scholar]
- Ben-Harb, S.; Irlinger, F.; Saint-Eve, A.; Panouillé, M.; Souchon, I.; Bonnarme, P. Versatility of microbial consortia and sensory properties induced by the composition of different milk and pea protein-based gels. LWT 2020, 118, 108720. [Google Scholar] [CrossRef]
- Murat, C.; Bard, M.-H.; Dhalleine, C.; Cayot, N. Characterisation of odour active compounds along extraction process from pea flour to pea protein extract. Int. Food Res. J. 2013, 53, 31–41. [Google Scholar] [CrossRef]
- Blagden, T.D.; Gilliland, S.E. Reduction of levels of volatile components associated with the “beany” flavor in soymilk by lactobacilli and streptococci. J. Food Sci. 2005, 70, M186–M189. [Google Scholar] [CrossRef]
- Pinthong, R.; Macrae, R.; Rothwell, J. The development of a soya-based yoghurt: I. Acid production by lactic acid bacteria. Int. J. Food Sci. Tech. 1980, 15, 647–652. [Google Scholar] [CrossRef]
- Zheng, Y.; Fei, Y.; Yang, Y.; Jin, Z.; Yu, B.; Li, L. A potential flavor culture: Lactobacillus harbinensis M1 improves the organoleptic quality of fermented soymilk by high production of 2,3-butanedione and acetoin. Food Microbiol. 2020, 91, 103540. [Google Scholar] [CrossRef]
- Schindler, S.; Zelena, K.; Krings, U.; Bez, J.; Eisner, P.; Berger, R.G. Improvement of the aroma of pea (Pisum sativum) protein extracts by lactic acid fermentation. Food Biotechnol. 2012, 26, 58–74. [Google Scholar] [CrossRef]
- Schindler, S.; Wittig, M.; Zelena, K.; Krings, U.; Bez, J.; Eisner, P.; Berger, R.G. Lactic fermentation to improve the aroma of protein extracts of sweet lupin (Lupinus angustifolius). Food Chem. 2011, 128, 330–337. [Google Scholar] [CrossRef]
- Ayad, E.H.; Verheul, A.; Engels, W.J.; Wouters, J.; Smit, G. Enhanced flavour formation by combination of selected lactococci from industrial and artisanal origin with focus on completion of a metabolic pathway. J. Appl. Microbiol. 2001, 90, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Yvon, M.; Berthelot, S.; Gripon, J. Adding α-ketoglutarate to semi-hard cheese curd highly enhances the conversion of amino acids to aroma compounds. Int. Dairy J. 1998, 8, 889–898. [Google Scholar] [CrossRef]
- Smit, G.; Smit, B.A.; Engels, W.J. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 2005, 29, 591–610. [Google Scholar] [CrossRef] [PubMed]
- Crafack, M.; Mikkelsen, M.B.; Saerens, S.; Knudsen, M.; Blennow, A.; Lowor, S.; Takrama, J.; Swiegers, J.H.; Petersen, G.B.; Heimdal, H. Influencing cocoa flavour using Pichia kluyveri and Kluyveromyces marxianus in a defined mixed starter culture for cocoa fermentation. Int. J. Food Microbiol. 2013, 167, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Rui, X.; Zhang, Y.; Cai, F.; Chen, X.; Jiang, M. Production of tofu by lactic acid bacteria isolated from naturally fermented soy whey and evaluation of its quality. LWT 2017, 82, 227–234. [Google Scholar] [CrossRef]
- Laaksonen, O.; Kahala, M.; Marsol-Vall, A.; Blasco, L.; Järvenpää, E.; Rosenvald, S.; Virtanen, M.; Tarvainen, M.; Yang, B. Impact of lactic acid fermentation on sensory and chemical quality of dairy analogues prepared from lupine (Lupinus angustifolius L.) seeds. Food Chem. 2021, 346, 128852. [Google Scholar] [CrossRef]
- Schlegel, K.; Lidzba, N.; Ueberham, E.; Eisner, P.; Schweiggert-Weisz, U. Fermentation of Lupin Protein Hydrolysates—Effects on Their Functional Properties, Sensory Profile and the Allergenic Potential of the Major Lupin Allergen Lup an 1. Foods 2021, 10, 281. [Google Scholar] [CrossRef]
- Yi, C.; Li, Y.; Zhu, H.; Liu, Y.; Quan, K. Effect of Lactobacillus plantarum fermentation on the volatile flavors of mung beans. LWT 2021, 146, 111434. [Google Scholar] [CrossRef]
