High-Shear De-Gassing and De-Ironing of an Aluminum Casting Alloy Made Directly from Aluminum End-of-Life Vehicle Scrap
Abstract
:1. Introduction
2. Experimental Procedures
3. Results and Discussion
3.1. Melting of the Zorba Cast Fraction Scrap in the As-Received Shredded Form

3.2. De-Gassing by HSMC of the Zorba Cast Fraction Re-Melted Ingots
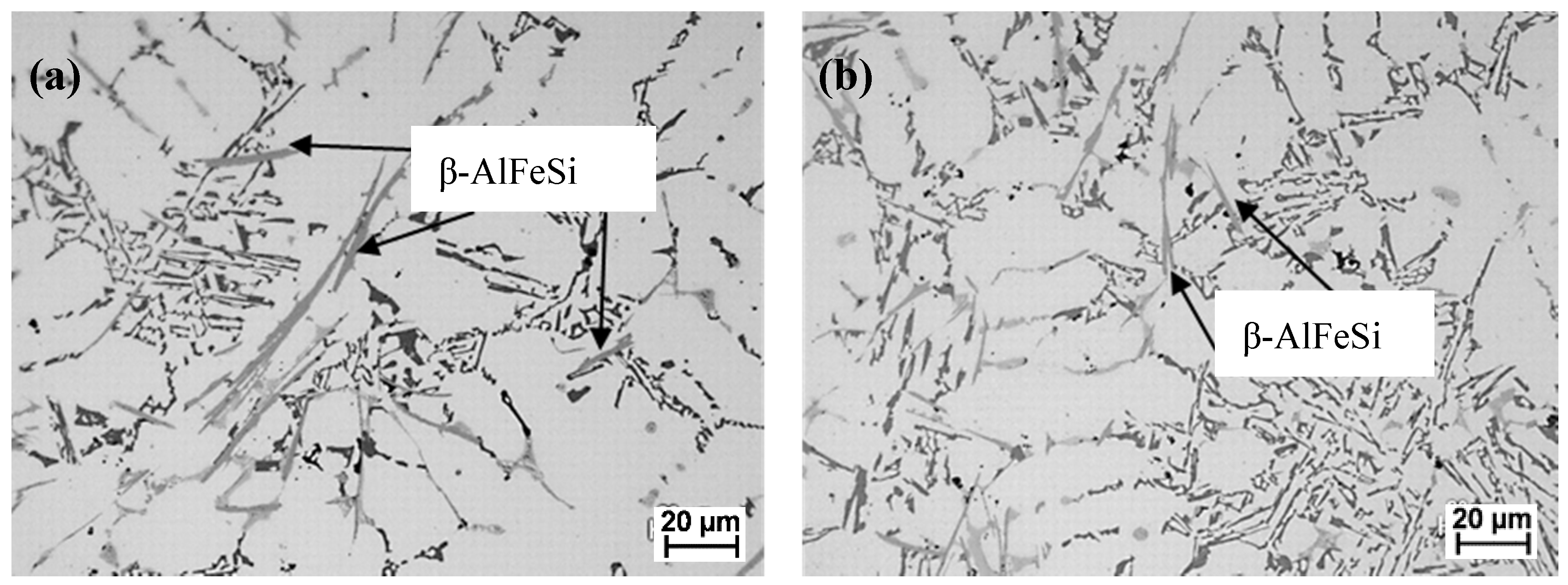
3.3. De-Ironing by HSMC of the Zorba Cast Fraction Remelted Ingots
3.3.1. Effect of HSMC on the as Recovered Zorba Ingots
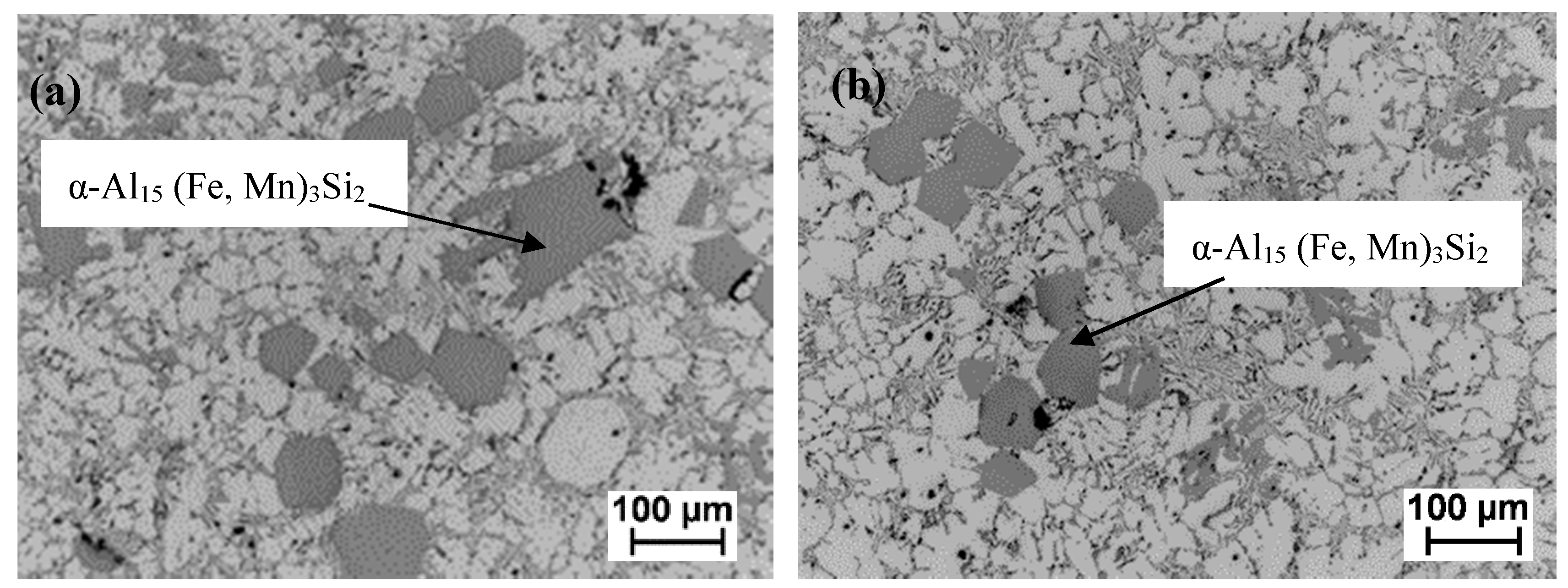
3.3.2. Effect of HSMC on De-Ironing after Mn Addition
4. Conclusions
- The recovery yield of the cast aluminum alloy was over 80%.
- Applying high-shear melt conditioning improved the degassing melt treatment in comparison with the traditional treatment of adding a degassing tablet. In addition, it provided grain refinement and reduced porosity content and size.
- The technology provided refinement of the β-AlFeSi plate-like phase present in the high-Fe aluminum cast fraction scrap.
- Adding Mn to the aluminum ingot melt enhanced the formation of the compact α-Al15 (Fe, Mn)3Si2 phase.
- HSMC after a Mn addition provided enhanced formation of the compact intermetallics and an effective de-ironing process up to 23%. It also helped the recovery of the added Mn with 28% effectiveness.
- There is potential for further Fe and Mn removal from this scrap stream by adjusting the processing and holding temperature after HSMC.
- The treated melt after the HSMC de-ironing process has a composition within the specifications of commercial alloys, such as LM24 and LM27, making it suitable for direct commercial use.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brooks, L.; Gaustad, G.; Gesting, A.; Mortvedt, T.; Freire, F. Ferrous and non-ferrous Recycling: Challenges and Potential Technology. Waste Manag. 2019, 85, 519. [Google Scholar] [CrossRef]
- Chang, I.; Luo, D.; Patel, J.B.; Al-Helal, K.; Huang, Y.; Scamans, G.; Fan, Z. Processing of Recycled AA6111 Aluminium Alloy from Two Different Feedstock of Aluminium Metal Scraps. J. Light Met. Weld. 2020, 58, 29–32. [Google Scholar]
- Zorba. The Key to Low Carbon Aluminium Supply? Available online: https://www.innovaltec.com/zorba-scrap-stream-blog/ (accessed on 9 October 2021).
