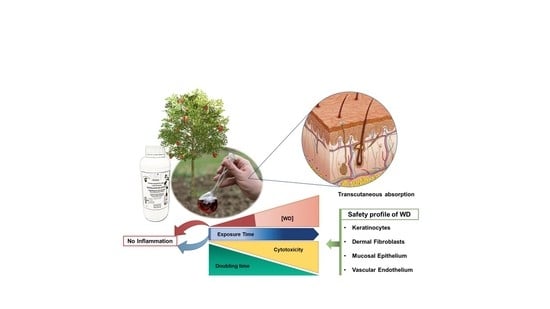
Characterization of the Safety Profile of Sweet Chestnut Wood Distillate Employed in Agriculture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wood Distillate
2.2. Cell Cultures
2.3. Cell Viability Assay
2.4. Expression of Inflammatory Markers
2.5. Statistical Analysis
3. Results
3.1. Acidity of Wood Distillate Was Differentially Buffered by Culture Media
3.2. WD Differentially Affected Cell Viability
3.3. The Longer Doubling Time Improved Cell Resistance to WD
3.4. The Exposure of Cells to WD Did Not Induce an Inflammatory Phenotype
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grewal, A.; Lokanadha, L.A.; Gunupuru, R. Production, prospects and potential application of pyroligneous acid in agriculture. J. Anal. Appl. Pyrol. 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Zhu, K.; Gu, S.; Liu, J.; Luo, T.; Khan, Z.; Zhang, K.; Hu, L. Wood Vinegar as a Complex Growth Regulator Promotes the Growth, Yield, and Quality of Rapeseed. Agronomy 2021, 11, 510. [Google Scholar] [CrossRef]
- BioDea. Datasheet—Distillato di Legno. 2020. Available online: https://www.biodea.bio/website/wp-content/uploads/2019/11/BioDea-scheda-informativa-Distillato-di-Legno-BIO.pdf (accessed on 1 January 2020).
- Vannini, A.; Moratelli, F.; Monaci, F.; Loppi, S. Effects of wood distillate and soy lecithin on the photosynthetic performance and growth of lettuce (Lactuca sativa L.). SN Appl. Sci. 2021, 3, 113. [Google Scholar] [CrossRef]
- Cardelli, R.; Becagli, M.; Marchini, F.; Saviozzi, A. Soil biochemical activities after the application of pyroligneous acid to soil. J. Compil. CSIRO Soil Res. 2020, 58, 461–467. [Google Scholar] [CrossRef]
- Mmojieje, J.; Hornung, A. The Potential Application of Pyroligneous Acid in the UK Agricultural Industry. J. Crop. Improv. 2015, 29, 2. [Google Scholar] [CrossRef]
- Balat, M.; Balat, M.; Kırtay, E.; Balat, H. Main routes for the thermoconversion of biomass into fuels and chemicals. Part 1: Pyrolysis systems. Energy Convers. Manag. 2009, 50, 3147–3157. [Google Scholar] [CrossRef]
- Mansur, D.; Yoshikawa, T.; Norinaga, K.; Hayashi, J.; Tago, T.; Masuda, T. Production of ketones from pyroligneous acid of woody biomass pyrolysis over an iron-oxide catalyst. Fuel 2013, 103, 130–134. [Google Scholar] [CrossRef] [Green Version]
- Mathew, S.; Zakaria, Z.A. Pyroligneous acid-the smoky acidic liquid from plant biomass. Appl. Microbiol. Biotechnol. 2015, 99, 611–622. [Google Scholar] [CrossRef]
- Hou, X.; Qiu, L.; Luo, S.; Kang, K.; Zhu, M.; Yao, Y. Chemical constituents and antimicrobial activity of wood vinegars at different pyrolysis temperature ranges obtained from Eucommia ulmoides Olivers branches. RSC Adv. 2018, 8, 40941–40949. [Google Scholar] [CrossRef] [Green Version]
- Misuri, F.; Marri, L. Antibacterial activity of wood distillate from residual virgin chestnut biomass. Eur. J. Wood Prod. 2020, 79, 237–239. [Google Scholar] [CrossRef]
- Yang, J.F.; Yang, C.H.; Liang, M.T.; Gao, Z.J.; Wu, Y.W.; Chuang, L.Y. Chemical Composition, Antioxidant, and Antibacterial Activity of Wood Vinegar from Litchi chinensis. Molecules 2016, 30, 1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loo, A.; Jain, K.; Darah, I. Antioxidant activity of compounds isolated from the pyroligneous acid, Rhizophora apiculata. Food Chem. 2008, 107, 1151–1160. [Google Scholar] [CrossRef]
- Fačkovcová, Z.; Vannini, A.; Monaci, F.; Grattacaso, M.; Paoli, L.; Loppi, S. Effects of wood distillate (pyroligneous acid) on sensitive bioindicators (lichen and moss). Ecotoxicol. Environ. Saf. 2020, 204, 111117. [Google Scholar] [CrossRef]
