Modeling of the Van Der Waals Forces during the Adhesion of Capsule-Shaped Bacteria to Flat Surfaces
Abstract
:1. Introduction
2. Materials and Methods
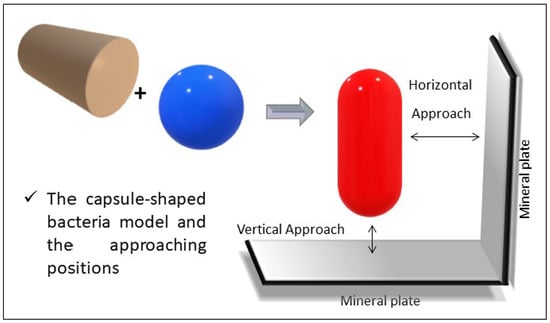
2.1. Capsule Model
2.2. Hamaker’s Microscopic Approach
2.2.1. Molecule-Plate Interaction Potential
2.2.2. Sphere-Flat Plate vdW Interaction Potential
2.2.3. Cylinder to Flat Plate vdW Interaction Potential
A Horizontal Cylindrical Particle Approach
A Vertical Cylindrical Particle Approach
2.2.4. Retardation Effect in van der Waals Interaction
2.3. Modeling Method and Software
- Spherical shell bacteria to spherical mineral particle;
- Horizontal capsule shaped bacteria to flat-plate mineral surface;
- Vertical capsule shaped bacteria to flat-plate mineral surface;
- Spherical shell bacteria to semi-infinite mineral wall.
3. Results and Discussion
3.1. Capsule-Flat Plate Interaction Potential
3.1.1. A Vertical Capsule-shaped Particle Approach
3.1.2. A Horizontal Capsule-shaped Particle Approach
3.2. Hamaker Interaction Constant for the Capsule-Shaped Particle
3.3. The van der Waals Interaction Potential of P. Putida to Hematite and Quartz
3.4. The Effect of Geometrical Shape to the van der Waals Interaction Potentials
4. Conclusions
- The density difference between each type of bacterial shape (capsule, cylinder, and sphere) require different amounts of energy to adhere to hematite and quartz surfaces;
- The orientations of bacteria approaching the mineral surface influences the adhesion of the cells to the mineral surface;
- The type of mineral has an effect. In the case of P. putida, adherence is stronger to quartz than hematite;
- The geometrical shape and curvature effect of the bacterial and mineral surfaces shows a greater interaction between spheres and spherical shell than other geometries and thus particle surface topography will have a considerable influence on the settlement of bacteria.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, J.; Sharma, V.K.; Parmar, S.; Singh, P.; Singh, R.K. Biofilm: A microbial assemblage on the surface—A boon or bane. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Biofilms; Elsevier: Amsterdam, The Netherlands, 2020; pp. 139–150. [Google Scholar]
- Bos, R.; Van der Mei, H.C.; Busscher, H.J. Physico-chemistry of initial microbial adhesive interactions–its mechanisms and methods for study. FEMS Microbiol. Rev. 1999, 23, 179–230. [Google Scholar] [CrossRef]
- Filipović, U.; Dahmane, R.G.; Ghannouchi, S.; Zore, A.; Bohinc, K. Bacterial adhesion on orthopedic implants. Adv. Colloid Interface Sci. 2020, 283, 102228. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wang, Z.; Zhao, Y.; Gu, Y.; Wang, Y.; Yu, J.; Xu, H. The performance of biochar-microbe multiple biochemical material on bioremediation and soil micro-ecology in the cadmium aged soil. Sci. Total Environ. 2019, 686, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Ng, T.C.A.; Sun, Q.; Elshahawy, A.M.K.; Lyu, Z.; He, Z.; Zhang, L.; Ng, H.Y.; Zeng, K.; Wang, J. Heterogeneous ZIF-L membranes with improved hydrophilicity and anti-bacterial adhesion for potential application in water treatment. RSC Adv. 2019, 9, 1591–1601. [Google Scholar] [CrossRef] [Green Version]
- Pichardo-Romero, D.; Garcia-Arce, Z.P.; Zavala-Ramírez, A.; Castro-Muñoz, R. Current advances in biofouling mitigation in membranes for water treatment: An overview. Processes 2020, 8, 182. [Google Scholar] [CrossRef] [Green Version]
- Dhanker, R.; Tyagi, P.; Kamble, S.S.; Gupta, D.; Hussain, T. Advances in fungi: Rejuvenation of polluted sites. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 251–275. [Google Scholar]