- Su, L.-W.; Cheng, Y.-H.; Hsiao, F.S.-H.; Han, J.-C.; Yu, Y.-H. Optimization of mixed solid-state fermentation of soybean meal by Lactobacillus species and Clostridium butyricum. Polish J. Microbiol. 2018, 67, 297. [Google Scholar] [CrossRef] [Green Version]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Siedler, S.; Balti, R.; Neves, A.R. Bioprotective mechanisms of lactic acid bacteria against fungal spoilage of food. Curr. Opin. Biotechnol. 2019, 56, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Shimelis, E.A.; Rakshit, S.K. Influence of natural and controlled fermentations on α-galactosides, antinutrients and protein digestibility of beans (Phaseolus vulgaris L.). Int. J. Food Sci. Tech. 2008, 43, 658–665. [Google Scholar] [CrossRef]


| Legume Type | Protein Ingredients Treated | LAB Strains (Fermentation Technique) | Nutritional Properties | References | |||
|---|---|---|---|---|---|---|---|
| Total Phenolic Content | Antioxidant Activity | Nutritive and Non-Nutritive Compounds | Digestibility | ||||
| Chickpea | Protein concentrate | P. pentosaceus P. acidilactici (SSF) | Increased | _ | Decrease in phytic acid Decrease in α-galactosides | _ | [10] |
| Milk | Lb. plantarum (LSF) | Increased | Increased | [29] | |||
| Cracked grain | Lb. plantarum (LSF) | Increased | Decreased | Increase in γ- and β-tocopherols Decrease in vitamin E | _ | [27] | |
| Flour | Lb. plantarum (LSF) | _ | _ | Decrease in TIA, phytate content, and protease inhibitor Increase in alpha-galactosidase enzyme | _ | [30] | |
| Seed | Lb. plantarum (SSF) | Increased | _ | Decrease in saponin content Decrease in TIA and phytic acid | Increased | [5] | |
| Cracked grain | Lb. plantarum (LSF) | Increased | Decreased | Increase in γ- and β-tocopherols Decrease in vitamin E | _ | [27] | |
| Flour | Lb. plantarum (LSF) | _ | _ | Decrease in TIA, phytate content, and protease inhibitor Increase in alpha-galactosidase enzyme | _ | [30] | |
| Flour | Lb. plantarum (SSF) | Increased | _ | Decrease in saponin content Decrease in phytic acid Decrease in TIA | Increased | [5] | |
| Flour | W. paramesenteroides Lb. plantarum | Increased | Increased | Decrease in tannin Decrease in TIA and α-chymotrypsin Decrease in α-amylase inhibitors | _ | [31] | |
| Milk | Lb. plantarum Lb. brevis Lb. reuteri (LSF) | _ | Increased No difference between the strains | Decrease in raffinose by Lb. reuteri | _ | [32] | |
| Milk | Lb. plantarum (LSF) | Increased | Increased | [29] | |||
| Protein concentrate | P. pentosaceus, P. acidilactici (SSF) | Increased | _ | Decrease in phytic acid Decrease in α-galactosides | _ | [10] | |
| Protein isolate | Lb. plantarum (LSF) | _ | _ | Decrease in IgE reactivity Decrease in B-conglycinin Decrease in Glycinin | _ | [33] | |
| Protein isolate | Lb. paracasei Lb. fermentum Lb. delbruecki Lb. plantarum Lb. helveticus Lb. reuteri P. pentosaceus (LSF) | _ | _ | Decrease in soybean allergenicity Glycinin degradation | _ | [34] | |
| Faba bean | Flour | Lb. plantarum (LSF) | Increased | _ | Decrease in vicine and convicine Decrease in TIA Decrease in condensed tannins No changes in phytic acid content | Increased | [35] |
| Flour | Lb. sakei Lc. lactis Lc. mesenteroides P. Pediococcus P. pentosaceus W. cibaria Weissella koreensis (LSF) | _ | Increased No difference between strains except for P. pentosaceus | The highest β-glucosidase activity for Pediococcus and W. koreensis The highest phytase activity for Lc. mesenteroides and P. pentosaceus Decrease in phytic acid for Lc. mesenteroides and P. pentosaceus No difference in raffinose concentration except for Lc. mesenteroides No changes in the concentration of condensed tannins except for P. pentosaceus, Lb. sakei | _ | [36] | |
