The Global Rise and the Complexity of Sesame Allergy: Prime Time to Regulate Sesame in the United States of America?
Abstract
:1. Introduction
2. Mapping the Origin and the Global Rise of Sesame Allergy: The 1950s to the Present
3. The Natural History of Sesame Allergy vs. Other Major Food Allergies
4. Sesame Allergy: A Complex Spectrum of Clinical Presentations
5. The Biochemical Diversity of Sesame Allergens: Proteins and Lipids
6. Pathogenesis of Sesame Allergy: Gaps in the Cellular and Molecular Mechanisms
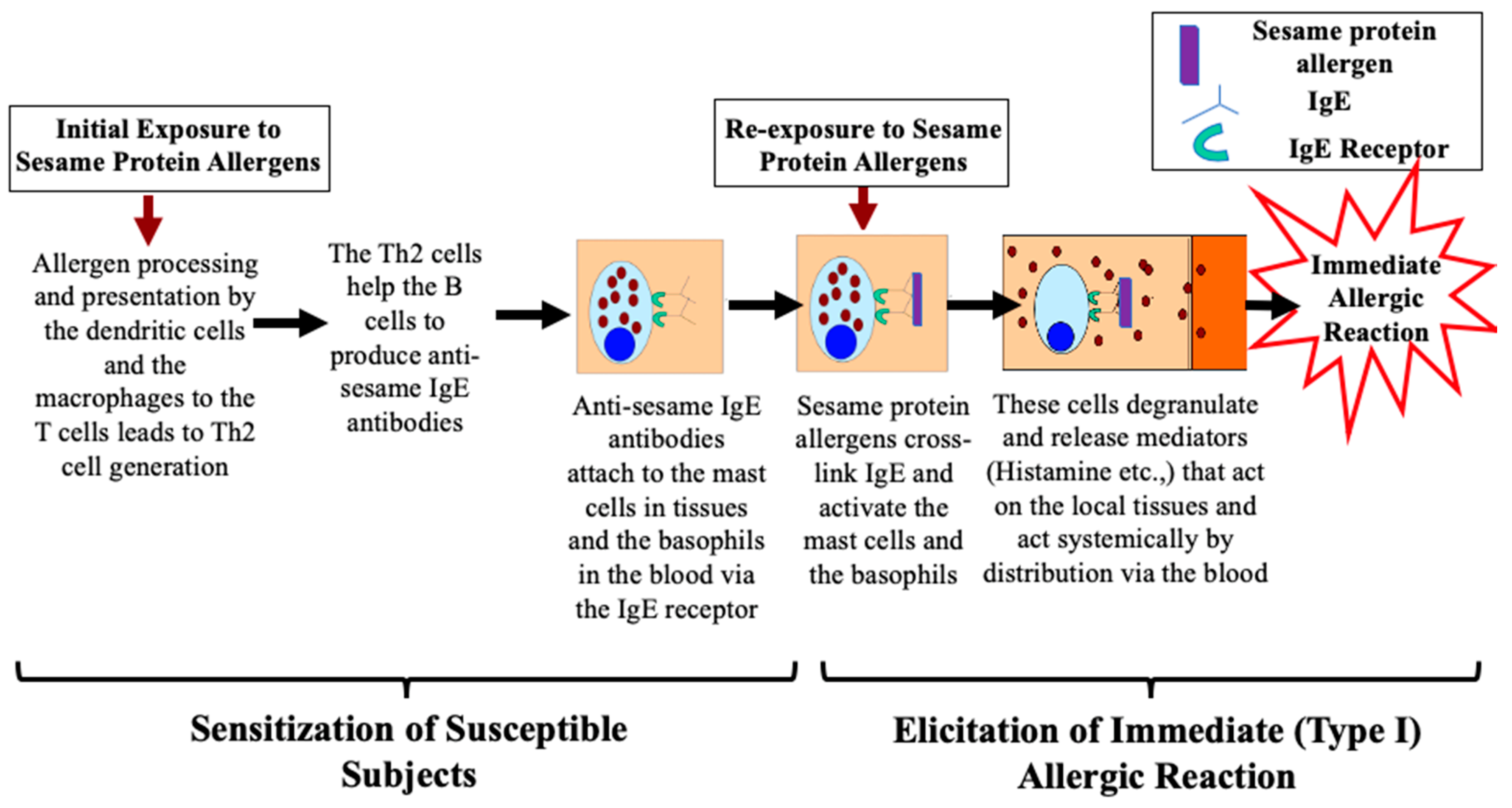
6.1. Sesame Seed Protein Allergens Cause Type I Hypersensitivity Reactions: Mechanisms
6.1.1. Phase One: Mechanism of Sensitization upon Initial Exposure to Protein Allergens
6.1.2. Phase Two: Mechanism of Elicitation of Allergic Reaction upon Re-Exposure to Protein Allergens
6.2. Sesame Seed Oil Lipid Allergens Cause Type IV Hypersensitivity Reaction: Mechanisms
6.2.1. Phase One: Mechanisms of Sensitization to Lipid Allergens
6.2.2. Phase Two: Mechanisms of Elicitation of Allergic Reaction to Lipid Allergens
6.3. Does Sesame Use Additional Immune Mechanisms to Trigger Allergic Reactions?
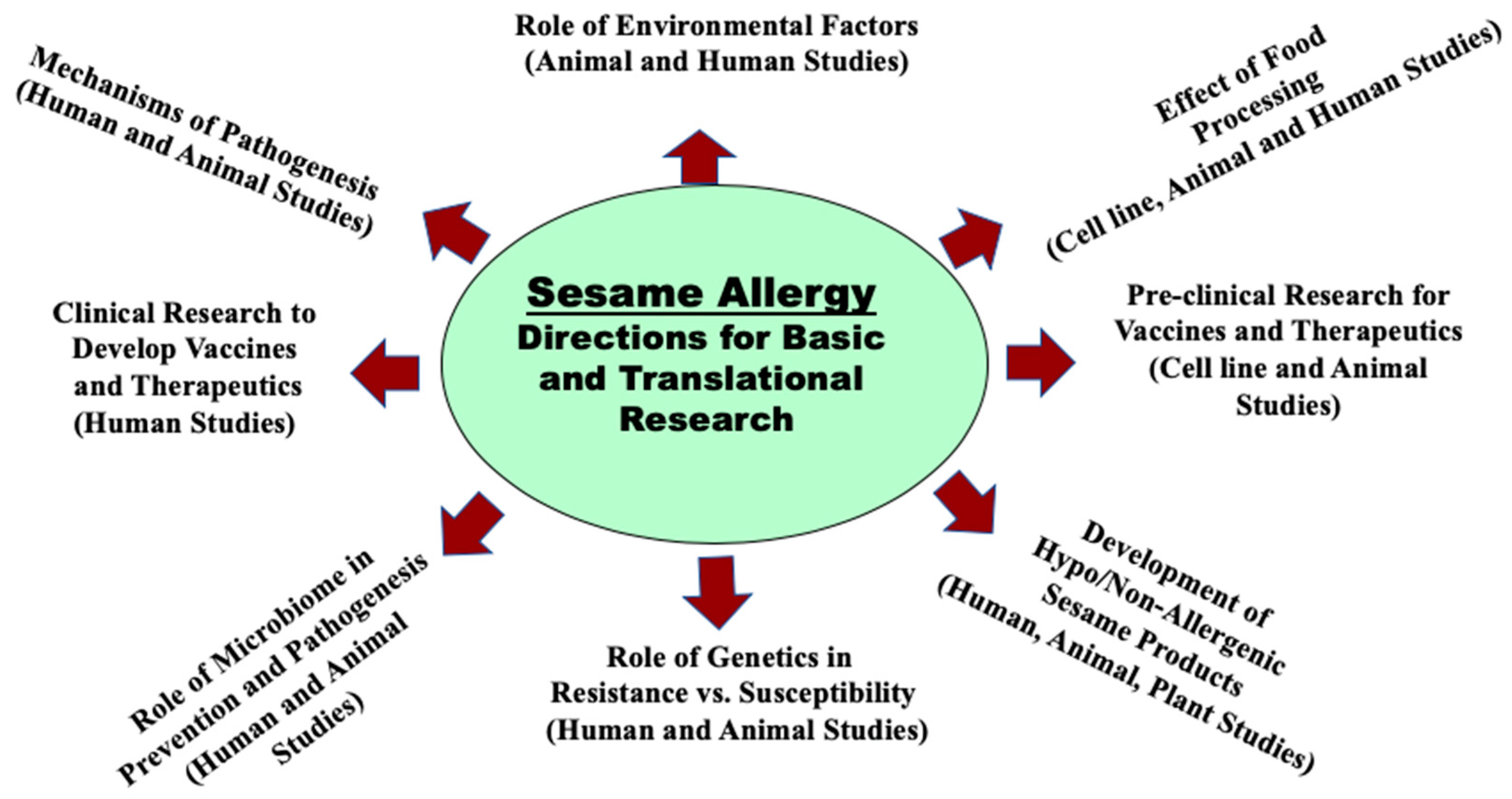
7. How to Advance the Basic, Preclinical, and Clinical Research in Sesame Allergy? An Urgent Need
8. The Global Regulation of Sesame for Food Safety: Does Sesame Need to Be Regulated in the United States of America?
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Ginaldi, L. New Perspectives in Food Allergy. Int. J. Mol. Sci. 2020, 21, 1474. [Google Scholar] [CrossRef] [Green Version]
- Warren, C.M.; Chadha, A.S.; Sicherer, S.H.; Jiang, J.; Gupta, R.S. Prevalence and Severity of Sesame Allergy in the United States. JAMA Netw. Open 2019, 2, e199144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food allergy. Nat. Rev. Dis. Primers 2018, 4, 17098. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prevalence and Severity of Food Allergies Among US Adults. JAMA Netw. Open 2019, 2, e185630. [Google Scholar] [CrossRef]
