Phenotyping Brown Rot Susceptibility in Stone Fruit: A Literature Review with Emphasis on Peach
Abstract
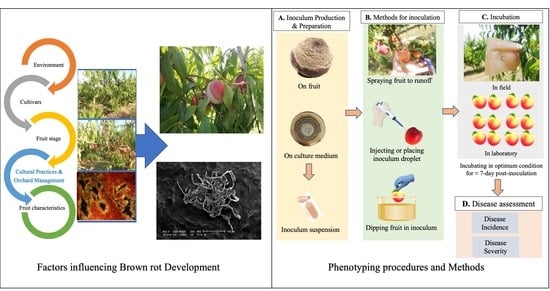
:1. Introduction
2. Factors Influencing Brown Rot Development
2.1. Environment
2.2. Cultivars
2.3. Fruit Stage
2.4. Cultural Practices and Orchard Management
2.5. Fruit Characteristics
3. Protocols for BR Susceptibility Evaluation
3.1. Fruit Preparations before Inoculation
3.2. Strain Conservation and Inoculum Production
| Fruit Species | Monilinia spp. | Maturity Determination | Wounded or Unwounded (Intact) | Production of Inoculum | Mode of Inoculation | Inoculum Concentration (conidia/mL) | Incubation Condition | Assessment Time | Disease Assessment | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Peach | M. fructicola | Fruit color determinations by spectrophotometer | Unwounded, wounded | V8A | Drop 10 µL | 2.5 × 104 | Humidified plastic containers at room temperature | 3 days | Disease incidence, disease severity (lesion diameter) | [7] |
| Peach | M. fructicola | Mature (firm ripe) and mature green | Unwounded, wounded | PDA | Drop 10 µL and a 5-mm mycelial disk | 2 × 105 | 23–25 °C/90% RH in dark | (24, 48 and 73, 96 h), rote diameter (48, 72, and 96 h) and sporulation 7 days | Disease incidence, disease severity (rot diameter), sporulation amount | [47] |
| Peach | M. fructicola | Commercial maturity | Unwounded | PDA | Drop 10 µL | 2 × 104 | 22–25 °C/95% RH, in dark | 3 days | Disease incidence | [46] |
| Peach, Nectarine, Plum | M. fructicola | Commercial maturity | Unwounded, wounded | PDA + acidified lactic acid | Drop 20 µL | 1 × 106, 105, 104, 103, 102 | 20 °C/95% RH in plastic cardboard boxes | 5 to 7 days | Disease incident and severity (lesion diameter) | [57] |
| Peach, Nectarine | M. fructicola | Maturity classes based on (IAD) | Unwounded, wounded | PDA supplemented with tomato pulp | Drop | 2.5 × 104 | 20 °C and 85% RH storage boxes | 3 and 5 days | Brown rot incidence (%), lesion diameter | [11] |
| Peach, Nectarine | M. fructicola, M. laxa | Commercial maturity | Wounded | PDA | Drop 15 µL | 1 × 104 | 0, 4, 10, 15, 20, 25, 30, 33 °C with ±1 °C/85% RH, dark or 12-h light photoperiod | 12 h for M. fructicola and 5–7 days for M. laxa | Lesion diameter, presence or absence of sporodochia | [36] |
| Peach, Apricot, Sweet cherry, Plum | M. fructicola, M. laxa | Commercial maturity | Wounded | V8A | Drop 30 µL | 1 × 105 | 22 °C/high RH, in containers | 6 days | Disease severity (rot diameter) | [39] |
| Peach, Nectarine | M. fructicola, M. laxa, M. fructigena | NA | Wounded | PDA | Drop 25 µL | 1 × 104 | 22 ± 2 °C/light and in humidity chambers lined with a moist paper | 7 days | % brown rot incidence, lesion diameter, sporulation, spore germination, mycelium length | [37] |
| Peach, Nectarine, Apricot, Plum | M. fructicola, M. laxa | Commercial maturity, immature fruit | Unwounded, wounded | V8A, PDA | Filter paper disks soaked in suspension, drop 10 µL | 1 × 104 | 22–25 °C/(90–100%) in plastic boxes lined with a damp paper towel and the lids closed | 7 days | Pathogenicity and disease incidence | [65] |
| Peach, Sweet cherry | M. Fructicola | Different maturity date | Unwounded | PDA | Drop 30 µL | 1 × 105, 106 | 15 to 30 °C with 2.5 °C intervals, then at 20 °C/>95% RH, in plastic boxes | 6 days | Disease severity (scaling 0 to 3) and percentage of fruit infection | [35] |
| Peach | M. laxa | Maturity at 0.6 IAD | Unwounded | NA | Spray | 1 × 105 | Fruit left on the tree bagged in plastic or paper bags | 7 days | Disease incidence% in the field | [14] |
| Peach | M. laxa | NA | Unwounded | NA | Spray | 1 × 105 | at 25 ± 2 °C/95–100% RH | 7 days | Brown rot infection number, percent of rotted skin (lesion) | [50] |
| Peach, Nectarine | M. laxa | NA | Unwounded | NA | Sprayed to runoff | 1 × 104, 106 | 23 °C/in trays lined with moist paper and plastic film. 16-h photoperiod | 7 days | Incidence (%) of fruit rot | [4] |
| Peach, Nectarine | M. laxa | Optimum maturity | Unwounded, wounded | Peach fruit | Drop | 25 × 103 | 23 °C/40–60% RH, in darkness | 5 days | Measuring brown rot incidence (%), lesion diameter (mm) and colonization extent (mm) | [10] |
