New Photomagnetic Ionic Salts Based on [MoIV(CN)8]4− and [WIV(CN)8]4− Anions †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. Crystal Structure Description of the Compounds
2.2.1. [{Cu(tren)}3(μ-tren)]4[Mo(CN)8]6·45H2O·2CH3OH (1)
2.2.2. [{Zn(tren)}3(μ-tren)]2[Mo(CN)8]3·18H2O (2)
2.2.3. [{Zn(tren)}3(μ-tren)]2[W(CN)8]3·17H2O (3)
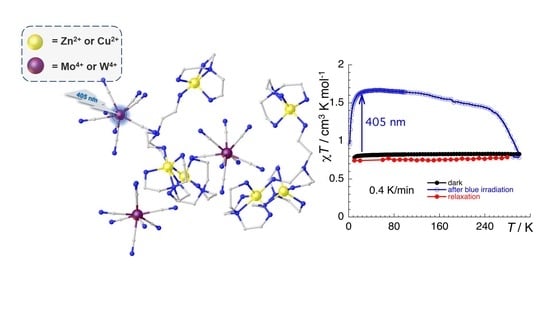
2.3. Magnetic and Photomagnetic Properties
2.3.1. CutrenMo Compound (1)
2.3.2. ZntrenMo Compound (2)
2.3.3. ZntrenW Compound (3)
3. Discussion and Conclusions
4. Materials and Methods
4.1. General Remarks
4.2. Synthesis
4.2.1. Preparation of CutrenMo (1)
4.2.2. Preparation of ZntrenMo (2)
4.2.3. Preparation of ZntrenW (3)
4.3. Physical Measurements
4.3.1. Infrared Spectroscopy
4.3.2. UV-Visible Spectroscopy
4.3.3. Magnetic Measurements
4.3.4. Powder X-ray Diffraction (PXRD)
4.3.5. Elemental Analysis
4.3.6. Single-Crystal X-ray Crystallography
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Compound/CCDC Number | 1/2083835 | 2/2083836 | 3/2083837 |
|---|---|---|---|
| Formula | Mo6Cu12C146H386N112O47 | Mo3Zn6C72H180N56O18 | W3Zn6C72H178N56O17 |
| Dcalc./g cm−3 | 1.424 | 1.406 | 1.537 |
| μmm−1 | 4.121 | 4.036 | 3.760 |
| Formula Weight | 5801.61 | 2798.68 | 3044.39 |
| T/K | 130(2) | 130(2) | 150(2) |
| Crystal System | monoclinic | monoclinic | monoclinic |
| Space Group | P21/c | Cc | Cc |
| a/Å | 30.4150(4) | 32.1487(9) | 31.8599(9) |
| b/Å | 37.1820(5) | 16.8699(3) | 16.9574(3) |
| c/Å | 22.5401(2) | 25.4013(6) | 25.3107(6) |
| β/° | 98.0410(10) | 106.487(3) | 106.013(3) |
| V/Å3 | 25239.8(5) | 13209.8(6) | 13143.8(6) |
| Z | 4 | 4 | 4 |
| Z’ | 1 | 1 | 1 |
| Wavelength/Å | 1.54184 | 1.54184 | 0.71073 |
| Radiation type | CuKa | CuKa | MoKa |
| Qmin/° | 2.309 | 2.867 | 1.701 |
| Qmax/° | 73.591 | 74.083 | 26.733 |
| Measured refl. | 199397 | 46339 | 151852 |
| Measured indep. refl. | 49550 | 19947 | 27860 |
| Observed indep. refl. | 35018 | 19214 | 22758 |
| Rint | 0.0760 | 0.0775 | 0.0796 |
| Parameters | 2908 | 1450 | 1438 |
| Restraints | 0 | 2 | 20 |
| Largest diff. peak (e-.A−3) | 19.876 | 1.7437 | 1.938 |
| Deepest diff. hole (e-.A−3) | −2.959 | −1.561 | −1.187 |
| GooF (S) | 1.764 | 1.042 | 1.018 |
| wR2 (all data) | 0.4624 | 0.2071 | 0.0959 |
| wR2 | 0.4298 | 0.2049 | 0.0894 |
| R1 (all data) | 0.2056 | 0.0791 | 0.0574 |
| R1 | 0.1830 | 0.0769 | 0.0397 |
| [{Cu(tren)}3(μ-tren)]12[Mo(CN)8]6·45H2O·2MeOH 1 | ||||||
| Mo(CN)8 | Mo1 | Mo2 | Mo3 | Mo4 | Mo5 | Mo6 |
| SAPR | 0.218 | 0.254 | 0.535 | 0.230 | 0.652 | 0.321 |
| TDD | 2.201 | 2.008 | 1.679 | 1.899 | 1.232 | 1.794 |
| JBTPR | 2.821 | 2.284 | 2.252 | 2.660 | 1.971 | 2.360 |
| BTPR | 2.220 | 1.709 | 1.649 | 2.077 | 1.384 | 1.738 |
| CuN5 | Cu1 | Cu2 | Cu3 | Cu4 | Cu5 | Cu6 |