- Sampson, H.A. Update on food allergy. J. Allergy Clin. Immunol. 2004, 113, 805–819. [Google Scholar] [CrossRef]
- Lee, A.J.; Shek, L.P. Food allergy in Singapore: Opening a new chapter. Singapore Med. J. 2014, 55, 244–247. [Google Scholar] [CrossRef]
- Loh, W.; Tang, M.L.K. The Epidemiology of Food Allergy in the Global Context. Int. J. Environ. Res. Public Health 2018, 15, 2043. [Google Scholar] [CrossRef] [Green Version]
- Kung, S.J.; Steenhoff, A.P.; Gray, C. Food allergy in Africa: Myth or reality? Clin. Rev. Allergy Immunol. 2014, 46, 241–249. [Google Scholar] [CrossRef]
- Levin, M.E.; Botha, M.; Basera, W.; Facey-Thomas, H.E.; Gaunt, B.; Gray, C.L.; Kiragu, W.; Ramjith, J.; Watkins, A.; Genuneit, J. Environmental factors associated with allergy in urban and rural children from the South African Food Allergy (SAFFA) cohort. J. Allergy Clin. Immunol. 2020, 145, 415–426. [Google Scholar] [CrossRef] [Green Version]
- Guidance & Regulation Food and Dietary Supplements. Available online: https://www.fda.gov/Food/GuidanceRegulation (accessed on 20 May 2020).
- EFSA Provides Scientific Basis for Labelling of Food Allergens: Current Evidence does not Allow Determination of Intake Thresholds. Available online: https://www.efsa.europa.eu/it/press/news/nda040325 (accessed on 21 June 2020).
- Allergy and Intolerance. Available online: https://www.food.gov.uk/science/allergy-intolerance (accessed on 22 January 2020).
- Food Labelling. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-labelling/ (accessed on 22 January 2020).
- Australia 2020. Available online: http://www.foodstandards.gov.au/consumer/foodallergies/allergies (accessed on 12 June 2020).
- Japan 2020. Available online: http://expatsguide.jp/features/everyday-life/food-allergy-labeling/ (accessed on 12 February 2020).
- FAO 2020. Available online: http://www.fao.org/faostat/en/#data/QD/visualize (accessed on 24 June 2020).