| Peach Apricot, plum | M. laxa | Commercial maturity | Unwounded, wounded | Fruit | Drop 20 µL | 1 × 106 | 23 °C/high RH | 10 days unwounded; 5 days wounded | Disease incidence, disease severity (lesion diameter) | [12] |
| Peach, Apricot | M. laxa | Commercial maturity | Unwounded, wounded | V8A | Dipping fruit for (1 min) inoculum | 1 × 105 | 20 C and 95% RH | 7 days | Brown rot incidence % | [58] |
| Peach, Plum | M. laxa | Mature fruit from the market | Wounded | PDA, canned peaches | Dipping for 30 sec in inoculum suspension or a drop | 1 × 10, 102, 103, 104, 105 spore/cm3 | 21 °C, wrapped in plastic bags | 5 days, or 4 to 6 days | Disease incidence % | [93] |
| Apricot | M. fructicola M. laxa | Mature apricots | Unwounded, wounded | Tinned apricot halves | Drop 30 µL | 1.5 × 104 | 15–22 °C | 48, 66, 72, 96 and 120 h | Lesion area, spore counts, storage rot, cuticle thickness | [13] |
| Apricot | M. laxa | Mature visually | Unwounded | PDA | Drop (drip) | 1 × 105 | 22 °C covered with polythene bags | 7 days | Percentage infection and scaling to resistant: 0–10%; moderately susceptible: 11–30%; susceptible: 31−50%; highly susceptible: >50% | [53] |
| Sweet and sour cherry | M. fructicola | NA | Unwounded | PDA | Drop 30 µL | 1 × 106, 105, 104, 103 | 20 °C/ 95%RH | 6 days | Percentage fruit infection, lesion development | [66] |
| Sweet cherry | M. fructicola | Commercial maturity | Unwounded | NA | Spraying | 1 × 104 | 13 °C 95–97% RH in the growth chamber | 8, 11 days | Disease incidence | [54] |
| Sweet cherry | M. laxa, M. fructigena | 5 to 6 weeks after blooming | Unwounded, wounded | PDA, Apple fruit | Spray | 1 × 105 | 20 °C under light | 7 days | Incidence of infection in field and polyethene tunnel | [56] |
| Prune | M. fructicola | Different stages | Wounded | Acidified PDA | Injecting ≈ 0.1 mL inoculum | 5 × 103 | Left on the tree | 27 days or more | Disease incidence (%), and natural infection in the field | [100] |
3.3. Inoculum Preparation
3.4. Field and Laboratory Protocols
3.5. Wounded or Unwounded Fruit
| Evaluating Environments | Advantages | Disadvantages | References |
|---|---|---|---|
| Field | Relatively faster in manipulation. Plenty of accessions can be evaluated in a short time. | High variability, which may lead to low repeatability of the result. Environmental factors may impair the level of the recorded susceptibility. | [14,99] |
| Laboratory or controlled condition | Enables fruit preparation before inoculation, such as disinfection, wounding. Facilitates the post-inoculation evaluation of traits such as fruit weight, acidity, Brix. Provides repeatable environmental conditions. Fruit manipulations relatively easier. Inoculum load could be precisely placed on fruit sides (cheeks). Allows recording of many parameters. | Not exactly representing the natural (field) condition. It is more laborious. | [7,55,102] |
3.6. Artificial Inoculum Application
3.7. Incubation
3.8. Infection Assessment
4. Inconsistency of Infection Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Byrde, R.J.W.; Willetts, H.J. The Brown Rot Fungi of Fruit: Their Biology and Control; Pergamon Press: Oxford, UK, 1977. [Google Scholar]
- Larena, I.; Torres, R.; De Cal, A.; Liñán, M.; Melgarejo, P.; Domenichini, P.; Bellini, A.; Mandrin, J.F.; Lichou, J.; De Eribe, X.O.; et al. Biological control of postharvest brown rot (Monilinia spp.) of peaches by field applications of Epicoccum nigrum. Biol. Control 2005, 32, 305–310. [Google Scholar] [CrossRef]
- Hong, C.; Michailides, T.J. Effect of Temperature on the Discharge and Germination of Ascospores by Apothecia of Monilinia fructicola. Plant Dis. 1998, 82, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Gell, I.; De Cal, A.; Torres, R.; Usall, J.; Melgarejo, P. Relationship between the incidence of latent infections caused by Monilinia spp. and the incidence of brown rot of peach fruit: Factors affecting latent infection. Eur. J. Plant Pathol. 2008, 121, 487–498. [Google Scholar] [CrossRef]