- Zhang, L.; Lv, X.; Torgerson, A.T.; Long, M. Removal of impurity elements from molten aluminium: A review. Miner. Process. Extr. Metall. Rev. 2011, 31, 150–228. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, J.; Damoah, L.N.W.; Robertson, D.G. Removal of iron from aluminium: A review. Miner. Process. Extr. Metall. Rev. 2012, 33, 99–157. [Google Scholar] [CrossRef]
- Mondolfo, L.F. Aluminum Alloys: Structure and Properties, 1st ed.; Butterworths: London, UK; Boston, MA, USA, 1976; p. 282. [Google Scholar]
- Ji, S.; Yang, W.; Gao, F.; Watson, D.; Fan, Z. Effect of iron on the microstructure and mechanical property of Al–Mg–Si–Mn and Al–Mg–Si diecast alloys. Mater. Sci. Eng. A 2013, 564, 130–139. [Google Scholar] [CrossRef]
- Yang, W.; Gao, F.; Ji, S. Formation and sedimentation of Fe-rich intermetallics in Al-Si- Cu-Fe alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 1704–1714. [Google Scholar] [CrossRef] [Green Version]
- Que, Z.; Wang, Y.; Fan, Z. Formation of the Fe-containing intermetallic compounds during solidification of Al-5Mg-2Si-0.7Mn-1.1Fe alloy. Mater. Metall. Trans. A 2018, 49, 2173–2181. [Google Scholar] [CrossRef] [Green Version]
- Ashtari, P.; Tezuka, H.; Sato, T. Modification of Fe-Containing Intermetallic Compounds by K Addition to Fe-Rich AA319 Aluminum Alloys. Scr. Mater. 2005, 53, 937–942. [Google Scholar] [CrossRef]
- Mbuya, T.O.; Odera, B.O.; Ng’ang’a, S.P. Influence of iron on castability and properties of aluminium silicon alloys: Literature review. Int. J. Cast Met. Res. 2003, 16, 451–465. [Google Scholar] [CrossRef]
- Lazaro-Nebreda, J.; Patel, J.B.; Scamans, G.M.; Fan, Z. Multi-Purpose High Shear Melt Conditioning Technology for Effective Melt Quality and for Recycling of Al-Alloy Scrap. In Proceedings of the 16th International Aluminium Alloys Conference (ICAA16), Montreal, QC, Canada, 17–21 June 2018; Canadian Institute of Mining, Metallurgy & Petroleum: Montreal, QC, Canada, 2018; p. 401623. [Google Scholar]
- Fan, Z.; Zuo, Y.B.; Jiang, B. Apparatus and Method for Liquid Metals Treatment. U.S. Patent No. 9,498,820, 22 November 2016. [Google Scholar]
- Patel, J.B.; Li, H.T.; Xia, M.X.; Jones, S.; Kumar, S.; O’Reilly, K.; Fan, Z. Melt Conditioned Direct Chill Casting (MC-DC) Process for Production of High Quality Aluminium Alloy Billets. Mater. Sci. Forum. 2014, 794, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Al-Helal, K.; Patel, J.B.; Fan, Z. Fe-Rich Intermetallic Formation and Mechanical Properties of Recycled AA6111 Alloy Strips Produced by Melt Conditioning Twin Roll Casting. JOM 2020, 72, 3753–3759. [Google Scholar] [CrossRef]
- Lazaro-Nebreda, J.; Patel, J.B.; Fan, Z. Improved degassing efficiency and mechanical properties of A356 aluminium alloy castings by high shear melt conditioning (HSMC) technology. J. Mater. Process. Technol. 2021, 294, 117146–117157. [Google Scholar] [CrossRef]
- Lazaro-Nebreda, J.; Patel, J.B.; Chang, I.T.H.; Stone, I.C.; Fan, Z. Solidification processing of scrap Al-alloys containing high levels of Fe. IOP Conf. Ser. Mater. Sci. Eng. 2019, 529, 012059–012065. [Google Scholar] [CrossRef]