| TBPY | 0.420 | 0.377 | 0.904 | 0.540 | 0.368 | 0.443 |
| SPY | 4.269 | 4.425 | 3.226 | 4.175 | 4.805 | 4.970 |
| JTBPY | 3.967 | 3.731 | 3.928 | 3.661 | 3.794 | 3.911 |
| CuN5 | Cu7 | Cu8 | Cu9 | Cu10 | Cu11 | Cu12 |
| TBPY | 0.380 | 0.321 | 2.225 | 0.348 | 0.514 | 0.789 |
| SPY | 4.975 | 4.832 | 1.491 | 5.009 | 4.016 | 3.401 |
| JTBPY | 3.966 | 3.639 | 4.730 | 3.705 | 4.016 | 3.977 |
| [{Zn(tren)}3(μ-tren)]2[Mo(CN)8]3·18H2O 2 | ||||||
| Mo(CN)8 | Mo1 | Mo2 | Mo3 | |||
| SAPR | 0.232 | 0.590 | 0.836 | |||
| TDD | 2.106 | 1.567 | 1.372 | |||
| JBTPR | 2.245 | 1.907 | 1.819 | |||
| BTPR | 1.663 | 1.422 | 1.111 | |||
| ZnN5 | Zn1 | Zn2 | Zn3 | Zn4 | Zn5 | Zn6 |
| TBPY | 0.817 | 0.773 | 1.076 | 0.771 | 0.814 | 0.760 |
| SPY | 5.582 | 4.987 | 5.149 | 5.715 | 4.698 | 5.558 |
| JTBPY | 2.594 | 2.368 | 2.152 | 2.654 | 2.510 | 2.548 |
| [{Zn(tren)}3(μ-tren)]2[W(CN)8]3·17H2O 3 | ||||||
| W(CN)8 | W1 | W2 | W3 | |||
| SAPR | 0.969 | 0.295 | 0.532 | |||
| TDD | 1.110 | 1.922 | 1.781 | |||
| JBTPR | 1.868 | 2.104 | 1.934 | |||
| BTPR | 1.151 | 1.540 | 1.355 | |||
| ZnN5 | Zn1 | Zn2 | Zn3 | Zn4 | Zn5 | Zn6 |
| TBPY | 0.792 | 0.916 | 0.742 | 0.976 | 0.852 | 0.889 |
| SPY | 5.393 | 4.487 | 5.890 | 4.707 | 4.803 | 5.930 |
| JTBPY | 2.514 | 2.535 | 2.449 | 2.285 | 2.290 | 2.413 |
References
- Adamson, A.; Perumareddi, J.R. Photochemistry of aqueous octacyanomolybdate(IV) ion, Mo(CN)8-4. Inorg. Chem. 1964, 4, 247–248. [Google Scholar] [CrossRef]
- Samotus, A.; Szklarzewicz, J. Photochemistry of transition metal octacyanides and related compounds. Past, present and future. Coord. Chem. Rev. 1993, 125, 63–74. [Google Scholar] [CrossRef]
- Mitra, R.; Mohan, H. Tetracyano-oxo(hydroxo) complexes of Mo(IV): Their interconverson ans relation o the primary photoaquation product of the Mo(CN)8-4 ion. J. Inorg. Nucl. Chem. 1974, 36, 3739–3743. [Google Scholar] [CrossRef]
- Sieklucka, B.; Podgajny, R.; Korzeniak, T.; Nowicka, B.; Pinkowicz, D.; Koziel, M. A decade of Octacyanides in Polynuclear Molecular materials. Eur. J. Inorg. Chem. 2011, 305–326. [Google Scholar] [CrossRef]
- Chorazy, S.; Zakrzewski, J.J.; Magott, M.; Korzeniak, T.; Nowicka, B.; Pinkowicz, D.; Podgajny, R.; Sieklucka, B. Octacyanidometallates for multifunctional molecule-based materials. Chem. Soc. Rev. 2020, 49, 5945–6001. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Pillet, S.; de Graaf, C.; Magott, M.; Bendeif, E.E.; Guionneau, P.; Rouzieres, M.; Marvaud, V.; Stefanczyk, O.; Pinkowicz, D.; et al. Photoinduced Mo-CN Bond Breakage in Octacyanomolybdate Leading to Spin Triplet Trapping. Angew. Chem. Int. Ed. Engl. 2020, 59, 3117–3121. [Google Scholar] [CrossRef] [PubMed]