- Chen, F.; Liu, X.; Chen, S.; Schnabel, E.; Schnabel, G. Characterization of Monilinia fructicola Strains Resistant to Both Propiconazole and Boscalid. Plant Dis. 2013, 97, 645–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egüen, B.; Melgarejo, P.; De Cal, A. Sensitivity of Monilinia fructicola from Spanish peach orchards to thiophanate-methyl, iprodione, and cyproconazole: Fitness analysis and competitiveness. Eur. J. Plant Pathol. 2015, 141, 789–801. [Google Scholar] [CrossRef]
- Martínez-García, P.J.; Parfitt, D.E.; Bostock, R.M.; Fresnedo-Ramírez, J.; Vazquez-Lobo, A.; Ogundiwin, E.A.; Gradziel, T.M.; Crisosto, C.H. Application of genomic and quantitative genetic tools to identify candidate resistance genes for brown rot resistance in peach. PLoS ONE 2013, 8, e78634. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, I.; Bassi, D.; Eduardo, I.; Ciacciulli, A.; Pirona, R.; Rossini, L.; Vecchietti, A. Qtl mapping for brown rot (Monilinia fructigena ) resistance in an intraspecific peach (Prunus persica L. Batsch) F1 progeny. Tree Genet. Genomes 2014, 10, 1223–1242. [Google Scholar] [CrossRef]
- Baró-Montel, N.; Eduardo, I.; Usall, J.; Casals, C.; Arús, P.; Teixidó, N.; Torres, R. Exploring sources of resistance to brown rot in an interspecific almond × peach population. J. Sci. Food Agric. 2019, 99, 4105–4113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obi, V.I.; Barriuso, J.J.; Moreno, M.A.; Giménez, R.; Gogorcena, Y. Optimizing protocols to evaluate brown rot (Monilinia laxa) susceptibility in peach and nectarine fruits. Australas. Plant Pathol. 2017, 46, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Baró-Montel, N.; Torres, R.; Casals, C.; Teixidó, N.; Segarra, J.; Usall, J. Developing a methodology for identifying brown rot resistance in stone fruit. Eur. J. Plant Pathol. 2019, 154, 287–303. [Google Scholar] [CrossRef]
- Pascal, T.; Levigneron, A.; Kervella, J.; Nguyen-The, C. Evaluation of two screening methods for resistance of apricot, plum and peach to Monilinia laxa. Euphytica 1994, 77, 19–23. [Google Scholar] [CrossRef]
- Walter, M.; McLaren, G.F.; Fraser, J.A.; Frampton, C.M.; Boyd-Wilson, K.S.H.; Perry, J.H. Methods of screening apricot fruit for resistance to brown rot caused by Monilinia spp. Australas. Plant Pathol. 2004, 33, 541–547. [Google Scholar] [CrossRef]
- Pacheco, I.; Perini, C.; Bassi, D.; Lama, M.; Foschi, S. Towards faster phenotyping methods for brown rot susceptibility by artificial inoculation in the orchard. In Proceedings of the VIII International Peach Symposium, Matera, Italy, 17–20 June 2013; Volume 1084, pp. 367–373. [Google Scholar]
- Ogawa, J.M.; Zehr, E.I.; Biggs, A.R. Brown rot. In Compendium of Stone Fruit Disease; Ogawa, J.M., Zehr, E.I., Bird, G.W., Ritchie, D.F., Uriu, K., Uyemoto, J.K., Eds.; American Phytopathological Society: Saint Paul, MN, USA, 1995; pp. 7–10. [Google Scholar]
- Jerome, S.M.R. Brown rot of stone fruits. Latent contamination in relation to spread of disease. J. Aust. Inst. Agric. Sci. 1958, 24, 132–140. [Google Scholar]
- Holtz, B.A.; Michailides, T.J.; Hong, C. Development of apothecia from stone fruit infected and stromatized by Monilinia fructicola in California. Plant Dis. 1998, 82, 1375–1380. [Google Scholar] [CrossRef] [Green Version]
- Biggs, A.R.; Northover, J. Inoculum sources for Monilinia fructicola in Ontario peach orchards. Can. J. Plant Pathol. 1985, 7, 302–307. [Google Scholar] [CrossRef]
- Gell, I.; De Cal, A.; Torres, R.; Usall, J.; Melgarejo, P. Conidial density of Monilinia spp. on peach fruit surfaces in relation to the incidences of latent infections and brown rot. Eur. J. Plant Pathol. 2009, 123, 415–424. [Google Scholar] [CrossRef]
- Casals, C.; Segarra, J.; De Cal, A.; Lamarca, N.; Usall, J. Overwintering of Monilinia spp. on mummified stone fruit. J. Phytopathol. 2015, 163, 160–167. [Google Scholar] [CrossRef]
- Landgraf, F.A.; Zehr, E.I. Inoculum sources for Monilinia fructicola in South Carolina peach orchards. Phytopathology 1982, 72, 185–190. [Google Scholar] [CrossRef]
- Villarino, M.; Melgarejo, P.; Usall, J.; Segarra, J.; De Cal, A. Primary inoculum sources of Monilinia spp. in Spanish peach orchards and their relative importance in brown rot. Plant Dis. 2010, 94, 1048–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Northover, J.; Cerkauskas, R.F. Detection and significance of symptomless latent infections of Monilinia fructicola in plums. Can. J. Plant Pathol. 1994, 16, 30–36. [Google Scholar] [CrossRef]