- Fačkovcová, Z.; Vannini, A.; Monaci, F.; Grattacaso, M.; Paoli, L.; Loppi, S. Uptake of Trace Elements in the Water Fern Azolla filiculoides after Short-Term Application of Chestnut Wood Distillate (Pyroligneous Acid). Plants 2020, 9, 1179. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils-a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Changhwan, A.; Jae-Hwan, L.; Mi-Jin, P.; Jae-Woo, K.; Jiyoon, Y.; Yeong-Min, Y.; Eui-Bae, J. Cytostatic effects of plant essential oils on human skin and lung cells. Exp. Ther. Med. 2020, 19, 2008–2018. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef] [PubMed]
- Morbidelli, L. Polyphenol-based nutraceuticals for the control of angiogenesis: Analysis of the critical issues for human use. Pharmacol. Res. 2016, 111, 384–393. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 21, 87. [Google Scholar] [CrossRef] [Green Version]
- Astiz, M.; de Alaniz, M.J.; Marra, C.A. The oxidative damage and inflammation caused by pesticides are reverted by lipoic acid in rat brain. Neurochem. Int. 2012, 61, 1231–1241. [Google Scholar] [CrossRef]
- Barbasz, A.; Kreczmer, B.; Skórka, M.; Czyżowska, A. Toxicity of pesticides toward human immune cells U-937 and HL-60. J. Environ. Sci. Health B 2020, 55, 719–725. [Google Scholar] [CrossRef]
- Shah, H.K.; Basu, T.S.; Banerjee, D. Organochlorine pesticides induce inflammation, ROS production, and DNA damage in human epithelial ovary cells: An in vitro study. Chemosphere Elsevier 2020, 246, 125691. [Google Scholar] [CrossRef]
- Lee, J.H. Chapter 3—Keratinocyte Differentiation and Epigenetics. In Epigenetics and Dermatology; Qianjin, L., Christopher, C., Chang, B., Richardson, C., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 37–52. [Google Scholar] [CrossRef]
- Smina, T.P.; Mohan, A.; Ayyappa, K.A.; Sethuraman, S.; Krishnan, U.M. Hesperetin exerts apoptotic effect on A431 skin carcinoma cells by regulating mitogen activated protein kinases and cyclins. Cell Mol. Biol. 2015, 30, 92–99. [Google Scholar] [CrossRef]
- Li, C.C.; Yu, F.S.; Fan, M.J.; Chen, Y.Y.; Lien, J.C.; Chou, Y.C.; Lu, H.F.; Tang, N.Y.; Peng, S.F.; Huang, W.W.; et al. Anticancer effects of cantharidin in A431 human skin cancer (Epidermoid carcinoma) cells in vitro and in vivo. Environ. Toxicol. 2017, 32, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Del Favero, G.; Woelflingseder, L.; Janker, L.; Neuditschko, B.; Seriani, S.; Gallina, P.; Sbaizero, O.; Gerner, C.; Marko, D. Deoxynivalenol induces structural alterations in epidermoid carcinoma cells A431 and impairs the response to biomechanical stimulation. Sci. Rep. 2018, 8, 11351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojtowicz, A.M.; Oliveira, S.; Carlson, M.W.; Zawadzka, A.; Rousseau, C.F.; Baksh, D. The importance of both fibroblasts and keratinocytes in a bilayered living cellular construct used in wound healing. Wound Repair Regen. 2014, 22, 246–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Q.; Ma, X.; Dong, J. Preparation, chemical constituents and antimicrobial activity of pyroligneous acids from walnut tree branches. J. Anal. Appl. Pyrolysis. 2010, 87, 24–28. [Google Scholar] [CrossRef]
- Bertuzzi, A.; Gandolfi, A.; Sinisgalli, C.; Starace, G.; Ubezio, P. Cell loss and the concept of potential doubling time. Cytometry 1997, 29, 34–40. [Google Scholar] [CrossRef]
- Roth, V. Doubling Time Computing. 2006. Available online: http://www.doubling-time.com/compute.php (accessed on 20 January 2020).
- Ciccone, V.; Filippelli, A.; Angeli, A.; Supuran, C.T.; Morbidelli, L. Pharmacological Inhibition of CA-IX Impairs Tumor Cell Proliferation, Migration and Invasiveness. Int. J. Mol. Sci. 2020, 21, 2983. [Google Scholar] [CrossRef] [Green Version]
- Quest Graph™ IC50 Calculator; AAT Bioquest, Inc.: Sunnyvale, CA, USA, 2021; Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 20 January 2020).
