Phytoremediation of Soil Contaminated with Lithium Ion Battery Active Materials—A Proof-of-Concept Study
Abstract
:1. Introduction
2. Results and Discussion
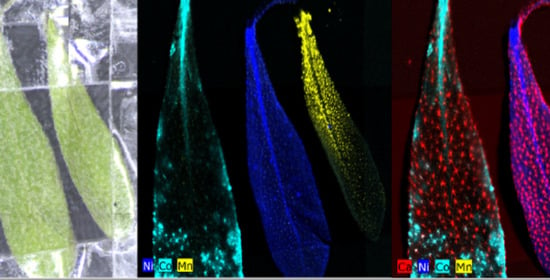
2.1. Hyperaccumulation of Ni, Co, and Mn in Alyssum Murale
2.2. Phytoremediation of Battery Materials—Proof-of-Concept
3. Materials and Methods
3.1. Contamination Studies with Alyssum Murale
Accumulation of Ni, Co, and Mn from Irrigation Water
3.2. Electrode Material Dissolution
Li, Ni, Co, and Mn Accumulation from Electrode Material
3.3. Inductively Coupled Plasma-Optical Emission Spectroscopy
3.3.1. Micro X-ray Fluorescence Spectroscopy
3.3.2. Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 23, 11433–11456. [Google Scholar] [CrossRef] [PubMed]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium Ion, Lithium Metal, and Alternative Rechargeable Battery Technologies: The Odyssey for High Energy Density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Betz, J.; Bieker, G.; Meister, P.; Placke, T.; Winter, M.; Schmuch, R. Theoretical versus Practical Energy: A Plea for More Transparency in the Energy Calculation of Different Rechargeable Battery Systems. Adv. Energy Mater. 2019, 9, 1–18. [Google Scholar] [CrossRef]
- Christophe Pillot. The Rechargeable Battery Market and Main Trends 2018–2030. In Proceedings of the Advanced Automotive Battery Conference, San Diego, CA, USA, 24 May 2019.
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and Cost of Materials for Lithium-Based Rechargeable Automotive Batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Rothermel, S.; Krueger, S.; Winter, M.; Nowak, S. Hydrometallurgical Processing and Thermal Treatment of Active Materials. In Recycling of Lithium-Ion Batteries; Springer International Publishing: Basel, Swiss, 2018; pp. 219–246. [Google Scholar]
- Diekmann, J.; Gruetzke, M.; Loellhoeffel, T.; Petermann, M.; Rothermel, S.; Winter, M.; Nowak, S.; Kwade, A. Recycling of Lithium Ion Batteries—Reapplication of the Recovered Materials as Lithium Ion Battery Materials. In Recycling of Lithium-Ion Batteries; Springer International Publishing: Basel, Swiss, 2018; pp. 39–51. [Google Scholar]
- Nowak, S.; Winter, M. The Role of Sub- and Supercritical CO2 as “Processing Solvent” for the Recycling and Sample Preparation of Lithium Ion Battery Electrolytes. Molecules 2017, 22, 403. [Google Scholar] [CrossRef]
- Rothermel, S.; Evertz, M.; Kasnascheetw, J.; Qi, X.; Grützke, M.; Winter, M.; Nowak, S. Graphite Recycling from Spent Lithium Ion Batteries Graphite Recycling from Spent Lithium Ion Batteries. ChemSusChem 2016, 9, 3473–3484. [Google Scholar] [CrossRef]
- Diekmann, J.; Rothermel, S.; Nowak, S.; Kwade, A. The LithoRec Process. In Recycling of Lithium-Ion Batteries; Springer International Publishing: Basel, Swiss, 2018; pp. 33–38. [Google Scholar]
- Krüger, S.; Hanisch, C.; Kwade, A.; Winter, M.; Nowak, S. Effect of Impurities Caused by a Recycling Process on the Electrochemical Performance of Li[Ni0.33Co0.33Mn0.33]O2. J. Electroanal. Chem. 2014, 726, 91–96. [Google Scholar] [CrossRef]