- Garcia-benitez, C.; Casals, C.; Usall, J.; Ismael, S.; Sánchez-Ramos, I.; Melgarejo, P.; De Cal, A. Impact of Postharvest Handling on Preharvest Latent Infections Caused by Monilinia spp. in Nectarines. J. Fungi 2020, 6, 266. [Google Scholar] [CrossRef] [PubMed]
- Förster, H.; Adaskaveg, J.E. Early brown rot infections in sweet cherry fruit are detected by Monilinia-specific DNA primers. Phytopathology 2000, 90, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adaskaveg, J.E.; Schnabel, G.; Förster, H. Diseases of Peach Caused by Fungi and Fungal-Like Organisms: Biology, Epidemiology and Management; CABI: Wallingford, UK, 2008; pp. 352–406. [Google Scholar]
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier: San Diego, CA, USA, 2005. [Google Scholar] [CrossRef]
- Lopresti, J.; Goodwin, I.; McGlasson, B.; Holford, P.; Golding, J. Variability in size and soluble solids concentration in peaches and nectarines. Hortic. Rev. (Am. Soc. Hortic. Sci). 2014, 42, 253–311. [Google Scholar]
- Lee, M.-H.H.; Bostock, R.M. Induction, regulation, and role in pathogenesis of appressoria in Monilinia fructicola. Phytopathology 2006, 96, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Lino, L.; Génard, M.; Signoret, V.; Quilot-Turion, B. Physical host factors for brown rot resistance in peach fruit. Acta Hortic. 2016, 1137, 105–112. [Google Scholar] [CrossRef]
- Cruickshank, R.H.; Wade, G.C. The activation of latent infections of Monilinia fructicola on apricots by volatiles from the ripening fruit. J. Phytopathol. 1992, 136, 107–112. [Google Scholar] [CrossRef]
- Willetts, H.J.; Harada, Y. A review of apothecial production by Monilinia fungi in Japan. Mycologia 1984, 76, 314–325. [Google Scholar] [CrossRef]
- Weaver, L.O. Effect of temperature and relative humidity on occurrence of blossom blight of stone fruits. Phytopathology 1950, 40, 1136–1153. [Google Scholar]
- McCallan, S.E.A. Studies on fungicides. II. Testing protective fungicides in the laboratory. Cornell Univ. Agric. Exp. Stn. Man. 1930, 128, 14. [Google Scholar]
- Biggs, A.R.; Northover, J. Influence of temperature and wetness duration on infection of peach and sweet cherry fruits by Monilinia fructicola. Phytopathology 1988, 78, 1352. [Google Scholar] [CrossRef]
- Bernat, M.; Segarra, J.; Xu, X.-M.M.; Casals, C.; Usall, J. Influence of temperature on decay, mycelium development and sporodochia production caused by Monilinia fructicola and M. laxa on stone fruits. Food Microbiol. 2017, 64, 112–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villarino, M.; Melgarejo, P.; De Cal, A. Growth and aggressiveness factors affecting Monilinia spp. survival peaches. Int. J. Food Microbiol. 2016, 227, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Bernat, M.; Casals, C.; Torres, R.; Teixidó, N.; Usall, J. Infection risk of Monilinia fructicola on stone fruit during cold storage and immersion in the dump tank. Sci. Hortic. 2019, 256, 108589. [Google Scholar] [CrossRef]
- Papavasileiou, A.; Testempasis, S.; Michailides, T.J.; Karaoglanidis, G.S. Frequency of brown rot fungi on blossoms and fruit in stone fruit orchards in Greece. Plant Pathol. 2015, 64, 416–424. [Google Scholar] [CrossRef]
- Wellman, R.H.; McCallan, S.E.A. An analysis of factors causing variation in spore germination tests of fungicides. IV. Time Temp. Contrib. Boyce Thompson Inst 1942, 12, i1. [Google Scholar]
- Tamm, L.; Minder, C.E.; Fluckiger, W.; Minder, E.; Fluckiger, W. Phenological analysis of brown rot blossom blight of sweet cherry caused by Monilinia laxa. Phytopathology 1995, 85, 401–408. [Google Scholar] [CrossRef]
- Fourie, P.H.; Holzh, G. Germination of dry, airborne conidia of Monilinia laxa and disease expression on nectarine fruit. Australas. Plant Pathol. 2003, 32, 9–18. [Google Scholar] [CrossRef]
- Corbin, J.B.; Ogawa, J.M.; Schultz, H.B. Fluctuations in numbers of Monilinia laxa conidia in an apricot orchard during the 1966 season. Phytopathology 1968, 58, 1387. [Google Scholar]