- Ciccone, V.; Monti, M.; Antonini, G.; Mattoli, L.; Burico, M.; Marini, F.; Maidecchi, A.; Morbidelli, L. Efficacy of AdipoDren® in Reducing Interleukin-1-Induced Lymphatic Endothelial Hyperpermeability. J. Vasc. Res. 2016, 53, 255–268. [Google Scholar] [CrossRef]
- Ciccone, V.; Zazzetta, M.; Morbidelli, L. Comparison of the Effect of Two Hyaluronic Acid Preparations on Fibroblast and Endothelial Cell Functions Related to Angiogenesis. Cells 2019, 8, 1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GraphPad Prism Version 8.3.0 for Windows; GraphPad Software: San Diego, CA, USA, 2019; Available online: www.graphpad.com (accessed on 15 October 2019).
- Wang, Q.; Cui, K.; Espin-Garcia, O.; Cheng, D.; Qiu, X.; Chen, Z.; Moore, M.; Bristow, R.G.; Xu, W.; Der, S.; et al. Resistance to bleomycin in cancer cell lines is characterized by prolonged doubling time, reduced DNA damage and evasion of G2/M arrest and apoptosis. PLoS ONE 2013, 8, e82363. [Google Scholar] [CrossRef] [Green Version]
- Eigenmann, M.J.; Frances, N.; Lavé, T.; Walz, A.C. PKPD modeling of acquired resistance to anti-cancer drug treatment. J. Pharmacokinet. Pharmacodyn. 2017, 44, 617–630. [Google Scholar] [CrossRef] [Green Version]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riemann, A.; Rauschner, M.; Gießelmann, M.; Reime, S.; Haupt, V.; Thews, O. Extracellular Acidosis Modulates the Expression of Epithelial-Mesenchymal Transition (EMT) Markers and Adhesion of Epithelial and Tumor Cells. Neoplasia 2019, 21, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Terzuoli, E.; Ziche, M.; Morbidelli, L. H2S dependent and independent anti-inflammatory activity of zofenoprilat in cells of the vascular wall. Pharmacol. Res. 2016, 113 Pt A, 426–437. [Google Scholar] [CrossRef]
- Donnini, S.; Finetti, F.; Solito, R.; Terzuoli, E.; Sacchetti, A.; Morbidelli, L.; Patrignani, P.; Ziche, M. EP2 prostanoid receptor promotes squamous cell carcinoma growth through epidermal growth factor receptor transactivation and iNOS and ERK1/2 pathways. FASEB J. 2007, 21, 2418–2430. [Google Scholar] [CrossRef] [Green Version]
- Bazzani, L.; Donnini, S.; Finetti, F.; Christofori, G.; Ziche, M. PGE2/EP3/SRC signaling induces EGFR nuclear translocation and growth through EGFR ligands release in lung adenocarcinoma cells. Oncotarget 2017, 8, 31270–31287. [Google Scholar] [CrossRef] [Green Version]
- Terzuoli, E.; Bellan, C.; Aversa, S.; Ciccone, V.; Morbidelli, L.; Giachetti, A.; Donnini, S.; Ziche, M. ALDH3A1 Overexpression in Melanoma and Lung Tumors Drives Cancer Stem Cell Expansion, Impairing Immune Surveillance through Enhanced PD-L1 Output. Cancers 2019, 11, 1963. [Google Scholar] [CrossRef] [Green Version]
- Safety Sheet of Wood Distillate; BioDea© and Esperia s.r.l. (RM Group Energy Solutions): Arezzo, Italy, 2020; Available online: https://www.biodea.bio/website/wp-content/uploads/2020/01/SCHEDA-DI-SICUREZZA-DISTILLATO-DI-LEGNO.pdf (accessed on 1 January 2020).






| Cell Type | IC50 WD 24 h (%, v/v) | IC50 WD 48 h (%, v/v) | Doubling Time (h) |
|---|---|---|---|
| HaCaT | 0.12 ± 0.02 | 0.08 ± 0.002 | 15.7 ± 2.08 |
| A431 | 0.19 ± 0.05 | 0.17 ± 0.03 | 20.6 ± 3.05 |
| NHDF | 0.31 ± 0.02 | 0.18 ± 0.07 | 29.6 ± 6.02 |
| HUVEC | 0.25 ± 0.003 | 0.18 ± 0.04 | 27.3 ± 3.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippelli, A.; Ciccone, V.; Loppi, S.; Morbidelli, L. Characterization of the Safety Profile of Sweet Chestnut Wood Distillate Employed in Agriculture. Safety 2021, 7, 79. https://0-doi-org.brum.beds.ac.uk/10.3390/safety7040079
Filippelli A, Ciccone V, Loppi S, Morbidelli L. Characterization of the Safety Profile of Sweet Chestnut Wood Distillate Employed in Agriculture. Safety. 2021; 7(4):79. https://0-doi-org.brum.beds.ac.uk/10.3390/safety7040079
Chicago/Turabian StyleFilippelli, Arianna, Valerio Ciccone, Stefano Loppi, and Lucia Morbidelli. 2021. "Characterization of the Safety Profile of Sweet Chestnut Wood Distillate Employed in Agriculture" Safety 7, no. 4: 79. https://0-doi-org.brum.beds.ac.uk/10.3390/safety7040079