- Gangur, V.; Kelly, C.; Navuluri, L. Sesame allergy: A growing food allergy of global proportions? Ann. Allergy Asthma Immunol. 2005, 95, 4–11. [Google Scholar] [CrossRef]
- Johnson, W., Jr.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Amended safety assessment of Sesamum indicum (sesame) seed oil, hydrogenated sesame seed oil, Sesamum indicum (sesame) oil unsaponifiables, and sodium sesameseedate. Int. J. Toxicol. 2011, 30 (Suppl. 3), 40S–53S. [Google Scholar] [CrossRef] [PubMed]
- Askari, A.; Ravansalar, S.A.; Naghizadeh, M.M.; Mosavat, S.H.; Khodadoost, M.; Jazani, A.M.; Hashempur, M.H. The efficacy of topical sesame oil in patients with knee osteoarthritis: A randomized double-blinded active-controlled non-inferiority clinical trial. Complement. Ther. Med. 2019, 47, 102183. [Google Scholar] [CrossRef] [PubMed]
- Afroz, M.; Zihad, S.M.N.K.; Uddin, S.J.; Rouf, R.; Rahman, M.S.; Islam, M.T.; Khan, I.N.; Ali, E.S.; Aziz, S.; Shilpi, J.A.; et al. A systematic review on antioxidant and antiinflammatory activity of Sesame (Sesamum indicum L.) oil and further confirmation of antiinflammatory activity by chemical profiling and molecular docking. Phytother. Res. 2019, 33, 2585–2608. [Google Scholar] [CrossRef]
- Alday, E.; Curiel, G.; Lopez-Gil, M.J.; Carreño, D.; Moneo, I. Occupational hypersensitivity to sesame seeds. Allergy 1996, 51, 69–70. [Google Scholar] [CrossRef]
- Keskinen, H.; Ostman, P.; Vaheri, E.; Tarvainen, K.; Grenquist-Norden, B.; Karppinen, O.; Nordman, H. A case of occupational asthma, rhinitis and urticaria due to sesame seed. Clin. Exp. Allergy 1991, 21, 623–624. [Google Scholar] [CrossRef]
- Caimmi, S.; Marseglia, A.; Caimmi, D.; Marseglia, G.L. Friday asthma crisis in the daughter of two bakers. Int. J. Immunopathol. Pharmacol. 2011, 24, 517–518. [Google Scholar] [CrossRef]
- Kägi, M.K.; Wüthrich, B. Falafel burger anaphylaxis due to sesame seed allergy. Ann. Allergy 1993, 71, 127–129. [Google Scholar] [CrossRef]
- Rubenstein, L. Sensitivity to sesame seed and sesame oil. N. Y. State J. Med. 1950, 50, 343. [Google Scholar]
- Uvitsky, I.H. Sensitivity to sesame seed. J. Allergy 1951, 22, 377–378. [Google Scholar] [CrossRef]
- Batterman, R.C.; Grossman, A.J.; Leifer, P. Skin eruptions with gold therapy; influence of sesame oil. Arthritis Rheum. 1958, 1, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Neering, H.; Vitányi, B.E.; Malten, K.E.; van Ketel, W.G.; van Dijk, E. Allergens in sesame oil contact dermatitis. Acta Derm.-Venereol. 1975, 55, 31–34. [Google Scholar] [PubMed]
- Malish, D.; Glovsky, M.M.; Hoffman, D.R.; Ghekiere, L.; Hawkins, J.M. Anaphylaxis after sesame seed ingestion. J. Allergy Clin. Immunol. 1981, 67, 35–38. [Google Scholar] [CrossRef]
- United Kingdom Ministry of Agriculture Fisheries and Food. Sesame Seed Allergy Food Safety Information Bulletin; UK Ministry of Agriculture Fisheries and Food: London, UK, 1996.
- Hill, D.J.; Hosking, C.S.; Zhie, C.Y.; Leung, R.; Baratwidjaja, K.; Iikura, Y.; Iyngkaran, N.; Gonzalez-Andaya, A.; Wah, L.B.; Hsieh, K.H. The frequency of food allergy in Australia and Asia. Environ. Toxicol. Pharmacol. 1997, 4, 101–110. [Google Scholar] [CrossRef]
- Dalal, I.; Binson, I.; Reifen, R.; Amitai, Z.; Shohat, T.; Rahmani, S.; Levine, A.; Ballin, A.; Somekh, E. Food allergy is a matter of geography after all: Sesame as a major cause of severe IgE-mediated food allergic reactions among infants and young children in Israel. Allergy 2002, 57, 362–365. [Google Scholar] [CrossRef]
- Navuluri, L.; Parvataneni, S.; Hassan, H.; Birmingham, N.P.; Kelly, C.; Gangur, V. Allergic and anaphylactic response to sesame seeds in mice: Identification of Ses i 3 and basic subunit of 11s globulins as allergens. Int. Arch. Allergy Immunol. 2006, 140, 270–276. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Muñoz-Furlong, A.; Godbold, J.H.; Sampson, H.A. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J. Allergy Clin. Immunol. 2010, 125, 1322–1326. [Google Scholar] [CrossRef]
- Ben-Shoshan, M.; Harrington, D.W.; Soller, L.; Fragapane, J.; Joseph, L.; St Pierre, Y.; Godefroy, S.B.; Elliott, S.J.; Clarke, A.E. A population-based study on peanut, tree nut, fish, shellfish, and sesame allergy prevalence in Canada. J. Allergy Clin. Immunol. 2010, 125, 1327–1335. [Google Scholar] [CrossRef] [Green Version]
- Irani, C.; Maalouly, G.; Germanos, M.; Kazma, H. Food allergy in Lebanon: Is sesame seed the “middle eastern” peanut. World Allergy Organ J. 2011, 4, 1–3. [Google Scholar] [CrossRef] [Green Version]
- CSPI. Available online: https://cspinet.org/new/201411181.html (accessed on 20 June 2020).
- Sheikh, F.; Amin, R.; Rehan Khaliq, A.M.; Al Otaibi, T.; Al Hashim, S.; Al Gazlan, S. First study of pattern of anaphylaxis in a large tertiary care hospital in Saudi Arabia. Asia Pac. Allergy 2015, 5, 216–221. [Google Scholar] [CrossRef]
- Bedolla-Barajas, M.; Bedolla-Pulido, T.R.; Macriz-Romero, N.; Morales-Romero, J.; Robles-Figueroa, M. Prevalence of Peanut, Tree Nut, Sesame, and Seafood Allergy in Mexican Adults. Rev. Invest. Clin. 2015, 67, 379–386. [Google Scholar]
- Nabavi, M.; Lavavpour, M.; Arshi, S.; Bemanian, M.H.; Esmaeilzadeh, H.; Molatefi, R.; Rekabi, M.; Ahmadian, J.; Eslami, N.; Shokri, S.; et al. Characteristics, Etiology and Treatment of Pediatric and Adult Anaphylaxis in Iran. Iran J. Allergy Asthma Immunol. 2017, 16, 480–487. [Google Scholar] [PubMed]
- Ali, F. A Survey of Self-Reported Food Allergy and Food-Related Anaphylaxis among Young Adult Students at Kuwait University, Kuwait. Med. Princ. Pract. 2017, 26, 229–234. [Google Scholar] [CrossRef] [PubMed]
- US FDA. Available online: https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-fdas-new-consideration-labeling-sesame-allergies (accessed on 20 June 2020).
- All Information (Except Text) for H.R.2117—FASTER Act of 2019. Available online: https://www.congress.gov/bill/116th-congress/house-bill/2117/all-info (accessed on 10 June 2020).
- Illinois Requires Food Manufacturers to Label Sesame Allergens. Available online: https://will.illinois.edu/news/story/illinois-requires-food-manufacturers-to-label-sesame-allergen (accessed on 15 June 2020).