- Holb, I.J. The brown rot fungi of fruit crops (Monilinia spp.): II. Important features of their epidemiology (Review paper). Int. J. Hortic. Sci. 2004, 10, 31–49. [Google Scholar] [CrossRef]
- Rungjindamai, N.; Jeffries, P.; Xu, X.M. Epidemiology and management of brown rot on stone fruit caused by Monilinia laxa. Eur. J. Plant Pathol. 2014, 140, 1–17. [Google Scholar] [CrossRef]
- Gradziel, T.M.; Thorpe, M.A.; Bostock, R.M.; Wilcox, S. Breeding for brown rot (Monilinia fructicola) resistance in clingstone peach with emphasis on the role of fruit phenolics. Acta Hortic. 1998, 465, 161–170. [Google Scholar] [CrossRef]
- Feliciano, A.; Feliciano, F.A.; Ogawa, J.M. Monilinia fructicola Resistance in the Peach Cultivar Bolinha. Phytopathology 1987, 77, 776. [Google Scholar] [CrossRef]
- Bostock, R.M.; Wilcox, S.M.; Wang, G.; Adaskaveg, J.E. Suppression of Monilinia fructicola cutinase production by peach fruit surface phenolic acids. Physiol. Mol. Plant Pathol. 1999, 54, 37–50. [Google Scholar] [CrossRef]
- Baccichet, I.; Chiozzotto, R.; Bassi, D.; Gardana, C.; Cirilli, M.; Spinardi, A. Characterization of fruit quality traits for organic acids content and profile in a large peach germplasm collection. Sci. Hortic. 2021, 278, 109865. [Google Scholar] [CrossRef]
- Bassi, D.; Rizzo, M.; Cantoni, L. Assaying brown rot [(Monilinia laxa Aderh. et Ruhl. (Honey)] susceptibility in peach cultivars and progeny. Acta Hortic. 1998, 465, 715–721. [Google Scholar] [CrossRef]
- Brown, A.F.; Yousef, G.G.; Guzman, I.; Chebrolu, K.K.; Werner, D.J.; Parker, M.; Gasic, K.; Perkins-Veazie, P. Variation of carotenoids and polyphenolics in peach and implications on breeding for modified phytochemical profiles. J. Am. Soc. Hortic. Sci. 2014, 139, 676–686. [Google Scholar] [CrossRef] [Green Version]
- Fu, W.; Burrell, R.; Linge, C.d.S.; Schnabel, G.; Gasic, K. Breeding for brown rot (Monilinia spp.) tolerance in Clemson University peach breeding program. J. Am. Pomol. Soc. 2018, 72, 94–100. [Google Scholar]
- Nicotra, A.; Conte, L.; Moser, L.; Fantechi, P.; Barbagiovanni, I.; Corazza, L.; Vitale, S.; Magnotta, A. Breeding programme for Monilinia laxa (Aderh. et Ruhl.) resistance on apricot. Acta Hortic. 2006, 701, 307–311. [Google Scholar] [CrossRef]
- Kappel, F.; Sholberg, P.L. Screening sweet cherry cultivars from the Pacific Agri-Food Research Centre Summerland breeding program for resistance to brown rot (Monilinia fructicola). Can. J. Plant Sci. 2008, 88, 747–752. [Google Scholar] [CrossRef]
- Northover, J.; Biggs, A.R. Effect of conidial concentration of Monilinia fructicola on brown rot development in detached cherries. Can. J. Plant Pathol. 1995, 17, 205–214. [Google Scholar] [CrossRef]
- Xu, X.M.; Bertone, C.; Berrie, A. Effects of wounding, fruit age and wetness duration on the development of cherry brown rot in the UK. Plant Pathol. 2007, 56, 114–119. [Google Scholar] [CrossRef]
- Hong, C.; Michailides, T.J.; Holtz, B.A. Effects of wounding, inoculum density, and biological control agents on postharvest brown rot of stone fruits. Plant Dis. 1998, 82, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Mari, M.; Casalini, L.; Baraldi, E.; Bertolini, P.; Pratella, G.C. Susceptibility of apricot and peach fruit to Monilinia laxa during phenological stages. Postharvest Biol. Technol. 2003, 30, 105–109. [Google Scholar] [CrossRef]
- Luo, Y.; Michailides, T.J. Factors Affecting Latent Infection of Prune Fruit by Monilinia fructicola. Phytopathology 2001, 91, 864–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira Lino, L.; Quilot-Turion, B.; Dufour, C.; Corre, M.-N.; Lessire, R.; Génard, M.; Poëssel, J.-L. Cuticular waxes of nectarines during fruit development in relation to surface conductance and susceptibility to Monilinia laxa. J. Exp. Bot. 2020. [Google Scholar] [CrossRef] [PubMed]
- Thomidis, T.; Tsipouridis, C.; Darara, V. Seasonal variation of nutrient elements in peach fruits (cv. May Crest) and its correlation with development of Brown rot (Monilinia laxa). Sci. Hortic. 2007, 111, 300–303. [Google Scholar] [CrossRef]
- Curtis, K.M. The morphological aspect of resistance to brown rot in stone fruit. Ann. Bot. 1928, 42, 39–68. [Google Scholar] [CrossRef]
