Synthesis, Crystal Structure and Magnetic Properties of 1D Chain Complexes Based on Azo Carboxylate Oxime Ligand
Abstract
:1. Introduction
2. Results
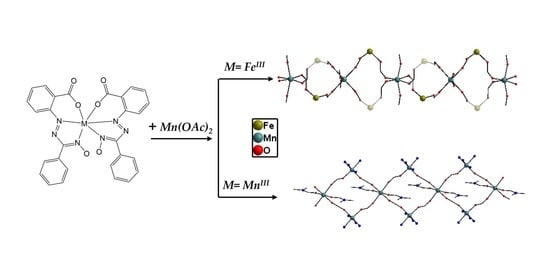
2.1. Crystal Structures
2.2. Magnetic Properties
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Physical Measurements
4.3. X-ray Crystallography
4.4. The Preparation of Complexes 1 and 2
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.-L.; Wang, Z.-M.; Gao, S. Strategies towards single-chain magnets. Coord. Chem. Rev. 2010, 254, 1081–1100. [Google Scholar] [CrossRef]
- Wang, S.; Ding, X.-H.; Li, Y.-H.; Huang, W. Dicyanometalate chemistry: A type of versatile building block for the construction of cyanide-bridged molecular architectures. Coord. Chem. Rev. 2012, 256, 439–464. [Google Scholar] [CrossRef]
- Li, Y.-H.; He, W.-R.; Ding, X.-H.; Wang, S.; Cui, L.-F.; Huang, W. Cyanide-bridged assemblies constructed from capped tetracyanometalate building blocks [MA(ligand)(CN)4]1−/2− (MA = Fe or Cr). Coord. Chem. Rev. 2012, 256, 2795–2815. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S.-Q.; Cui, A.-L.; Kou, H.-Z. A cyano-bridged single-chain magnet featuring alternate high- and low-spin manganese(III) porphyrins. Chem. Commun. 2014, 50, 2120–2122. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Zhao, H.-H.; Funck, E.; Dunbar, K.R. A Single-Chain Magnet Tape Based on Hexacyanomanganate(III). Angew. Chem. Int. Ed. 2015, 54, 5583–5587. [Google Scholar] [CrossRef]
- Palii, A.; Ostrovsky, S.M.; Klokishner, S.I.; Tsukerblat, B.S.; Dunbar, K.R. Highly Anisotropic Orbitally Dependent Superexchange in Cyano-Bridged Clusters Containing Mn(III) and Mn(II) Ions. ChemPhysChem 2006, 7, 871–879. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnets; Wiley-VCH: NewYork, NY, USA, 1993; pp. 175–181. [Google Scholar]
- Miyasaka, H.; Madanbashi, T.; Saitoh, A.; Motokawa, N.; Ishikawa, R.; Yamashita, M.; Bahr, S.; Wernsdorfer, W.; Clerac, R. Cyano-Bridged MnIII-MIII Single-Chain Magnets with MIII=CoIII, FeIII, MnIII, and CrIII. Chem. Eur. J. 2012, 18, 3942–3954. [Google Scholar] [CrossRef] [PubMed]
- Buades, A.B.; Arderiu, V.S.; Maxwell, L.; Amoza, M.; Choquesillo-Lazarte, D.; Aliaga-Alcalde, N.; Vinas, C.; Teixidor, F.; Ruiz, E. Slow-spin relaxation of a low-spin S = 1/2 FeIII carborane complex. Chem. Commun. 2019, 55, 3825–3828. [Google Scholar] [CrossRef] [PubMed]
- Cirera, J.; Ruiz, E.; Alvarez, S.; Neese, F.; Kortus, J. How to Build Molecules with Large Magnetic Anisotropy. Chem. Eur. J. 2009, 15, 4078–4087. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, R.; Madalan, A.M.; Costes, J.-P.; Wernsdorfer, W.; Andruh, M. A heterotrimetallic 3d–3d′–4f single chain magnet constructed from anisotropic high-spin 3d–4f nodes and paramagnetic spacers. Dalton Trans. 2010, 39, 4734–4736. [Google Scholar] [CrossRef]
- Dhers, S.; Feltham, H.L.C.; Rouzieres, M.; Clerac, R.; Brooker, S. Discrete versus Chain Assembly: Hexacyanometallate Linkers and Macrocyclic {3d–4f} Single-Molecule Magnet Building Blocks. Inorg. Chem. 2019, 58, 5543–5554. [Google Scholar] [CrossRef] [PubMed]