- Sokol, K.; Rasooly, M.; Dempsey, C.; Lassiter, S.; Gu, W.; Lumbard, K.; Frischmeyer-Guerrerio, P.A. Prevalence and diagnosis of sesame allergy in children with IgE-mediated food allergy. Pediatric Allergy Immunol. 2020, 31, 214–218. [Google Scholar] [CrossRef] [PubMed]
- NIH. Available online: https://www.nih.gov/news-events/nih-research-matters/sesame-allergy-common-among-children-food-allergies (accessed on 13 July 2020).
- Kahveci, M.; Koken, G.; Şahiner, Ü.M.; Soyer, Ö.; Şekerel, B.E. Immunoglobulin E-Mediated Food Allergies Differ in East Mediterranean Children Aged 0-2 Years. Int. Arch. Allergy Immunol. 2020, 181, 365–374. [Google Scholar] [CrossRef]
- Emmett, S.E.; Angus, F.J.; Fry, J.S.; Lee, P.N. Perceived prevalence of peanut allergy in Great Britain and its association with other atopic conditions and with peanut allergy in other household members. Allergy 1999, 54, 380–385, Erratum in 1999, 54, 891. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.; Peckitt, C.; Northstone, K.; Strachan, D.; Lack, G.; Henderson, J.; Golding, J. ALSPAC Study Team. Relationship between aeroallergen and food allergen sensitization in childhood. Clin. Exp. Allergy 2005, 35, 933–940. [Google Scholar] [CrossRef]
- Pereira, B.; Venter, C.; Grundy, J.; Clayton, C.B.; Arshad, S.H.; Dean, T. Prevalence of sensitization to food allergens, reported adverse reaction to foods, food avoidance, and food hypersensitivity among teenagers. J. Allergy Clin. Immunol. 2005, 116, 884–892. [Google Scholar] [CrossRef]
- Venter, C.; Pereira, B.; Grundy, J.; Clayton, C.B.; Arshad, S.H.; Dean, T. Prevalence of sensitization reported and objectively assessed food hypersensitivity amongst six-year-old children: A population-based study. Pediatric Allergy Immunol. 2006, 17, 356–363. [Google Scholar] [CrossRef]
- Osborne, N.J.; Koplin, J.J.; Martin, P.E.; Gurrin, L.C.; Lowe, A.J.; Matheson, M.C.; Ponsonby, A.L.; Wake, M.; Tang, M.L.; Dharmage, S.C.; et al. HealthNuts Investigators. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J. Allergy Clin. Immunol. 2011, 127, 668–676.e2. [Google Scholar] [CrossRef]
- Peters, R.L.; Koplin, J.J.; Gurrin, L.C.; Dharmage, S.C.; Wake, M.; Ponsonby, A.L.; Tang, M.L.K.; Lowe, A.J.; Matheson, M.; Dwyer, T.; et al. HealthNuts Study. The prevalence of food allergy and other allergic diseases in early childhood in a population-basedstudy: HealthNuts age 4-year follow-up. J. Allergy Clin. Immunol. 2017, 140, 145–153.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nachshon, L.; Schwartz, N.; Elizur, A.; Schon, Y.; Cheryomukhin, M.; Katz, Y.; Goldberg, M.R. The Prevalence of Food Allergy in Young Israeli Adults. J. Allergy Clin. Immunol. Pract. 2019, 7, 2782–2789.e4. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.C.; Kidon, M.I.; Liew, W.K.; Goh, A.; Tang, J.P.; Chay, O.M. The changing face of food hypersensitivity in an Asian community. Clin. Exp. Allergy 2007, 37, 1055–1061. [Google Scholar] [CrossRef]
- Cohen, A.; Goldberg, M.; Levy, B.; Leshno, M.; Katz, Y. Sesame food allergy and sensitization in children: The natural history and long-term follow-up. Pediatric Allergy Immunol. 2007, 18, 217–223. [Google Scholar] [CrossRef]
- Warren, C.M.; Jiang, J.; Gupta, R.S. Epidemiology and Burden of Food Allergy. Curr. Allergy Asthma Rep. 2020, 20, 6. [Google Scholar] [CrossRef]
- Permaul, P.; Stutius, L.M.; Sheehan, W.J.; Rangsithienchai, P.; Walter, J.E.; Twarog, F.J.; Young, M.C.; Scott, J.E.; Schneider, L.C.; Phipatanakul, W. Sesame allergy: Role of specific IgE and skin-prick testing in predicting food challenge results. Allergy Asthma Proc. 2009, 30, 643–648. [Google Scholar] [CrossRef]
- Aaronov, D.; Tasher, D.; Levine, A.; Somekh, E.; Serour, F.; Dalal, I. Natural history of food allergy in infants and children in Israel. Ann. Allergy Asthma Immunol. 2008, 101, 637–640. [Google Scholar] [CrossRef]
- Chiu, J.T.; Haydik, I.B. Sesame seed oil anaphylaxis. J. Allergy Clin. Immunol. 1991, 88 (3 Pt 1), 414–415. [Google Scholar] [CrossRef]
- Stevens, W.J.; Ebo, D.G.; Bridts, C.H.; De Clerck, L.S. Anaphylaxis tos sesame (Sesamum indicum) seed and sesame oil. J. Allergy Clin. Immunol. 2002, 109, 650. [Google Scholar] [CrossRef]
- Van Dijk, E.; Dijk, E.; Neering, H.; Vitányi, B.E. Contact hypersensitivity to sesame oil in patients with leg ulcers and eczema. Acta Derm. Venereol. 1973, 53, 133–135. [Google Scholar]
- Kubo, Y.; Nonaka, S.; Yoshida, H. Contact sensitivity to unsaponifiable substances in sesame oil. Contact Dermat. 1986, 15, 215–217. [Google Scholar] [CrossRef]
- Oiso, N.; Yamadori, Y.; Higashimori, N.; Kawara, S.; Kawada, A. Allergic contact dermatitis caused by sesame oil in a topical Chinese medicine, shi-un-ko. Contact Dermat. 2008, 58, 109. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Warren, C.M.; Brown-Whitehorn, T.; Cianferoni, A.; Schultz-Matney, F.; Gupta, R.S. Food protein-induced enterocolitis syndrome in the US population-based study. J. Allergy Clin. Immunol. 2019, 144, 1128–1130. [Google Scholar] [CrossRef] [Green Version]
- Ovadia, A.; Nahum, A.; Tasher, D.; Abiri, S.; Epov, L.; Kessel, A.; Dalal, I. Sesame: An unrecognized trigger of food protein-induced enterocolitis syndrome. J. Allergy Clin. Immunol. Pract. 2019, 7, 305–306. [Google Scholar] [CrossRef]
- Momen, T.; Saneian, H.; Amini, N. Demographic, Clinical, and Allergic Characteristics of Children with Eosinophilic Esophagitis in Isfahan, Iran. Iran J. Allergy Asthma Immunol. 2018, 17, 533–539. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Choi, S.S.; Gupta, S.K. Eosinophilic esophagitis: Current status and future directions. Pediatr. Res. 2020, in press. [Google Scholar] [CrossRef] [Green Version]