- Emery, K.M.; Michailides, T.J.; Scherm, H. Incidence of latent infection of immature peach fruit by Monilinia fructicola and relationship to brown rot Georgia. Plant Dis. 2000, 84, 853–857. [Google Scholar] [CrossRef] [Green Version]
- Guidarelli, M.; Zubini, P.; Nanni, V.; Bonghi, C.; Rasori, A.; Bertolini, P.; Baraldi, E. Gene expression analysis of peach fruit at different growth stages and with different susceptibility to Monilinia laxa. Eur. J. Plant Pathol. 2014, 140, 503–513. [Google Scholar] [CrossRef]
- Kreidl, S.; Edwards, J.; Villalta, O.N. Assessment of pathogenicity and infection requirements of Monilinia species causing brown rot of stone fruit in Australian orchards. Australas. Plant Pathol. 2015, 44, 419–430. [Google Scholar] [CrossRef]
- Northover, J.; Biggs, A.R. Susceptibility of immature and mature sweet and sour cherries to Monilinia fructicola. Plant Dis. 1990, 74, 280. [Google Scholar] [CrossRef]
- Li, S.-H.; Huguet, J.-G.; Schoch, P.G.; Orlando, P. Response of peach tree growth and cropping to soil water deficit at various phenological stages of fruit development. J. Hortic. Sci. 1989, 64, 541–552. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, Z.; Michailides, T.J. Analysis of factors affecting latent infection and sporulation of Monilinia fructicola on prune fruit. Plant Dis. 2001, 85, 999–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercier, V.; Bussi, C.; Plenet, D.; Lescourret, F. Effects of limiting irrigation and of manual pruning on brown rot incidence in peach. Crop Prot. 2008, 27, 678–688. [Google Scholar] [CrossRef]
- Bussi, C.; Plenet, D.; Merlin, F.; Guillermin, A.; Mercier, V. Limiting brown rot incidence in peach with tree training and pruning. Fruits 2015, 70, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, C.; Chadœuf, J.; Vercambre, G.; Génard, M.; Lescourret, F. Cuticular cracking on nectarine fruit surface: Spatial distribution and development in relation to irrigation and thinning. J. Am. Soc. Hortic. Sci. 2007, 132, 583–591. [Google Scholar] [CrossRef] [Green Version]
- Bellingeri, M.; Quilot-Turion, B.; Lino, L.O.; Bevacqua, D. The crop load affects brown rot progression in fruit orchards: High fruit densities facilitate fruit exposure to spores but reduce the infection rate by decreasing fruit growth and cuticle cracking. Front. Ecol. Evol. 2018, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Elmer, P.A.G.; Spiers, T.M.; Wood, P.N. Effects of pre-harvest foliar calcium sprays on fruit calcium levels and brown rot of peaches. Crop Prot. 2007, 26, 11–18. [Google Scholar] [CrossRef]
- Thomidis, T.; Tsipouridis, C.; Michailides, Z.; Exadaktylou, E. Effect of zinc on the leaf mineral content, yield, fruit weight and susceptibility of peaches to Monilinia laxa. Aust. J. Exp. Agric. 2006, 46, 1203–1205. [Google Scholar] [CrossRef]
- Thomidis, T.; Karagiannidis, N.; Stefanou, S.; Paresidou, M.; Prodromou, I. Influence of boron applications on preharvest and postharvest nectarine fruit rot caused by brown rot. Australas. Plant Pathol. 2017, 46, 177–181. [Google Scholar] [CrossRef]
- De Melo, G.W.B.; Sete, P.B.; Ambrosini, V.G.; Freitas, R.F.; Basso, A.; Brunetto, G. Nutritional status, yield and composition of peach fruit subjected to the application of organic compost. Acta Sci. Agron. 2016, 38, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Gibert, C.; Chadœuf, J.; Nicot, P.; Vercambre, G.; Génard, M.; Lescourret, F. Modelling the effect of cuticular crack surface area and inoculum density on the probability of nectarine fruit infection by Monilinia laxa. Plant Pathol. 2009, 58, 1021–1031. [Google Scholar] [CrossRef]
- Lee, M.-H.H.; Bostock, R.M. Fruit exocarp phenols in relation to quiescence and development of Monilinia fructicola infections in Prunus spp.: A role for cellular redox? Phytopathology 2007, 97, 269–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Benitez, C.; Melgarejo, P.; Sandin-España, P.; Sevilla-Morán, B.; De Cal, A. Degrading enzymes and phytotoxins in Monilinia spp. Eur. J. Plant Pathol. 2019, 154, 305–318. [Google Scholar] [CrossRef]