- Pinkowicz, D.; Southerland, H.I.; Avendano, C.; Prosvirin, A.; Sanders, C.; Wernsdorfer, W.; Pedersen, K.S.; Dreiser, J.; Clerac, R.; Nehrkorn, J.; et al. Cyanide Single-Molecule Magnets Exhibiting Solvent Dependent Reversible “On” and “Off” Exchange Bias Behavior. J. Am. Chem. Soc. 2015, 137, 14406–14422. [Google Scholar] [CrossRef]
- Bai, Y.-L.; Tao, J.; Wernsdorfer, W.; Sato, O.; Huang, R.-B.; Zheng, L.-S. Coexistence of Magnetization Relaxation and Dielectric Relaxation in a Single-Chain Magnet. J. Am. Chem. Soc. 2006, 128, 16428–16429. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Biswas, R.; Drew, M.G.B.; Ida, Y.; Ishida, T.; Ghosh, A. Structure and magnetic properties of an unprecedented syn-anti μ-nitrito-1κO:2κO′ bridged Mn(III)-salen complex and its isoelectronic and isostructural formate analogue. Dalton Trans. 2011, 40, 3295–3304. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Chakravorty, A. Carboxyl-Bonded Low-Spin Iron(III). Chemistry of a Family of Coordination Type cis-FeN4O2. Inorg. Chem. 1996, 35, 1935–1939. [Google Scholar]
- Ganguly, S.; Karmakar, S.; Chakravorty, A. First Examples of Carboxyl-Bonded Low-Spin Manganese(III) Complexes. Inorg. Chem. 1997, 36, 116–118. [Google Scholar] [CrossRef]
- Chen, X.; Cui, A.-L.; Liu, C.-M.; Kou, H.-Z. Synthesis, structures, and magnetic properties of carboxylate-bridged trinuclear complexes based on low-spin manganese(III)/iron(III) building blocks. Transit. Met. Chem. 2013, 38, 683–688. [Google Scholar] [CrossRef]
- Kou, H.Z.; Zhang, Y.D.; Cui, A.L. Carboxylate-bridged helical chains based on an azo carboxylate oximate ligand. Sci. China Chem. 2012, 55, 1042–1046. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE, version 2.1; University of Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Carlin, R.L. Magnetochemistry; Springer: Berlin/Heidelberg, Germany, 1986; pp. 14–18. [Google Scholar]
- Pei, Y.; Nakatani, K.; Kahn, O.; Sletten, J.; Renard, J.P. Magnetism of alternating bimetallic chains: Application to triaqua(oxamido-N-benzoato-N-propionato)coppermanganese monohydrate. Inorg. Chem. 1989, 28, 3170–3175. [Google Scholar] [CrossRef]
- Fisher, M.E. Magnetism in One-Dimensional Systems—The Heisenberg Model for Infinite Spin. Am. J. Phys. 1964, 32, 343–346. [Google Scholar] [CrossRef]
- Chiari, B.; Cinti, A.; Piovesana, O.; Zanazzi, P.F. Exchange Interactions in the Bimetallic Chain Compound Cu(ethylenediamine)2MnCl4. Inorg. Chem. 1995, 34, 2652–2657. [Google Scholar] [CrossRef]
- Ni, Z.-H.; Kou, H.-Z.; Zhao, Y.-H.; Zheng, L.; Wang, R.-J.; Cui, A.-L. Fe(bpb)(CN)2]− as a Versatile Building Block for the Design of Novel Low-Dimensional Heterobimetallic Systems: Synthesis, Crystal Structures, and Magnetic Properties of Cyano-Bridged FeIII−NiII Complexes [(bpb)2− = 1,2-Bis(pyridine-2-carboxamido)benzenate]. Inorg. Chem. 2005, 44, 2050–2059. [Google Scholar]