- Van Ree, R.; Voitenko, V.; van Leeuwen, W.A.; Aalberse, R.C. Profilin is a cross-reactive allergen in pollen and vegetable foods. Int. Arch. Allergy Immunol. 1992, 98, 97–104. [Google Scholar] [CrossRef]
- Beyer, K.; Bardina, L.; Grishina, G.; Sampson, H.A. Identification of sesame seed allergens by 2 dimensional proteomics and Edmansequencing: Seed storage proteins as common food allergens. J. Allergy Clin. Immunol. 2002, 110, 154–159. [Google Scholar] [CrossRef]
- Beyer, K.; Grishina, G.; Bardina, L.; Sampson, H.A. Identification of 2 new sesame seed allergens: Ses i 6 and Ses i 7. J. Allergy Clin. Immunol. 2007, 119, 1554–1556. [Google Scholar] [CrossRef]
- Magni, C.; Ballabio, C.; Restani, P.; Fuggetta, D.; Alessandri, C.; Mari, A. Molecular insight into IgE mediated reactions to sesame (Sesamum indicumL.) seed proteins. Ann. Allergy Asthma Immunol. 2010, 105, 458–464. [Google Scholar] [CrossRef]
- Teodorowicz, M.; Terlouw, R.J.; Jansen, A.; Savelkoul, H.F.; Ruinemans-Koerts, J. Immunological Characterization of Dutch Sesame Seed-Allergic Patients. Int. Arch. Allergy Immunol. 2016, 169, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Leduc, V.; Moneret-Vautrin, D.A.; Tzen, J.T.C.; Morisset, M.; Guerin, L.; Kanny, G. Identification of oleosins as major allergens in sesame seed allergic patients. Allergy 2006, 61, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Jappe, U.; Schwager, C. Relevance of Lipophilic Allergens in Food Allergy Diagnosis. Curr. Allergy Asthma Rep. 2017, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, A.M.; Rossnagel, M.; Brix, B.; Blankestijn, M.A.; Le, T.M.; Suer, W.; Otten, H.G.; Knulst, A.C. Sesame oleosins are minor allergens. Clin. Transl. Allergy 2019, 9, 32. [Google Scholar] [CrossRef]
- Taylor, S.L.; Busse, W.W.; Sachs, M.I. Peanut oil is not allergenic to peanut-sensitive individuals. J. Allergy Clin. Immunol. 1981, 68, 372–375. [Google Scholar] [CrossRef]
- Kadlubowska, D.; Bargman, H.; Sasseville, D. Systemic contact dermatitis caused by inhaled cashew oil smoke. Contact Dermat. 2016, 75, 248–250. [Google Scholar] [CrossRef]
- Hirao, A.; Oiso, N.; Matsuda, H.; Kawara, S.; Kawada, A. Occupational allergic contact dermatitis due to cashew nut oil. Contact Dermat. 2008, 59, 131–132. [Google Scholar] [CrossRef]
- Hamilton, T.K.; Zug, K.A. Systemic contact dermatitis to raw cashew nuts in a pesto sauce. Am. J. Contact Dermat. 1998, 9, 51–54. [Google Scholar]
- Jin, Y.; Acharya, H.G.; Acharya, D.; Jorgensen, R.; Gao, H.; Secord, J.; Ng, P.K.W.; Gangur, V. Advances in Molecular Mechanisms of Wheat Allergenicity in Animal Models: A Comprehensive Review. Molecules 2019, 24, 1142. [Google Scholar] [CrossRef] [Green Version]
- Cartledge, N.; Chan, S. Atopic Dermatitis and Food Allergy: A Paediatric Approach. Curr. Pediatric Rev. 2018, 14, 171–179. [Google Scholar] [CrossRef]
- Wąsik, M.; Nazimek, K.; Nowak, B.; Askenase, P.W.; Bryniarski, K. Delayed-Type Hypersensitivity Underlying Casein Allergy Is Suppressed by Extracellular Vesicles Carrying miRNA-150. Nutrients 2019, 11, 907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampson, H.A.; O’Mahony, L.; Burks, A.W.; Plaut, M.; Lack, G.; Akdis, C.A. Mechanisms of food allergy. J. Allergy Clin. Immunol. 2018, 141, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, D.H.; Igyártó, B.Z.; Gaspari, A.A. Early immune events in the induction of allergic contact dermatitis. Nat. Rev. Immunol. 2012, 12, 114–124. [Google Scholar] [CrossRef]
- Kim, J.H.; Hu, Y.; Yongqing, T.; Kim, J.; Hughes, V.A.; Le Nours, J.; Marquez, E.A.; Purcell, A.W.; Wan, Q.; Sugita, M.; et al. CD1a on Langerhans cells controls inflammatory skin disease. Nat. Immunol. 2016, 17, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Casorati, G.; Dellabona, P. Of self-lipids, CD1-restricted T cells, and contact sensitization. Eur. J. Immunol. 2017, 47, 1119–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betts, R.J.; Perkovic, A.; Mahapatra, S.; Del Bufalo, A.; Camara, K.; Howell, A.R.; Martinozzi Teissier, S.; De Libero, G.; Mori, L. Contact sensitizers trigger human CD1-autoreactive T-cell responses. Eur. J. Immunol. 2017, 47, 1171–1180. [Google Scholar] [CrossRef] [Green Version]
- Nicolai, S.; Wegrecki, M.; Cheng, T.Y.; Bourgeois, E.A.; Cotton, R.N.; Mayfield, J.A.; Monnot, G.C.; Le Nours, J.; van Rhijn, I.; Rossjohn, J.; et al. Human T cell response to CD1a and contact dermatitis allergens in botanical extracts and commercial skin care products. Sci. Immunol. 2020, 5, eaax5430. [Google Scholar] [CrossRef]
- Nguyen, H.L.; Yiannias, J.A. Contact Dermatitis to Medications and Skin Products. Clin. Rev. Allergy Immunol. 2019, 56, 41–59. [Google Scholar] [CrossRef]
- Arias, Á.; Lucendo, A.J. Molecular basis and cellular mechanisms of eosinophilic esophagitis for the clinical practice. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 99–117. [Google Scholar] [CrossRef]
- Noguchi, E.; Akiyama, M.; Yagami, A.; Hirota, T.; Okada, Y.; Kato, Z.; Kishikawa, R.; Fukutomi, Y.; Hide, M.; Morita, E.; et al. HLA-DQ and RBFOX1 as susceptibility genes for an outbreak of hydrolyzed wheat allergy. J. Allergy Clin. Immunol. 2019, 144, 1354–1363. [Google Scholar] [CrossRef] [Green Version]