- Erickson, E.M.; Schipper, F.; Penki, T.R.; Shin, J.-Y.; Erk, C.; Chesneau, F.-F.F.; Markovsky, B.; Aurbach, D. Review—Recent Advances and Remaining Challenges for Lithium Ion Battery Cathodes. J. Electrochem. Soc. 2017, 164, A6341–A6348. [Google Scholar] [CrossRef]
- Schipper, F.; Erickson, E.M.; Erk, C.; Shin, J.-Y.; Chesneau, F.F.; Aurbach, D. Review—Recent Advances and Remaining Challenges for Lithium Ion Battery Cathodes. J. Electrochem. Soc. 2017, 164, A6220–A6228. [Google Scholar] [CrossRef]
- Watari, T.; McLellan, B.C.; Giurco, D.; Dominish, E.; Yamasue, E.; Nansai, K. Total Material Requirement for the Global Energy Transition to 2050: A Focus on Transport and Electricity. Resour. Conserv. Recycl. 2019, 148, 91–103. [Google Scholar] [CrossRef]
- Cappa, J.J.; Pilon-Smits, E.A.H. Evolutionary Aspects of Elemental Hyperaccumulation. Planta 2014, 239, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Pollard, A.J.; Reeves, R.D.; Baker, A.J.M. Facultative Hyperaccumulation of Heavy Metals and Metalloids. Plant Sci. 2014, 217–218, 8–17. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy Metal Hyperaccumulating Plants: How and Why Do They Do It? And What Makes Them so Interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Krämer, U. Metal Hyperaccumulation in Plants. Annu. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Tappero, R.; Peltier, E.; Gräfe, M.; Heidel, K.; Ginder-Vogel, M.; Livi, K.J.T.; Rivers, M.L.; Marcus, M.A.; Chaney, R.L.; Sparks, D.L. Hyperaccumulator Alyssum Murale Relies on a Different Metal Storage Mechanism for Cobalt than for Nickel. New Phytol. 2007, 175, 641–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Yu, F.; Chen, M.; Zhou, Z.; Chen, C.; Li, M.S.; Zhu, J. A Newly Found Manganese Hyperaccumulator—Polygonum Lapathifolium Linn. Int. J. Phytoremediation 2016, 18, 348–353. [Google Scholar] [CrossRef]
- Deng, T.-H.-B.; van der Ent, A.; Tang, Y.-T.; Sterckeman, T.; Echevarria, G.; Morel, J.-L.; Qiu, R.-L. Nickel Hyperaccumulation Mechanisms: A Review on the Current State of Knowledge. Plant Soil 2018, 423, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and Opportunities in the Phytoremediation of Heavy Metals Contaminated Soils: A Review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Role of Hyperaccumulators in Phytoextraction of Metals from Contaminated Mining Sites: A Review. Crit. Rev. Environ. Sci. Technol. 2011, 41, 168–214. [Google Scholar] [CrossRef]
- Sarma, H. Metal Hyperaccumulation in Plants: A Review Focusing on Phytoremediation Technology. J. Environ. Sci. Technol. 2011, 4, 118–138. [Google Scholar] [CrossRef] [Green Version]
- Sinkala, T. Integrated Phytomining and Ethanol Production in the Zambian Copperbelt to Minimize Mine Decontamination Costs and Environmental and Social Impacts: A Review. J. South. African Inst. Min. Metall. 2018, 118, 815–824. [Google Scholar] [CrossRef]