- Abate, D.; Pastore, C.; Gerin, D.; De Miccolis Angelini, R.M.; Rotolo, C.; Pollastro, S.; Faretra, F. Characterization of Monilinia spp. Populations on Stone Fruit in South Italy. Plant Dis. 2018, 102, 1708–1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Benitez, C.; Melgarejo, P.; De Cal, A.; Fontaniella, B. Microscopic analyses of latent and visible Monilinia fructicola infections in nectarines. PLoS ONE 2016, 11, e0160675. [Google Scholar] [CrossRef] [Green Version]
- Baró-Montel, N.; Vall-llaura, N.; Usall, J.; Teixidó, N.; Naranjo-Ortíz, M.A.; Gabaldón, T.; Torres, R. Pectin methyl esterases and rhamnogalacturonan hydrolases: Weapons for successful Monilinia laxa infection in stone fruit? Plant Pathol. 2019, 68, 1381–1393. [Google Scholar] [CrossRef]
- Garcia-Benitez, C.; Melgarejo, P.; De Cal, A. Fruit maturity and post-harvest environmental conditions influence the pre-penetration stages of Monilinia infections in peaches. Int. J. Food Microbiol. 2017, 241, 117–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, T.; Kudo, M.; Watanabe, S. Fruit cracking and characteristics of fruit thickening in ‘Satonishiki’cherry. J. Jpn. Soc. Hortic. Sci. 1990, 59, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Gibert, C.; Lescourret, F.F.; Génard, M.; Vercambre, G.; Perez Pastor, A.; Pérez Pastor, A. Modelling the effect of fruit growth on surface conductance to water vapour diffusion. Ann. Bot. 2005, 95, 673–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, J.V. Cracking in cherries: VI. Cracking susceptibility in relation to the growth rhythm of the fruit. Acta Agric. Scand. 1973, 23, 52–54. [Google Scholar] [CrossRef]
- Ohta, K.; Hosoki, T.; Matsumoto, K.; Ohya, M.; Ito, N.; Inaba, K. Relationships between fruit cracking and changes of fruit diameter associated with solute flow to fruit in cherry tomatoes. J. Jpn. Soc. Hortic. Sci. 1997, 65, 753–759. [Google Scholar] [CrossRef]
- Papavasileiou, A.; Tanou, G.; Samaras, A.; Samiotaki, M.; Molassiotis, A.; Karaoglanidis, G. Proteomic analysis upon peach fruit infection with Monilinia fructicola and Monilinia laxa identify responses contributing to brown rot resistance. Sci. Rep. 2020, 10, 7807. [Google Scholar] [CrossRef] [PubMed]
- Ziosi, V.; Noferini, M.; Fiori, G.; Tadiello, A.; Trainotti, L.; Casadoro, G.; Costa, G. A new index based on vis spectroscopy to characterize the progression of ripening in peach fruit. Postharvest Biol. Technol. 2008, 49, 319–329. [Google Scholar] [CrossRef]
- Zhang, B.; Peng, B.; Zhang, C.; Song, Z.; Ma, R. Determination of fruit maturity and its prediction model based on the pericarp index of absorbance difference (IAD) for peaches. PLoS ONE 2017, 12, e0177511. [Google Scholar] [CrossRef] [PubMed]
- Gradziel, T.M.; Bostock, R.M.; Adaskaveg, J.E. Resistance to brown rot disease in peach is determined by multiple structural and biochemical components. Acta Hortic. 2003, 622, 347–352. [Google Scholar] [CrossRef]
- Feliziani, E.; Lichter, A.; Smilanick, J.L.; Ippolito, A. Disinfecting agents for controlling fruit and vegetable diseases after harvest. Postharvest Biol. Technol. 2016, 122, 53–69. [Google Scholar] [CrossRef]
- Fourie, J.F.; Holz, G. Artificial inoculation of stone fruit with Botrytis cinerea, Monilinia laxa and Rhizopus stolonifer*. Phytophylactica 1985, 181, 179–181. [Google Scholar]
- Janisiewicz, W.J.; Biggs, A.R.; Jurick, W.M.; Vico, I.; Conway, W.S. Biological characteristics of Monilinia fructicola isolates from stone fruits in eastern West Virginia. Can. J. Plant Pathol. 2013, 35, 315–327. [Google Scholar] [CrossRef]
- Spadoni, A.; Cappellin, L.; Neri, F.; Algarra Alarcon, A.; Romano, A.; Guidarelli, M.; Gasperi, F.; Biasioli, F.; Mari, M. Effect of hot water treatment on peach volatile emission and Monilinia fructicola development. Plant Pathol. 2015, 64, 1120–1129. [Google Scholar] [CrossRef]
- De Cal, A.; M.-SAGASTA, E.; Melgarejo, P. Biological control of peach twig blight (Monilinia laxa) with Penicillium frequentans. Plant Pathol. 1990, 39, 612–618. [Google Scholar] [CrossRef]
- Tamm, L.; Fluckiger, W. Influence of temperature and moisture on growth, spore production, and conidial germination of Monilinia laxa. Phytopathology 1993, 83, 1321–1326. [Google Scholar] [CrossRef]