- Carter, C.A.; Frischmeyer-Guerrerio, P.A. The Genetics of Food Allergy. Curr. Allergy Asthma Rep. 2018, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Asai, Y.; Eslami, A.; van Ginkel, C.D.; Akhabir, L.; Wan, M.; Ellis, G.; Ben-Shoshan, M.; Martino, D.; Ferreira, M.A.; Allen, K.; et al. Genome-wide association study and meta-analysis in multiple populations identifies new loci for peanut allergy and establishes C11orf30/EMSY as a genetic risk factor for food allergy. J. Allergy Clin. Immunol. 2018, 141, 991–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashley, S.E.; Tan, H.T.; Peters, R.; Allen, K.J.; Vuillermin, P.; Dharmage, S.C.; Tang, M.L.K.; Koplin, J.; Lowe, A.; Ponsonby, A.L.; et al. Genetic variation at the Th2 immune gene IL13 is associated with IgE-mediated paediatric food allergy. Clin. Exp. Allergy 2017, 47, 1032–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.H.; Song, Y.; Wu, W.; Yu, K.; Zhang, G. The gut microbiota, environmental factors, and links to the development of food allergy. Clin. Mol. Allergy 2020, 18, 5. [Google Scholar] [CrossRef]
- Marrs, T.; Logan, K.; Craven, J.; Radulovic, S.; McLean, W.H.A.I.; Lack, G.; Flohr, C.; Perkin, M.R. EAT Study Team. Dog ownership at three months of age is associated with protection against food allergy. Allergy 2019, 74, 2212. [Google Scholar] [CrossRef] [PubMed]
- Metzler, S.; Frei, R.; Schmaußer-Hechfellner, E.; von Mutius, E.; Pekkanen, J.; Karvonen, A.M.; Kirjavainen, P.V.; Dalphin, J.C.; Divaret-Chauveau, A.; Riedler, J.; et al. PASTURE/EFRAIM study group. Association between antibiotic treatment during pregnancy and infancy and the development of allergic diseases. Pediatric Allergy Immunol. 2019, 30, 423–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, M.; Peters, R.L.; Koplin, J.J.; Field, M.J.; McWilliam, V.; Sawyer, S.M.; Vuillermin, P.J.; Pezic, A.; Gurrin, L.C.; Douglass, J.A.; et al. Risk Factors for Food Allergy in Early Adolescence: The SchoolNuts Study. J. Allergy Clin. Immunol. Pract. 2018, 6, 496–505. [Google Scholar] [CrossRef]
- Mitselou, N.; Hallberg, J.; Stephansson, O.; Almqvist, C.; Melén, E.; Ludvigsson, J.F. Cesarean delivery, preterm birth, and risk of food allergy: Nationwide Swedish cohort study of more than 1 million children. J. Allergy Clin. Immunol. 2018, 142, 1510–1514.e2. [Google Scholar] [CrossRef] [Green Version]
- Hennessy, Á.; Hourihane, J.O.; Malvisi, L.; Irvine, A.D.; Kenny, L.C.; Murray, D.M.; Kiely, M.E. Antenatal vitamin D exposure and childhood eczema, food allergy, asthma and allergic rhinitis at 2 and 5 years of age in the atopic disease-specific Cork BASELINE Birth Cohort Study. Allergy 2018, 73, 2182–2191. [Google Scholar] [CrossRef]
- DeMuth, K.; Stecenko, A.; Sullivan, K.; Fitzpatrick, A. Relationship between treatment with antacid medication and the prevalence of food allergy in children. Allergy Asthma Proc. 2013, 34, 227–232. [Google Scholar] [CrossRef]
- Silva, C.M.; Silva, S.A.D.; Antunes, M.M.C.; Silva, G.A.P.D.; Sarinho, E.S.C.; Brandt, K.G. Do infants with cow’s milk protein allergy have inadequate levels of vitamin D? J. Pediatr. 2017, 93, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.H.; Matsui, E.C.; Wood, R.A.; Keet, C.A. Urinary levels of triclosan and parabens are associated with aeroallergen and food sensitization. J. Allergy Clin. Immunol. 2012, 130, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.A.; Yuen, A.W.T.; Woo, E.; Chu, K.H.; Kwan, H.S.; Yang, G.X.; Yang, Y.; Leung, P.S.C. Microbiota and Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Feehley, T.; Plunkett, C.H.; Bao, R.; Choi Hong, S.M.; Culleen, E.; Belda-Ferre, P.; Campbell, E.; Aitoro, R.; Nocerino, R.; Paparo, L.; et al. Healthy infants harbor intestinal bacteria that protect against food allergy. Nat. Med. 2019, 25, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.; Novak, N. Effects of daily food processing on allergenicity. Crit. Rev. Food Sci. Nutr. 2019, 59, 31–42. [Google Scholar] [CrossRef]
- Meng, S.; Li, J.; Chang, S.; Maleki, S.J. Quantitative and kinetic analyses of peanut allergens as affected by food processing. Food Chem. X 2019, 1, 100004. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.; Li, K.; Li, X.; Yang, A.; Tong, P.; Chen, H. Allergenicity assessment on thermally processed peanut influenced by extraction and assessment methods. Food Chem. 2019, 281, 130–139. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, Y.; Zhao, Y.; Tang, G.; Niu, B.; Chen, Q. Boiling and roasting treatment affecting the peanut allergenicity. Ann. Transl. Med. 2018, 6, 357. [Google Scholar] [CrossRef]
- Ortiz, T.; Para, R.; Gonipeta, B.; Reitmeyer, M.; He, Y.; Srkalovic, I.; Ng, P.K.; Gangur, V. Effect of extrusion processing on immune activation properties of hazelnut protein in a mouse model. Int. J. Food Sci. Nutr. 2016, 67, 660–669. [Google Scholar] [CrossRef]
- Verhoeckx, K.C.M.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N.; et al. Food processing and allergenicity. Food Chem. Toxicol. 2015, 80, 223–240. [Google Scholar] [CrossRef]
- Blanc, F.; Vissers, Y.M.; Adel-Patient, K.; Rigby, N.M.; Mackie, A.R.; Gunning, A.P.; Wellner, N.K.; Skov, P.S.; Przybylski-Nicaise, L.; Ballmer-Weber, B.; et al. Boiling peanut Ara h 1 results in the formation of aggregates with reduced allergenicity. Mol. Nutr. Food Res. 2011, 55, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kulis, M. Hypoallergenic Proteins for the Treatment of Food Allergy. Curr. Allergy Asthma Rep. 2019, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Maslin, K.; Fox, A.T.; Chambault, M.; Meyer, R. Palatability of hypoallergenic formulas for cow’s milk allergy and healthcare professional recommendation. Pediatr. Allergy Immunol. 2018, 29, 857–862. [Google Scholar] [CrossRef]