- Zhang, X.; Houzelot, V.; Bani, A.; Morel, J.L.; Echevarria, G.; Simonnot, M.O. Selection and Combustion of Ni-Hyperaccumulators for the Phytomining Process. Int. J. Phytoremediat. 2014, 16, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Barbaroux, R.; Mercier, G.; Blais, J.F.; Morel, J.L.; Simonnot, M.O. A New Method for Obtaining Nickel Metal from the Hyperaccumulator Plant Alyssum Murale. Sep. Purif. Technol. 2011, 83, 57–65. [Google Scholar] [CrossRef]
- Broadhurst, C.L.; Chaney, R.L. Growth and Metal Accumulation of an Alyssum Murale Nickel Hyperaccumulator Ecotype Co-Cropped with Alyssum Montanum and Perennial Ryegrass in Serpentine Soil. Front. Plant Sci. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bani, A.; Echevarria, G.; Sulçe, S.; Morel, J.L. Improving the Agronomy of Alyssum Murale for Extensive Phytomining: A Five-Year Field Study. Int. J. Phytoremediat. 2015, 17, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Sellami, R.; Gharbi, F.; Rejeb, S.; Rejeb, M.N.; Henchi, B.; Echevarria, G.; Morel, J.L. Effects of Nickel Hyperaccumulation on Physiological Characteristics of Alyssum Murale Grown on Metal Contaminated Waste Amended Soil. Int. J. Phytoremediat. 2012, 14, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Galiová, M.V.; Száková, J.; Prokeš, L.; Čadková, Z.; Coufalík, P.; Kanický, V.; Otruba, V.; Tlustoš, P. Variability of Trace Element Distribution in Noccaea Spp., Arabidopsis Spp., and Thlaspi Arvense Leaves: The Role of Plant Species and Element Accumulation Ability. Environ. Monit. Assess. 2019, 191, 181. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.P.; Orlic, I.; Siegele, R.; Ashwath, N.; Baker, A.J.M.; Walsh, K.B. Elemental Mapping Using PIXE Shows the Main Pathway of Nickel Movement Is Principally Symplastic within the Fruit of the Hyperaccumulator Stackhousia Tryonii. New Phytol. 2003, 160, 479–488. [Google Scholar] [CrossRef]
- Punshon, T.; Guerinot, M.L.; Lanzirotti, A. Using Synchrotron X-Ray Fluorescence Microprobes in the Study of Metal Homeostasis in Plants. Ann. Bot. 2009, 103, 665–672. [Google Scholar] [CrossRef] [Green Version]
- Husted, S.; Persson, D.P.; Laursen, K.H.; Hansen, T.H.; Pedas, P.; Schiller, M.; Hegelund, J.N.; Schjoerring, J.K. Review: The Role of Atomic Spectrometry in Plant Science. J. Anal. At. Spectrom. 2011, 26, 52–79. [Google Scholar] [CrossRef]
- Keeling, S.M.; Stewart, R.B.; Anderson, C.W.N.; Robinson, B.H. Nickel and Cobalt Phytoextraction by the Hyperaccumulator Berkheya Coddii: Implications for Polymetallic Phytomining and Phytoremediation. Int. J. Phytoremediat. 2003, 5, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Broadhurst, C.L.; Chaney, R.L.; Angle, J.S.; Erbe, E.F.; Maugel, T.K. Nickel Localization and Response to Increasing Ni Soil Levels in Leaves of the Ni Hyperaccumulator Alyssum Murale. Plant Soil 2004, 265, 225–242. [Google Scholar] [CrossRef]