- Mobian, P.; Kern, J.-P.; Sauvage, J.-P. Light-Driven Machine Prototypes Based on Dissociative Excited States: Photoinduced Decoordination and Thermal Recoordination of a Ring in a Ruthenium(ii)-Containing [2]Catenane. Angew. Chem. Int. Ed. 2004, 43, 2392–2395. [Google Scholar] [CrossRef] [PubMed]
- Soupart, A.; Alary, F.; Heully, J.-L.; Elliott, P.I.P.; Dixon, I.M. Recent progress in ligand release reaction mechanims: Theortical insights focusing on Ru(II) 3MC states. Coord. Chem. Rev. 2020, 408, 231184. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Browne, W.R. Photochemistry of iron complexes. Coord. Chem. Rev. 2018, 374, 15–35. [Google Scholar] [CrossRef]
- Venkataramani, S.; Jana, U.; Dommaschk, M.; Sonnichsen, F.D.; Tuzcek, F.; Herges, R. Magnetic bistability of molecules in homogeneous solution at room temperature. Science 2011, 331, 445–448. [Google Scholar] [CrossRef]
- Su, S.-Q.; Wu, S.-Q.; Baker, M.L.; Bencok, P.; Azuma, N.; Miyazaki, Y.; Nakano, M.; Kang, S.; Shiota, Y.; Yoshizawa, K.; et al. Quenching and Restoration of Orbital Angular Momentum through a Dynamic Bond in a Cobalt(II) Complex. J. Am. Chem. Soc. 2020, 26, 11434–11441. [Google Scholar] [CrossRef] [PubMed]
- Guionneau, P.; Le Gac, F.; Kaiba, A.; Costa, J.S.; Chasseau, D.; Létard, J.-F. A reversible metal–ligand bond break associated to a spin-crossover. Chem. Commun. 2007, 3723, 3723–3725. [Google Scholar] [CrossRef]
- Aguila, D.; Dechambenoit, P.; Rouzières, M.; Mathonière, C.; Clérac, R. Direct crystallographic evidence of the reversible photo-formation and thermo-rupture of a coordination bond inducing spin-crossover phenomenon. Chem. Comm. 2017, 53, 11588–11591. [Google Scholar] [CrossRef]
- Perumareddi, J.R.; Liehr, A.; Adamson, A. Ligand Field Theory of Transition Metal Cyanide Complexes. Part I. The Zero, One and Two Electron or Hole Configuration. J. Am. Chem. Soc. 1963, 85, 249–259. [Google Scholar] [CrossRef]
- Duggan, M.; Ray, N.; Hataway, B.; Tomlison, G.; Brint, P.; Pelin, K. Crystal Structure and Electronic Properties of Ammine[tris(2-aminoethyl) amine]copper(II) Diperchlorate and Potassium Penta-amminecopper(II)Tris( hexaf luorophosphate). J. Chem. Soc. Dalton Trans. 1980, 1342–1348. [Google Scholar] [CrossRef]
- Shek, I.Y. A novel trinuclear copper(II) complex bridged by tren: [Cu3(tren)4][Pt(CN)4]3·2H2O. New J. Chem. 1999, 23, 1049–1050. [Google Scholar] [CrossRef]
- Cirera, J.; Ruiz, E.; Alvarez, S. Continuous Shape measures as a stereochemical tools in Organometallic Chemistry. Organometallics 2005, 24, 1556–1562. [Google Scholar] [CrossRef]
- Korzeniak, T.; Mathonière, C.; Kaiba, A.; Guionneau, P.; Koziel, M.; Sieklucka, B. First example of photomagnetic effects in ionic pairs [Ni(bipy)3]2[Mo(CN)8]·12H2O. Inorg. Chim. Acta 2008, 361, 3500–3504. [Google Scholar] [CrossRef]