- Phillips, D.J. Effect of Temperature on Monilinia fructicola Conidia Produced on Fresh Stone Fruits. Plant Dis. 1984, 68, 610. [Google Scholar] [CrossRef]
- Obi, V.I.; Barriuso, J.J.; Gogorcena, Y. Peach Brown Rot: Still in Search of an Ideal Management Option. Agriculture 2018, 8, 125. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Michailides, T.J.; Morgan, D.P.; Krueger, W.H.; Buchner, R.P. Inoculum dynamics, fruit infection, and development of brown rot in prune orchards in California. Phytopathology 2005, 95, 1132–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mwamburi, L.A.; Laing, M.D.; Miller, R.M. Effect of surfactants and temperature on germination and vegetative growth of Beauveria bassiana. Brazilian J. Microbiol. 2015, 46, 67–74. [Google Scholar] [CrossRef]
- Rodríguez-Pires, S.; Garcia-Companys, M.; Espeso, E.A.; Melgarejo, P.; de Cal, A. Influence of light on the Monilinia laxa–stone fruit interaction. Plant Pathol. 2021, 70, 326–335. [Google Scholar] [CrossRef]
- Tian, S.P.; Bertolini, P. Effect of temperature during conidial formation of Monilinia laxa on conidial size, germination and infection of stored nectarines. J. Phytopathol. 1999, 147, 635–641. [Google Scholar] [CrossRef]
- Peschel, S.; Knoche, M. Characterization of microcracks in the cuticle of developing sweet cherry fruit. J. Am. Soc. Hortic. Sci. 2005, 130, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Schumann, C.; Winkler, A.; Brüggenwirth, M.; Köpcke, K.; Knoche, M. Crack initiation and propagation in sweet cherry skin: A simple chain reaction causes the crack to ‘run’. PLoS ONE 2019, 14, e0219794. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Kumar, P.; Malik, A. Evaluation of Beauveria bassiana spore compatibility with surfactants. Int. J. Med. Heal. Sci. 2013, 7, 8–12. [Google Scholar]
- Oliveira, D.G.P.; Pauli, G.; Mascarin, G.M.; Delalibera, I. A protocol for determination of conidial viability of the fungal entomopathogens Beauveria bassiana and Metarhizium anisopliae from commercial products. J. Microbiol. Methods 2015, 119, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, A.D. Evolutionary Significance of Phenotypic Plasticity in Plants. Adv. Genet. 1965, 13, 115–155. [Google Scholar] [CrossRef]
- Pascal, T.; Kervella, J.; Pfeiffer, F.G.; Sauge, M.H.; Esmenjaud, D. Evaluation of the interspecific progeny Prunus persica cv Summergrand × Prunus davidiana for disease resistance and some agronomic features. Acta Hortic. 1998, 465, 185–191. [Google Scholar] [CrossRef]




| Inoculation Test | Correlation | p-Value |
|---|---|---|
| field 2013 vs. field 2014 | 0.2861 | 0.0063 * |
| field 2013 vs. field 2015 | −0.0819 | 0.5004 |
| field 2014 vs. field 2015 | 0.3148 | 0.003 * |
| lab 2013 vs. lab 2014 | 0.288 | 0.003 * |
| lab 2013 vs. lab 2015 1 | −0.0292 | 0.8162 |
| lab 2014 vs. lab 2015 1 | 0.2401 | 0.0522 |
| field 2013 vs. lab 2013 | 0.2947 | 0.0056 * |
| field 2013 vs. lab 2014 | −0.1151 | 0.3123 |
| field 2013 vs. lab 2015 1 | 0.049 | 0.7223 |
| field 2014 vs. lab 2014 | 0.1704 | 0.0884 |
| field 2014 vs. lab 2015 1 | 0.2301 | 0.1509 |
| field 2015 vs. lab 2015 1 | 0.4562 | 10−4 *** |
| field 2015 vs. natural infection 2015 | 0.3714 | 10−4 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, M.H.; Bassi, D.; Corre, M.-N.; Lino, L.O.; Signoret, V.; Quilot-Turion, B.; Cirilli, M. Phenotyping Brown Rot Susceptibility in Stone Fruit: A Literature Review with Emphasis on Peach. Horticulturae 2021, 7, 115. https://0-doi-org.brum.beds.ac.uk/10.3390/horticulturae7050115
Mustafa MH, Bassi D, Corre M-N, Lino LO, Signoret V, Quilot-Turion B, Cirilli M. Phenotyping Brown Rot Susceptibility in Stone Fruit: A Literature Review with Emphasis on Peach. Horticulturae. 2021; 7(5):115. https://0-doi-org.brum.beds.ac.uk/10.3390/horticulturae7050115
Chicago/Turabian StyleMustafa, Majid Hassan, Daniele Bassi, Marie-Noëlle Corre, Leandro Oliveira Lino, Véronique Signoret, Bénédicte Quilot-Turion, and Marco Cirilli. 2021. "Phenotyping Brown Rot Susceptibility in Stone Fruit: A Literature Review with Emphasis on Peach" Horticulturae 7, no. 5: 115. https://0-doi-org.brum.beds.ac.uk/10.3390/horticulturae7050115