- Palladino, C.; Breiteneder, H. Peanut allergens. Mol. Immunol. 2018, 100, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Kohno, K.; Takahashi, H.; Endo, T.R.; Matsuo, H.; Shiwaku, K.; Morita, E. Characterization of a hypoallergenic wheat line lacking ω-5 gliadin. Allergol. Int. 2016, 65, 400–405. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Jin, Y.; Jian, D.I.; Olson, E.; Ng, P.K.W.; Gangur, V. Development and validation of a mouse-based primary screening method for testing relative allergenicity of proteins from different wheat genotypes. J. Immunol. Methods 2019, 464, 95–104. [Google Scholar] [CrossRef]
- Gonipeta, B.; Kim, E.; Gangur, V. Mouse models of food allergy: How well do they simulate the human disorder? Crit. Rev. Food Sci. Nutr. 2015, 55, 437–452. [Google Scholar] [CrossRef]
- Shaker, D. An Analysis of “Natural” Food Litigation to Build a Sesame Allergy Consumer Class Action. Food Drug Law J. 2017, 72, 103–140. [Google Scholar]
- Blom, W.M.; Michelsen-Huisman, A.D.; van Os-Medendorp, H.; van Duijn, G.; de Zeeuw-Brouwer, M.L.; Versluis, A.; Castenmiller, J.J.M.; Noteborn, H.P.J.M.; Kruizinga, A.G.; Knulst, A.C.; et al. Accidental food allergy reactions: Products and undeclared ingredients. J. Allergy Clin. Immunol. 2018, 142, 865–875. [Google Scholar] [CrossRef] [Green Version]
- Caminiti, L.; Vita, D.; Passalacqua, G.; Arrigo, T.; Barberi, S.; Lombardo, F.; Pajno, G.B. Tahini, a little known sesame-containing food, as an unexpected cause of severe allergic reaction. J. Investig. Allergol. Clin. Immunol. 2006, 16, 308–310. [Google Scholar]
- Reese, I.; Holzhauser, T.; Schnadt, S.; Dölle, S.; Kleine-Tebbe, J.; Raithel, M.; Worm, M.; Zuberbier, T.; Vieths, S. Allergen and allergy risk assessment, allergen management, and gaps in the European Food Information Regulation (FIR): Are allergic consumers adequately protected by current statutory food safety and labeling regulations? Allergo J. Int. 2015, 24, 180–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segal, L.; Ben-Shoshan, M.; Alizadehfar, R.; Primeau, M.N.; Asai, Y.; Killorn, K.R.; Chan, E.; Cheuk, S.; Shand, G.; St-Pierre, Y.; et al. Initial and accidental reactions are managed inadequately in children with sesame allergy. J. Allergy Clin. Immunol. Pract. 2017, 5, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; Baumert, J.L. Worldwide food allergy labeling and detection of allergens in processed foods. Chem. Immunol. Allergy 2015, 101, 227–234. [Google Scholar] [PubMed]
- Eberlein-König, B.; Rueff, F.; Przybilla, B. Generalized urticaria caused by sesame seeds with negative prick test results and without demonstrable specific IgE antibodies. J. Allergy Clin. Immunol. 1995, 96, 560–561. [Google Scholar] [CrossRef]
- Kanny, G.; De Hauteclocque, C.; Moneret-Vautrin, D.A. Sesame seed and sesame seed oil contain masked allergens of growing importance. Allergy 1996, 51, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Kolopp-Sarda, M.N.; Moneret-Vautrin, D.A.; Gobert, B.; Kanny, G.; Brodschii, M.; Bene, M.C.; Faure, G.C. Specific humoral immune responses in 12 cases of food sensitization to sesame seed. Clin. Exp. Allergy 1997, 27, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Remington, B.C.; Westerhout, J.; Meima, M.Y.; Blom, W.M.; Kruizinga, A.G.; Wheeler, M.W.; Taylor, S.L.; Houben, G.F.; Baumert, J.L. Updated population minimal eliciting dose distributions for use in risk assessment of 14 priority food allergens. Food Chem. Toxicol. 2020, 139, 111259. [Google Scholar] [CrossRef]
- Kuroyama, M.; Kagawa, H.; Kitada, S.; Maekura, R.; Mori, M.; Hirano, H. Exogenous lipoid pneumonia caused by repeated sesame oil pulling: A report of two cases. BMC Pulm. Med. 2015, 15, 135. [Google Scholar] [CrossRef] [Green Version]
- Veysman, B.; Vlahos, I.; Oshva, L. Pneumonitis and eosinophilia after in vitro fertilization treatment. Ann. Emerg. Med. 2006, 47, 472–475. [Google Scholar] [CrossRef]
- Phy, J.L.; Weiss, W.T.; Weiler, C.R.; Damario, M.A. Hypersensitivity to progesterone-in-oil after in vitro fertilization and embryo transfer. Fertil. Steril. 2003, 80, 1272–1275. [Google Scholar] [CrossRef]
- Bouckaert, Y.; Robert, F.; Englert, Y.; De Backer, D.; De Vuyst, P.; Delbaere, A. Acute eosinophilic pneumonia associated with intramuscular administration of progesterone as luteal phase support after IVF: Case report. Hum. Reprod. 2004, 19, 1806–1810. [Google Scholar] [CrossRef] [PubMed]
- Darsow, U.; Bruckbauer, H.; Worret, W.I.; Hofmann, H.; Ring, J. Subcutaneous oleomas induced by self-injection of sesame seed oil for muscle augmentation. J. Am. Acad. Dermatol. 2000, 42 Pt 1, 292–294. [Google Scholar] [CrossRef]
- Khankhanian, N.K.; Hammers, Y.A.; Kowalski, P. Exuberant local tissue reaction to intramuscular injection of nandrolone decanoate (Deca-Durabolin)—A steroid compound in a sesame seed oil base--mimicking soft tissue malignant tumors: A case report and review of the literature. Mil. Med. 1992, 157, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Stern, A.; Wüthrich, B. Non-IgE-mediated anaphylaxis to sesame. Allergy 1998, 53, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Cano, R.; Picado, C.; Valero, A.; Bartra, J. Mechanisms of Anaphylaxis Beyond IgE. J. Investig. Allergol. Clin. Immunol. 2016, 26, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Marchisotto, M.J.; Harada, L.; Kamdar, O.; Smith, B.M.; Waserman, S.; Sicherer, S.; Allen, K.; Muraro, A.; Taylor, S.; Gupta, R.S. Food Allergen Labeling and Purchasing Habits in the United States and Canada. J. Allergy Clin. Immunol. Pract. 2017, 5, 345–351.e2. [Google Scholar] [CrossRef] [PubMed]


| Country | List of Federally Regulated Allergenic Foods |
|---|---|
| USA | 8 foods: wheat, milk, soybean, egg, peanut, tree nuts, fish, and crustacean shellfish # (sesame is not federally regulated). |