- Hunter, J.G.; Vergnano, O. Nickel and Cobalt Toxicities in Oat Plants. Ann. Bot. 1952, 17, 317–329. [Google Scholar]
- Cabello-Conejo, M.I.; Centofanti, T.; Kidd, P.S.; Prieto-Fernández, Á.; Chaney, R.L. Evaluation of Plant Growth Regulators To Increase Nickel Phytoextraction By Alyssum Species. Int. J. Phytoremediation 2013, 15, 365–375. [Google Scholar] [CrossRef]
- McNear, D.H.; Peltier, E.; Everhart, J.; Chaney, R.L.; Sutton, S.; Newville, M.; Rivers, M.; Sparks, D.L. Application of Quantitative Fluorescence and Absorption-Edge Computed Microtomography to Image Metal Compartmentalization in Alyssum Murale. Environ. Sci. Technol. 2005, 39, 2210–2218. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of Heavy Metal(Loid)s Contaminated Soils—To Mobilize or to Immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef]
- Diehl, M.; Evertz, M.; Winter, M.; Nowak, S. Deciphering the Lithium Ion Movement in Lithium Ion Batteries: Determination of the Isotopic Abundances of 6Li and 7Li. RSC Adv. 2019, 9, 12055–12062. [Google Scholar] [CrossRef]
- Harte, P.; Evertz, M.; Schwieters, T.; Diehl, M.; Winter, M.; Nowak, S. Adaptation and Improvement of an Elemental Mapping Method for Lithium Ion Battery Electrodes and Separators by Means of Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry. Anal. Bioanal. Chem. 2019, 411, 581–589. [Google Scholar] [CrossRef]





| Contamination Group | Sample | Ni/wt% | Co/wt% | Mn/wt% |
|---|---|---|---|---|
| Control | Leaf | <LOD | <LOD | 0.02 (±0.00) |
| Stem | <LOD | <LOD | <LOD | |
| Root | <LOD | <LOD | <LOD | |
| Soil | <LOD | <LOD | 0.02 (±0.00) | |
| Level 1 | Leaf | 0.04 (±0.00) | 0.06 (±0.00) | 0.06 (±0.00) |
| Stem | 0.02 (±0.00) | 0.01 (±0.00) | 0.03 (±0.00) | |
| Root | 0.02 (±0.00) | 0.08 (±0.00) | 0.03 (±0.00) | |
| Soil | 0.01 (±0.00) | 0.01 (±0.00) | 0.01 (±0.00) | |
| Level 2 | Leaf | 0.34 (±0.01) | 0.14 (±0.00) | 0.10 (±0.00) |
| Stem | 0.09 (±0.00) | 0.05 (±0.00) | 0.12 (±0.00) | |
| Root | 0.23 (±0.00) | 0.14 (±0.00) | 0.13 (±0.00) | |
| Soil | 0.72 (±0.01) | 1.66 (±0.03) | 0.53 (±0.01) | |
| Level 3 | Leaf | 1.83 (±0.03) | 0.58 (±0.01) | 0.34 (±0.01) |
| Stem | 1.60 (±0.03) | 0.68 (±0.01) | 0.28 (±0.01) | |
| Root | 0.88 (±0.02) | 0.80 (±0.01) | 0.82 (±0.02) | |
| Soil | 2.35 (±0.04) | 1.97 (±0.04) | 2.44 (±0.06) |
| Contamination Group | Sample | Nicon/wt% | Niacc/wt% | Cocon/wt% | Coacc/wt% | Mncon/wt% | Mnacc/wt% |
|---|---|---|---|---|---|---|---|
| Control | Leaf | <LOD | <LOD | <LOD | <LOD | 0.02 (±0.00) | 0.01 (±0.00) |
| Stem | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | |
| Root | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | |
| Soil | <LOD | <LOD | <LOD | <LOD | 0.02 (±0.00) | 0.02 (±0.00) | |
| Level 1 | Leaf | 0.04 (±0.00) | 0.09 (±0.00) | 0.06 (±0.00) | 0.15 (±0.00) | 0.06 (±0.00) | 0.10 (±0.00) |
| Stem | 0.02 (±0.00) | 0.02 (±0.00) | 0.01 (±0.00) | 0.01 (±0.00) | 0.03 (±0.00) | 0.02 (±0.00) | |
| Root | 0.02 (±0.00) | 0.02 (±0.00) | 0.08 (±0.00) | 0.01 (±0.00) | 0.03 (±0.00) | 0.02 (±0.00) | |