- Zara, G.; Zeidan, M.B.; Fancello, F.; Sanna, M.L.; Mannazzu, I.; Budroni, M.; Zara, S. The administration of l-cysteine and l-arginine inhibits biofilm formation in wild-type biofilm-forming yeast by modulating FLO11 gene expression. Appl. Microbiol. Biotechnol. 2019, 103, 7675–7685. [Google Scholar] [CrossRef]
- Miller, E.; Menashe, O.; Dosoretz, C.G. A tailored permeable reactive bio-barrier for in situ groundwater remediation: Removal of 3-chlorophenol as a case study. Environ. Technol. 2020. [Google Scholar] [CrossRef]
- Hu, J.; Lin, J.; Zhang, Y.; Lin, Z.; Qiao, Z.; Liu, Z.; Yang, W.; Liu, X.; Dong, M.; Guo, Z. A new anti-biofilm strategy of enabling arbitrary surfaces of materials and devices with robust bacterial anti-adhesion via a spraying modified microsphere method. J. Mater. Chem. A 2019, 7, 26039–26052. [Google Scholar] [CrossRef]
- Benn, G.; Pyne, A.L.B.; Ryadnov, M.G.; Hoogenboom, B.W. Imaging live bacteria at the nanoscale: Comparison of immobilisation strategies. Analyst 2019, 144, 6944–6952. [Google Scholar] [CrossRef] [Green Version]
- Fomina, M.; Skorochod, I. Microbial Interaction with Clay Minerals and Its Environmental and Biotechnological Implications. Minerals 2020, 10, 861. [Google Scholar] [CrossRef]
- Poortinga, A.T.; Bos, R.; Norde, W.; Busscher, H.J. Electric double layer interactions in bacterial adhesion to surfaces. Surf. Sci. Rep. 2002, 47, 1–32. [Google Scholar] [CrossRef]
- Li, B.; Yin, J.; Liu, X.; Wu, H.; Li, J.; Li, X.; Guo, W. Probing van der Waals interactions at two-dimensional heterointerfaces. Nat. Nanotechnol. 2019, 14, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, J.; Duong, D.L.; Cho, S.; Kim, S.W.; Yang, H. Proximity Engineering of the van der Waals Interaction in Multilayered Graphene. ACS Appl. Mater. Interfaces 2019, 11, 42528–42533. [Google Scholar] [CrossRef] [PubMed]
- Tantardini, C. When does a hydrogen bond become a van der Waals interaction? a topological answer. J. Comput. Chem. 2019, 40, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Brooks, B.R. A double exponential potential for van der Waals interaction. AIP Adv. 2019, 9, 065304. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Yang, S.; Jiang, Y.; Li, J.; Li, S.; Ren, J.-C.; Liu, W. Modeling chemical reactions on surfaces: The roles of chemical bonding and van der Waals interactions. Prog. Surf. Sci. 2019, 94, 100561. [Google Scholar] [CrossRef]
- Elimelech, M.G.; Jia, X.; Williams, R.J. Particle Deposition & Aggregation: Measurement, Modelling and Simulation; Butterworth-Heinemann: Oxford, UK, 1995. [Google Scholar]
- Russel, W.B.; Saville, D.A.; Schowalter, W.R. Colloidal dispersions; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Unni, H.N.; Yang, C. Kinetics of Colloidal Particle Deposition to a Solid Surface from Pressure Driven Microchannel Flows. Can. J. Chem. Eng. 2008, 85, 609–616. [Google Scholar] [CrossRef]
- Sharma, P.; Flury, M.; Zhou, J. Detachment of colloids from a solid surface by a moving air–water interface. J. Colloid Interface Sci. 2008, 326, 143–150. [Google Scholar] [CrossRef]
- Eltaboni, F.B.; Caseley, E.; Katsikogianni, M.; Swanson, L.; Swift, T.; Romero-González, M. Fluorescence Spectroscopy Analysis of the Bacteria–Mineral Interface: Adsorption of Lipopolysaccharides to Silica and Alumina. Langmuir 2020, 36, 1623–1632. [Google Scholar] [CrossRef]
- Gu, Y.; Li, D. The van der Waals interaction between a spherical particle and a cylinder. J. Colloid Interface Sci. 1999, 217, 60–69. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Istraelachvili, J. Intermolecular and Surface Forces, with Special Applications to Colloidal and Biological Systems; Academic Press: London, UK, 1985. [Google Scholar]