| Canada | 12 foods: wheat and triticale (hybrid of wheat and rye grains), milk, soy, egg, peanut, tree nuts, fish, shellfish (crustaceans, mollusks *), sesame, mustard, sulfites. |
| European Union ** & UK | 14 foods: Cereals that contain gluten including wheat, rye, barley and oats, milk, soy, egg, fish, shellfish (crustaceans including prawns, crabs, and lobsters, mollusks including mussels and oysters), peanut, tree nuts, sesame seed, mustard, sulfur dioxide, and sulfites, celery, lupine. |
| Australia & New Zealand | 10 foods: wheat, milk, soybean, egg, peanut, tree nuts, fish, shellfish (crustacean and mollusks), sesame, and lupine. |
| Japan | 7 foods: wheat, buckwheat, milk, egg, peanut, shrimp, and crab; Recommends manufacturers to display 20 additional foods: abalone, squid, salmon roe, orange, cashew nut, kiwi, beef, walnut, sesame, salmon, mackerel, soybean, chicken, banana, pork, matsutake mushroom, peach, yam, apple, and gelatin. |
| Year | Milestone |
|---|---|
| 1950 | The first report, sesame seed allergy and anaphylaxis, USA [26]. |
| 1951 | The first report of asthma due to sesame, USA [27]. |
| 1958 | The first report, sesame oil allergic dermatitis (urticaria and rash upon injection), USA [28]. |
| 1975 | The first identification of sesamin, sesamol, and sesamolin as lipid allergens in contact dermatitis [29]. |
| 1981 | The first identification of sesame seed proteins of 8–62 kDa as protein allergens [30]. |
| 1991 | The first report of occupational (baker) allergy (dermatitis, asthma, rhinitis) to sesame seed [23]. |
| 1996 | The first prevalence study of sesame allergy in the UK general population, 0.05% [31]. |
| 1997 | The first prevalence study of sesame allergy in the pediatric population in Australia, 0.42% [32]. |
| 1999 | Sesame regulated by the European Union, Canada, Australia, and New Zealand [12,13,14,15]. |
| 2002 | The first report showing that the sesame allergy is more prevalent in Israel (0.18%) than peanut allergy (0.04%) [33]. |
| 2005 | The first report asking whether sesame allergy might be growing globally; concern on the absence of sesame regulation in the USA [18]. |
| 2006 | FALCPA 2004 Act implemented in USA; did not include sesame as a major allergen [11]. |
| 2006 | The first and the only animal model of sesame allergy and anaphylaxis [34]. |
| 2010 | The first report to show the population prevalence of sesame allergy in USA at 0.1% [35]. |
| 2011 | The first prevalence study of sesame allergy in Canada (children, 0.23%, adults, 0.05%) [36]. |
| 2011 | The first prevalence of sesame allergy in Lebanon (children, 2.6–3.9%, adults, 1.9%) [37]. |
| 2014 | 18 November, Citizen Petition by Center for Science in Public Interest asking the US FDA to regulate sesame as an allergen [38]. |
| 2015 | The first report that in Saudi Arabia, sesame was the third most common cause of anaphylaxis (22.7% of all anaphylaxis cases) [39]. |
| 2015 | The first study to show the prevalence of sesame allergy in Mexican adults (0.1%) [40] |
| 2017 | The first report that sesame anaphylaxis in Iran is common (children, 1.3%, adults, 9.3% of all anaphylaxis cases) [41]. |
| 2017 | The first study to show the prevalence of sesame allergy in Kuwait (young adults, 0.46%) [42]. |
| 2018 | 29 October, US FDA requests for public information on sesame allergy in the US [43]. |
| 2019 | A second study shows prevalence of sesame allergy in the USA at 0.23–0.49% [2]. |
| 2019 | 8 April, FASTER Act introduced in USA; it proposes to regulate sesame as an allergen [44]. |
| 2019 | Illinois (USA) enacts a new state law mandating sesame labeling on food packages [45]. |
| 2019 | NIH/USA study: 17% of food allergic children have sesame allergy [46]. |
| 2019 | NIH (USA) news release on the above story; highlights rising concerns on lack of sesame regulation in the USA [47]. |
| 2020 | The first report from Turkey: 20.2% of food allergic children have sesame allergy [48]. |
| Name | Biochemical Nature | Size (Da) | Solubility | Function |
|---|---|---|---|---|
| Ses i 1 | 2S Albumin | 9000 | Hydrophilic | Seed storage |
| Ses i 2 | 2S Albumin | 7000 | Hydrophilic | Seed storage |
| Ses i 3 | 7S Vicilin-like globulin | 45,000 | Hydrophilic | Seed storage |
| Ses i 4 | Oleosin | 17,000 | Hydrophobic | Oil-body structure |
| Ses i 5 | Oleosin | 15,000 | Hydrophobic | Oil-body structure |
| Ses i 6 | 11S Globulin | 52,000 | Hydrophilic | Seed storage |
| Ses i 7 | 11S Globulin | 57,000 | Hydrophilic | Seed storage |
| Ses i 8 * | Profilin | 14,000 | Hydrophilic | Actin-binding |
| Sesamin | Lipid allergen, lignan | 354 | Hydrophobic | Pro-antioxidant |
| Sesamol ** | Lipid allergen, lignan | 134 | Hydrophobic | Antioxidant |
| Sesamolin | Lipid allergen, lignan | 370 | Hydrophobic | Antioxidant |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangur, V.; Acharya, H.G. The Global Rise and the Complexity of Sesame Allergy: Prime Time to Regulate Sesame in the United States of America? Allergies 2021, 1, 1-21. https://0-doi-org.brum.beds.ac.uk/10.3390/allergies1010001
Gangur V, Acharya HG. The Global Rise and the Complexity of Sesame Allergy: Prime Time to Regulate Sesame in the United States of America? Allergies. 2021; 1(1):1-21. https://0-doi-org.brum.beds.ac.uk/10.3390/allergies1010001
Chicago/Turabian StyleGangur, Venugopal, and Harini G. Acharya. 2021. "The Global Rise and the Complexity of Sesame Allergy: Prime Time to Regulate Sesame in the United States of America?" Allergies 1, no. 1: 1-21. https://0-doi-org.brum.beds.ac.uk/10.3390/allergies1010001