| Soil | 0.53 (±0.00) | 0.31 (±0.01) | 0.50 (±0.01) | 0.28 (±0.01) | 0.29 (±0.01) | 0.35 (±0.01) | |
| Level 2 | Leaf | 0.34 (±0.01) | 0.40 (±0.01) | 0.14 (±0.00) | 0.49 (±0.01) | 0.10 (±0.00) | 0.28 (±0.01) |
| Stem | 0.02 (±0.00) | 0.08 (±0.00) | 0.05 (±0.00) | 0.13 (±0.00) | 0.12 (±0.00) | 0.11 (±0.00) | |
| Root | 0.23 (±0.00) | 0.15 (±0.00) | 0.14 (±0.00) | 0.31 (±0.01) | 0.13 (±0.00) | 0.10 (±0.00) | |
| Soil | 2.97 (±0.05) | 2.50 (±0.04) | 1.23 (±0.02) | 1.30 (±0.02) | 1.16 (±0.03) | 1.07 (±0.03) | |
| Level 3 | Leaf | 1.83 (±0.03) | 2.19 (±0.04) | 0.58 (±0.01) | 0.66 (±0.01) | 0.34 (±0.01) | 0.41 (±0.01) |
| Stem | 1.60 (±0.03) | 3.22 (±0.06) | 0.68 (±0.01) | 1.97 (±0.04) | 0.28 (±0.01) | 0.61 (±0.02) | |
| Root | 0.88 (±0.02) | 1.20 (±0.02) | 0.80 (±0.01) | 2.53 (±0.05) | 0.82 (±0.02) | 1.50 (±0.04) | |
| Soil | 3.11 (±0.05) | 2.72 (±0.05) | 2.62 (±0.05) | 2.34 (±0.04) | 3.11 (±0.08) | 2.72 (±0.07) |
| Contamination Group | Sample | Ni/mg kg−1 | Co/mg kg−1 | Mn/mg kg−1 | Li/mg kg−1 |
|---|---|---|---|---|---|
| Soil contamination Start | Calculated | 1449 (±25) | 1455 (±27) | 1356 (±35) | 514 (±12) |
| Measured | 1975 (±34) | 1925 (±35) | 2150 (±55) | 575 (±14) | |
| After 100 days | Measured | 2.73 (±0.05) | 2.69 (±0.05) | 2.69 (±0.07) | 0.91 (±0.02) |
| Alyssum murale after 100 days | Leaf | 0.49 (±0.01) | 0.63 (±0.01) | 0.78 (±0.02) | 5.90 (±0.14) |
| Stem | <LOD | <LOD | 0.30 (±0.01) | 0.30 (±0.01) | |
| Root | <LOD | <LOD | <LOD | 0.32 (±0.01) |
| Transition Metal | Contamination Day | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Addition | 0. | 2. | 4. | 6. | 8. | 10. | 12. | 14. | 16. | 18. | 20. |
| ∑ Level 1/g | 0.00 | 0.01 | 0.02 | 0.03 | 0.04 | 0.05 | 0.06 | 0.07 | 0.08 | 0.09 | 0.10 |
| ∑ Level 2/g | 0.00 | 0.05 | 0.10 | 0.15 | 0.20 | 0.25 | 0.30 | 0.35 | 0.40 | 0.45 | 0.50 |
| ∑ Level 3/g | 0.00 | 0.25 | 0.50 | 0.75 | 1.00 | 1.25 | 1.50 | 1.75 | 2.00 | 2.25 | 2.50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henschel, J.; Mense, M.; Harte, P.; Diehl, M.; Buchmann, J.; Kux, F.; Schlatt, L.; Karst, U.; Hensel, A.; Winter, M.; et al. Phytoremediation of Soil Contaminated with Lithium Ion Battery Active Materials—A Proof-of-Concept Study. Recycling 2020, 5, 26. https://0-doi-org.brum.beds.ac.uk/10.3390/recycling5040026
Henschel J, Mense M, Harte P, Diehl M, Buchmann J, Kux F, Schlatt L, Karst U, Hensel A, Winter M, et al. Phytoremediation of Soil Contaminated with Lithium Ion Battery Active Materials—A Proof-of-Concept Study. Recycling. 2020; 5(4):26. https://0-doi-org.brum.beds.ac.uk/10.3390/recycling5040026
Chicago/Turabian StyleHenschel, Jonas, Maximilian Mense, Patrick Harte, Marcel Diehl, Julius Buchmann, Fabian Kux, Lukas Schlatt, Uwe Karst, Andreas Hensel, Martin Winter, and et al. 2020. "Phytoremediation of Soil Contaminated with Lithium Ion Battery Active Materials—A Proof-of-Concept Study" Recycling 5, no. 4: 26. https://0-doi-org.brum.beds.ac.uk/10.3390/recycling5040026