- Bridonneau, N.; Long, J.; Cantin, J.L.; Von Bardeleben, J.; Pillet, S.; Bendeif, E.E.; Aravena, D.; Ruiz, E.; Marvaud, V. First Evidence of Light-induced Spin Transition in Molybdenum(IV). Chem. Commun. 2015, 51, 8229–8232. [Google Scholar] [CrossRef]
- Magott, M.; Stefanczyk, O.; Sieklucka, B.; Pinkowicz, D. Octacyanidotungstate(IV) Coordination Chains Demonstrate a Light-Induced Excited Spin State Trapping Behavior and Magnetic Exchange Photoswitching. Angew. Chem. Int. Ed. 2017, 56, 13283–13287. [Google Scholar] [CrossRef] [PubMed]
- Magott, M.; Reczyński, M.; Gaweł, B.; Sieklucka, B.; Pinkowicz, D. A photomagnetic sponge: High-temperature light-induced ferrimagnet controlled by water sorption. J. Am. Chem. Soc. 2018, 140, 15876–15882. [Google Scholar] [CrossRef]
- Ohkoshi, S.I.; Tokoro, H.; Hozumi, T.; Zhang, Y.; Hashimoto, K.; Mathonière, C.; Bord, I.; Rombaut, G.; Verelst, M.; Cartier Dit Moulin, C. Photoinduced magnetization in copper octacyanomolybdate. J. Am. Chem. Soc. 2006, 128, 270–277. [Google Scholar] [CrossRef]
- Chastanet, G.; Desplanches, C.; Baldé, C.; Rosa, P.; Marchivie, M.; Guionneau, P.A. Critical review of the T(LIESST) temepeature in soun crrossover materails—What it is and what it is not. Chem. Sq. 2018, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Szklarzewicz, J.; Matoga, D.; Lewiński, K. Photocatalytical Decomposition of Hydrazine in K4[Mo(CN)8] Solution: X-ray Crystal Structure of (PPh4)2[Mo(CN)4O(NH3)]·2H2O. Inorg. Chim. Acta 2007, 360, 2002–2008. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Acta Crystallogr, Sect. A. A Found Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolomanov, O.V.; Blake, A.J.; Champness, N.R.; Schroder, M. OLEX: New software for visualization and analysis of extended crystal structures. J. Appl. Crystallogr. 2003, 36, 1283–1284. [Google Scholar] [CrossRef] [Green Version]
- Llunell, M.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE v. 2.1.; University of Barcelona: Barcelona, Spain, 2013. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, X.; Guionneau, P.; Lafon, E.; Perot, S.; Kauffmann, B.; Mathonière, C. New Photomagnetic Ionic Salts Based on [MoIV(CN)8]4− and [WIV(CN)8]4− Anions. Magnetochemistry 2021, 7, 97. https://0-doi-org.brum.beds.ac.uk/10.3390/magnetochemistry7070097
Qi X, Guionneau P, Lafon E, Perot S, Kauffmann B, Mathonière C. New Photomagnetic Ionic Salts Based on [MoIV(CN)8]4− and [WIV(CN)8]4− Anions. Magnetochemistry. 2021; 7(7):97. https://0-doi-org.brum.beds.ac.uk/10.3390/magnetochemistry7070097
Chicago/Turabian StyleQi, Xinghui, Philippe Guionneau, Enzo Lafon, Solène Perot, Brice Kauffmann, and Corine Mathonière. 2021. "New Photomagnetic Ionic Salts Based on [MoIV(CN)8]4− and [WIV(CN)8]4− Anions" Magnetochemistry 7, no. 7: 97. https://0-doi-org.brum.beds.ac.uk/10.3390/magnetochemistry7070097