| Seeds | Lb. plantarum (LSF) | Increased | Increased | Decrease in condensed tannins concentration and TIA Degradation of vicine Degradation of verbascose Degradation of stachyose Degradation of raffinose | Increased | [37] | |
| Flour | Lb. plantarum (LSF) | _ | _ | Decrease in phytic acid | Increased | [38] | |
| Flour | Lb. plantarum (LSF) | _ | _ | _ | Increase in digestibility and quality of protein | [39] | |
| Flour | Lb. plantarum (LSF) | _ | _ | Degradation of vicine and convicine Degradation of aglycones Low toxicity | _ | [40] | |
| Flour | Lb. plantarum | _ | _ | Decrease in vicine and convicine | _ | [41] | |
| Pea | Flour | Lb. plantarum (LSF) | Increased | No difference | Decrease in TIA Decrease in inositol phosphate content | [42] | |
| Protein concentrate | Lb. plantarum (LSF) | Increased | _ | Decrease in TIA Decrease in chymotrypsin inhibitory activity Decrease in tannin | No differences | [43] | |
| Flour | Co-culture Lb. delbrueckii, S. thermophilus, and Lb. acidophilus (LSF) | Increased | _ | Decrease in tannin content Decrease in TIA | Increased | [44] | |
| Protein isolate | Lb. rhamnosus (LSF) | _ | Increased | _ | _ | [45] | |
| Bean and pea | Flour | Lb. plantarum (LSF) | _ | _ | Decrease in oligosaccharides | No difference in digestibility of pea Increase in digestibility of bean | [46] |
| Pea and whey | Protein isolate | Lb. fermentum Lactobacillus gasseri Lactobacillus oris Lb. reuteri Lb. acidophilus Lb. plantarum Lb. helveticus (LSF) | _ | _ | _ | No changes in ACE inhibitor for pea protein Increase in ACE inhibitor for whey protein | [47] |
| Yellow and red lentils, white and black beans, chickpeas, and pea grains | Raw flour and gelatinized flour | Lb. plantarum P. acidilactici Lc. mesenteroides Lb. rossiae Lb. brevis (LSF) | Increased The lowest for fermented raw flour red lentil The highest for fermented raw flour chickpea | Increased The highest for black bean The lowest for chickpea | Increase in TIA, saponin, and condensed tannins Degradation of phytic acid Decrease in raffinose, the highest for red and yellow lentils, the lowest for white and black beans | _ | [48] |
| Chickpea, lentil, wheat, barley, and quinoa | Flour | Lb. rossiae Lb. plantarum Lactobacillus sanfranciscensis (LSF) | Increased | Increased The highest for quinoa, barley, and lentil The lowest for wheat and chickpea | Decrease in condensed tannin concentration, the highest decrease for lentil Decrease in TIA Decrease in raffinose concentration Decrease in phytic acid Decrease in phytase activity | Increased | [49] |
| Lupine seeds (sweet and bitter flour of lupin) | Flour and Protein isolate | Lb. plantarum Lb. brevis Lactobacillus curvatus W. cibaria Lb. parabuchneri Lb. helveticus P. pentosaceus (LSF) | _ | _ | Decrease in raffinose, the highest reduction in raffinose for Lb. acidophilus Decrease in phytic acid, the highest reduction belongs to Lb. plantarum | _ | [50] |
| Lupine and soya beans | Flour | Lb. sakei P. acidilactici P. pentosaceus (SSF) | _ | _ | _ | Increased Higher digestibility for soybean | [6] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emkani, M.; Oliete, B.; Saurel, R. Effect of Lactic Acid Fermentation on Legume Protein Properties, a Review. Fermentation 2022, 8, 244. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation8060244
Emkani M, Oliete B, Saurel R. Effect of Lactic Acid Fermentation on Legume Protein Properties, a Review. Fermentation. 2022; 8(6):244. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation8060244
Chicago/Turabian StyleEmkani, Mehrsa, Bonastre Oliete, and Rémi Saurel. 2022. "Effect of Lactic Acid Fermentation on Legume Protein Properties, a Review" Fermentation 8, no. 6: 244. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation8060244