- Montgomery, S.W.; Franchek, M.A.; Goldschmidt, V.W. Analytical Dispersion Force Calculations for Nontraditional Geometries. J. Colloid Interface Sci. 2000, 227, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N. The calculation of van der Waals dispersion forces between macroscopic bodies. Proc. R. Soc. London. Ser. A. Math. Phys. Sci. 1972, 331, 39–55. [Google Scholar]
- Bhattacharjee, P. Elimelech Surface Element Integration: A Novel Technique for Evaluation of DLVO Interaction between a Particle and a Flat Plate. J. Colloid Interface Sci. 1997, 193, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Tadmor, R. The London-van der Waals interaction energy between objects of various geometries. J. Physics: Condens. Matter 2001, 13, L195–L202. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Sharma, A. Lifshitz-van der Waals Energy of Spherical Particles in Cylindrical Pores. J. Colloid Interface Sci. 1995, 171, 288–296. [Google Scholar] [CrossRef]
- Young, K.D. Bacterial morphology: Why have different shapes? Curr. Opin. Microbiol. 2007, 10, 596–600. [Google Scholar] [CrossRef] [Green Version]
- Hamaker, H.C. The London—van der Waals attraction between spherical particles. Physica 1937, 4, 1058–1072. [Google Scholar] [CrossRef]
- Cao, T.; Elimelech, M. Colloidal stability of cellulose nanocrystals in aqueous solutions containing monovalent, divalent, and trivalent inorganic salts. J. Colloid Interface Sci. 2021, 584, 456–463. [Google Scholar] [CrossRef]
- Erbil, H.Y. Solid and Liquid Interfaces; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- Bhattacharjee, S.; Elimelech, M.; Borkovec, M. DLVO interaction between colloidal particles: Beyond Derjaguin’s approximation. Croat. Chem. Acta 1998, 71, 883–903. [Google Scholar]
- Gregory, J. Interaction of unequal double layers at constant charge. J. Colloid Interface Sci. 1975, 51, 44–51. [Google Scholar] [CrossRef]
- Fathiah, M.Z.; Edyvean, R.G. The Role of Ionic Strength and Mineral Size to Zeta-Potential for the Adhesion of P. putida to Mineral Surfaces. World Acad. Sci. Eng. Technol. Int. J. Biotechnol. Bioeng. 2015, 9, 805–810. [Google Scholar]
















| Surface | Pseudomonas putida | Hematite | Quartz |
|---|---|---|---|
| Hamaker Constant, A | N/A | 9.91 × 10−20 | 1.22 × 10−19 |
| Sphere radius, Rsphere | 0.5 µm | - | - |
| Cylinder radius, Rcylinder | 0.5 µm | - | - |
| Shell thickness, hc | 0.2 µm | - | - |
| Length of cylinder, L | 2.0 µm | - | - |
| Mineral Radius: | |||
| Radius of ring, RRing | - | 0.5 cm | 0.5 cm |
| Separation Distance, D | - | 0.5–10 nm | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed Zuki, F.; Edyvean, R.G.J.; Pourzolfaghar, H.; Kasim, N. Modeling of the Van Der Waals Forces during the Adhesion of Capsule-Shaped Bacteria to Flat Surfaces. Biomimetics 2021, 6, 5. https://0-doi-org.brum.beds.ac.uk/10.3390/biomimetics6010005
Mohamed Zuki F, Edyvean RGJ, Pourzolfaghar H, Kasim N. Modeling of the Van Der Waals Forces during the Adhesion of Capsule-Shaped Bacteria to Flat Surfaces. Biomimetics. 2021; 6(1):5. https://0-doi-org.brum.beds.ac.uk/10.3390/biomimetics6010005
Chicago/Turabian StyleMohamed Zuki, Fathiah, Robert G. J. Edyvean, Hamed Pourzolfaghar, and Norherdawati Kasim. 2021. "Modeling of the Van Der Waals Forces during the Adhesion of Capsule-Shaped Bacteria to Flat Surfaces" Biomimetics 6, no. 1: 5. https://0-doi-org.brum.beds.ac.uk/10.3390/biomimetics6010005