- Zhang, Y.; Patel, J.B.; Lazaro-Nebreda, J.; Fan, Z. Improved Defect Control and Mechanical Property Variation in High-Pressure Die Casting of A380 Alloy by High Shear Melt Conditioning. JOM 2018, 70, 2726–2730. [Google Scholar] [CrossRef] [Green Version]
- Dispinar, D.; Campbell, J. Critical assessment of reduced pressure test. Part 1: Porosity phenomena. Int. J. Cast Met. Res. 2004, 17, 280–286. [Google Scholar] [CrossRef]
- Al-Helal, K.; Lazaro-Nebreda, J.; Patel, J.B.; Scamans, G.M.; Fan, Z. Melt Conditioned Direct Chill (MC-DC) Casting of AA-6111 Aluminium Alloy Formulated from Incinerator Bottom Ash (IBA). Recycling 2019, 4, 37. [Google Scholar] [CrossRef] [Green Version]
- Snopinski, P.; Krol, M.; Tanski, T.; Krupinska, B. Effect of cooling rate on microstructural development in alloy ALMG9. J. Therm. Anal. Calorim. 2018, 133, 379–390. [Google Scholar] [CrossRef] [Green Version]




| Wt.% | Cu | Mg | Si | Fe | Mn | Ni | Zn | Pb | Sn | Ti | Cr | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zorba Ingot | 2.34 | 0.55 | 6.32 | 0.80 | 0.21 | 0.08 | 0.89 | 0.07 | 0.03 | 0.06 | 0.03 | Bal. |
| LM24 | 3.0–4.0 | <0.3 | 7.5–9.5 | <1.3 | <0.5 | <0.5 | <3.0 | <0.3 | <0.2 | <0.2 | - | Bal. |
| LM27 | 1.5–2.5 | <0.35 | 6.0–8.0 | 0.5–0.7 | 0.2–0.6 | <0.3 | <1.0 | <0.2 | <0.1 | <0.2 | <0.05 | Bal. |
| Sample | Density Index DI% | Porosity Fraction (%) | Porosity Size (μm) | Grain Size (μm) |
|---|---|---|---|---|
| Before Degassing | 13.1 | 3.8 | 50 ± 14 | 1750 ± 313 |
| After Tablet Degassing | 6.4 | 1.8 | 35 ± 14 | 1560 ± 235 |
| After HSMC Degassing | 2.6 | 0.5 | 18 ± 3 | 1160 ± 287 |
| Wt.% | Cu | Mg | Si | Fe | Mn | Ni | Zn | Pb | Sn | Ti | Cr | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zorba Ingot | 2.34 | 0.55 | 6.32 | 0.80 | 0.21 | 0.08 | 0.89 | 0.07 | 0.03 | 0.06 | 0.03 | Bal. |
| After HSMC and holding 30 min at 610 °C | 2.32 | 0.57 | 6.36 | 0.81 | 0.22 | 0.08 | 0.86 | 0.07 | 0.06 | 0.06 | 0.05 | Bal. |
| Wt.% | Cu | Mg | Si | Fe | Mn | Ni | Zn | Pb | Sn | Ti | Cr | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zorba with Mn addition | 2.32 | 0.54 | 6.30 | 0.79 | 0.78 | 0.07 | 0.87 | 0.06 | 0.03 | 0.05 | 0.03 | Bal. |
| After HSMC and holding 30 min at 630 °C | 2.41 | 0.57 | 6.28 | 0.61 | 0.56 | 0.08 | 0.90 | 0.07 | 0.04 | 0.06 | 0.05 | Bal. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Helal, K.; Lazaro-Nebreda, J.; Patel, J.B.; Scamans, G.M. High-Shear De-Gassing and De-Ironing of an Aluminum Casting Alloy Made Directly from Aluminum End-of-Life Vehicle Scrap. Recycling 2021, 6, 66. https://0-doi-org.brum.beds.ac.uk/10.3390/recycling6040066
Al-Helal K, Lazaro-Nebreda J, Patel JB, Scamans GM. High-Shear De-Gassing and De-Ironing of an Aluminum Casting Alloy Made Directly from Aluminum End-of-Life Vehicle Scrap. Recycling. 2021; 6(4):66. https://0-doi-org.brum.beds.ac.uk/10.3390/recycling6040066
Chicago/Turabian StyleAl-Helal, Kawther, Jaime Lazaro-Nebreda, Jayesh B. Patel, and Geoff M. Scamans. 2021. "High-Shear De-Gassing and De-Ironing of an Aluminum Casting Alloy Made Directly from Aluminum End-of-Life Vehicle Scrap" Recycling 6, no. 4: 66. https://0-doi-org.brum.beds.ac.uk/10.3390/recycling6040066