- Li, L.; Liao, D.; Jiang, Z.; Yan, S. A 3-D Polymer, Mn(NITpPy)2(tp)(H2O)2: Crystal Structure and Magnetic Properties. Inorg. Chem. 2002, 41, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-L.; Liu, N.; Zhang, J.-Y.; Gao, E.-Q. Assembling the Cage-Based Metal−Organic Network from a Cubic Metalloligand. Inorg. Chem. 2007, 46, 1034–1035. [Google Scholar] [CrossRef] [PubMed]






| 1 | 2 | |
|---|---|---|
| Formula | C58H44Fe2MnN12O14 | C65H57Mn3N15O15 |
| Fw | 1299.69 | 1453.07 |
| T/K | 293(2) | 293(2) |
| Crystal system | Tetragonal | Triclinic |
| Space group | P4(2)/n | P-1 |
| a/Å | 20.651(3) | 9.2247(6) |
| b/Å | 20.651(3) | 13.2300(7) |
| c/Å | 13.641(3) | 14.2577(8) |
| α/° | 90 | 82.3377(15) |
| β/° | 90 | 79.666(2) |
| γ/° | 90 | 85.466(2) |
| V/Å3 | 5818(2) | 1693.83(17) |
| Z | 4 | 1 |
| ρcalcd/g cm−1 | 1.484 | 1.496 |
| Reflections collected | 6637 | 7655 |
| GOF on F2 | 1.090 | 1.153 |
| R1 [I > 2σ(I)] | 0.0579 | 0.0485 |
| wR2 (all data) | 0.1972 | 0.1631 |
| CCDC | 2044110 | 2044111 |
| Complex 1 (M = Fe) | Et4N[Fe(L)2] [16] (M = Fe) | Complex 2 (M = Mn) | Et4N[Mn(L)2] [17] (M = Mn) | |
|---|---|---|---|---|
| M1-O1 | 1.935(3) | 1.919(6) | 1.939(3) | 1.906(7) |
| M1-O5 | 1.929(2) | 1.879(6) | 1.929(3) | 1.906(7) |
| M1-N1 | 1.895(3) | 1.892(6) | 1.924(3) | 1.929(6) |
| M1-N3 | 1.887(3) | 1.907(6) | 1.950(3) | 1.950(7) |
| M1-N4 | 1.904(3) | 1.888(6) | 1.934(3) | 1.929(6) |
| M1-N6 | 1.895(3) | 1.899(7) | 1.953(3) | 1.950(7) |
| M1-O5-C15 | 127.8(2) | 129.0(3) | 131.3(2) | 132.7(3) |
| M1-O1-C1 | 127.6(2) | 130.3(3) | 131.7(2) | 132.7(3) |
| O1-M1-N3 | 172.54(13) | 170.7(2) | 170.11(12) | 168.4(3) |
| O5-M1-N6 | 172.36(13) | 165.9(3) | 170.33(12) | 168.4(3) |
| N1-M1-N4 | 178.44(13) | 178.5(3) | 164.92(12) | 173.4(5) |
| 1 | 2 | ||
|---|---|---|---|
| Mn1-O2A | 2.183(3) | Mn2-O2 | 2.151(3) |
| Mn1-O2B | 2.183(3) | Mn2-O2A | 2.151(3) |
| Mn1-O6 | 2.192(3) | Mn2-O4 | 2.162(3) |
| Mn1-O6A | 2.192(3) | Mn2-O4A | 2.162(3) |
| Mn1-O7A | 2.181(7) | Mn2-O7 | 2.167(3) |
| Mn1-O7B | 2.193(5) | Mn2-O7A | 2.167(3) |
| C1A-O2A-Mn1 | 144.6(3) | Mn2-O2-C1 | 167.8(3) |
| C15-O6-Mn1 | 139.6(3) | Mn2-O4A-C15A | 169.3(3 |
| O7A-Mn1-O7B | 164.58(25) | O2B-Mn1-O6 | 172.65(11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, M.; Chen, X.; Kou, H.-Z. Synthesis, Crystal Structure and Magnetic Properties of 1D Chain Complexes Based on Azo Carboxylate Oxime Ligand. Magnetochemistry 2021, 7, 105. https://0-doi-org.brum.beds.ac.uk/10.3390/magnetochemistry7070105
Zeng M, Chen X, Kou H-Z. Synthesis, Crystal Structure and Magnetic Properties of 1D Chain Complexes Based on Azo Carboxylate Oxime Ligand. Magnetochemistry. 2021; 7(7):105. https://0-doi-org.brum.beds.ac.uk/10.3390/magnetochemistry7070105
Chicago/Turabian StyleZeng, Min, Xi Chen, and Hui-Zhong Kou. 2021. "Synthesis, Crystal Structure and Magnetic Properties of 1D Chain Complexes Based on Azo Carboxylate Oxime Ligand" Magnetochemistry 7, no. 7: 105. https://0-doi-org.brum.beds.ac.uk/10.3390/magnetochemistry7070105