Is It Worth Considering Multicentric High-Grade Glioma a Surgical Disease? Analysis of Our Clinical Experience and Literature Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Study
2.2. Review of Literature
2.3. Statistical Analysis
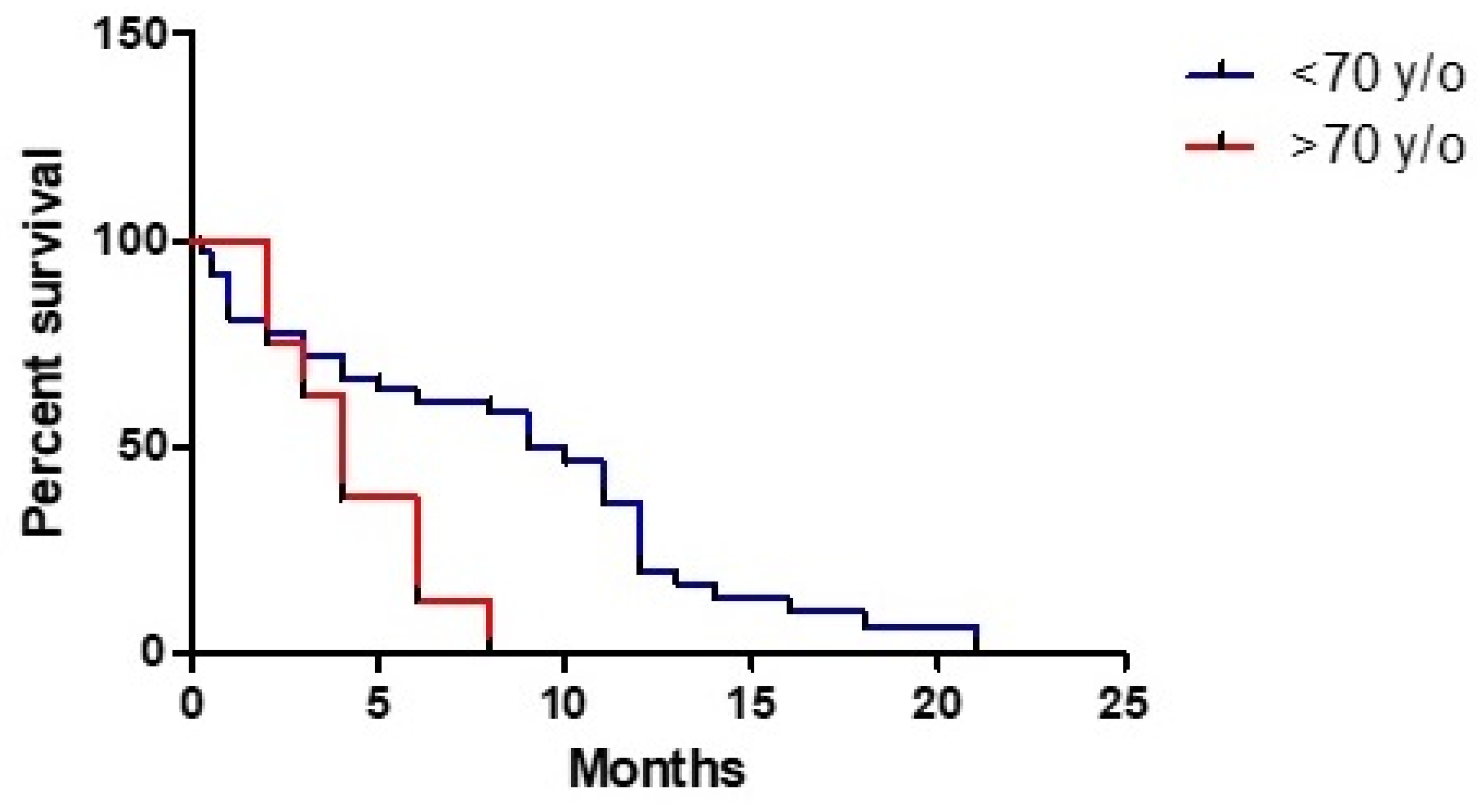
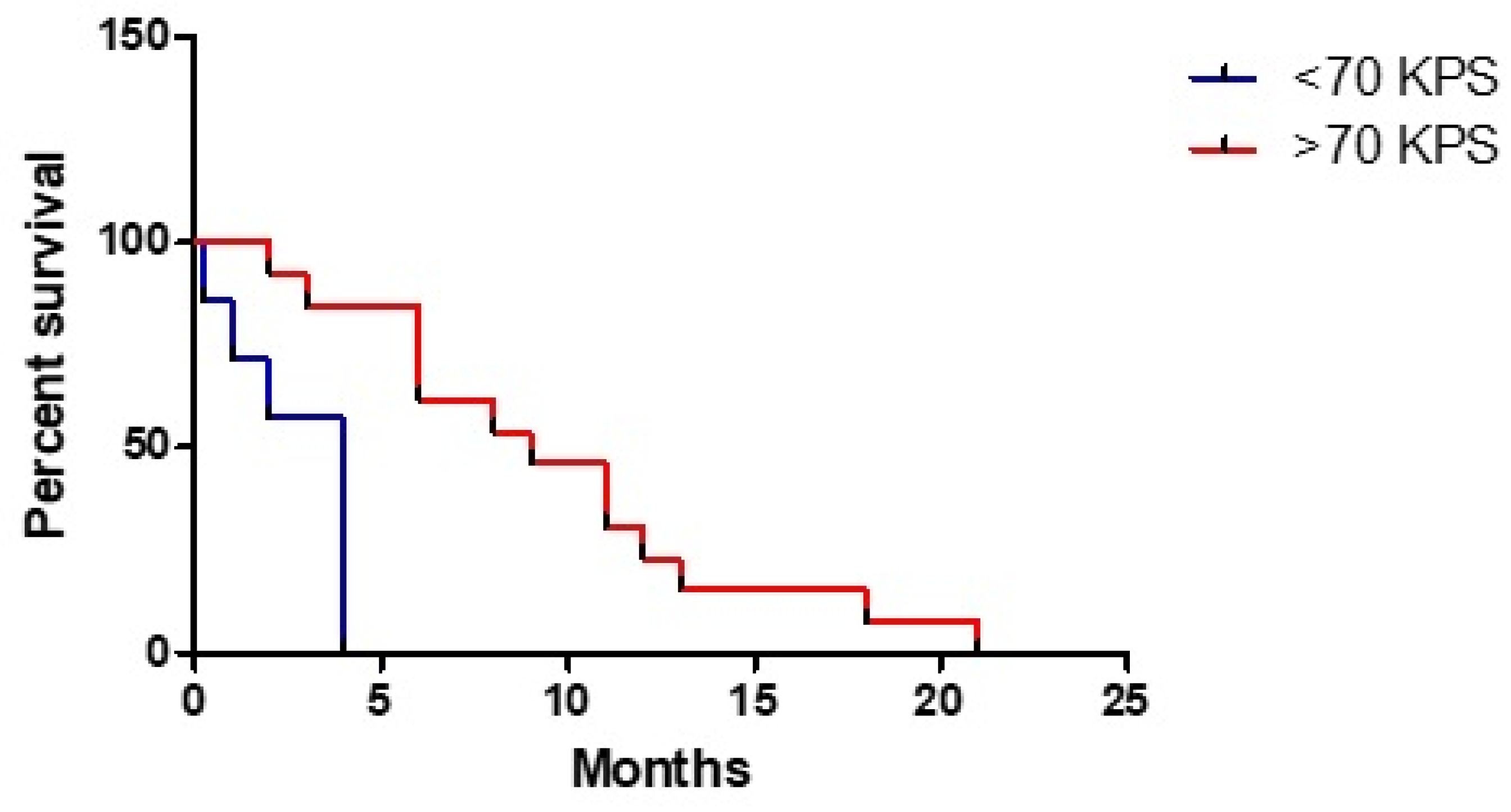
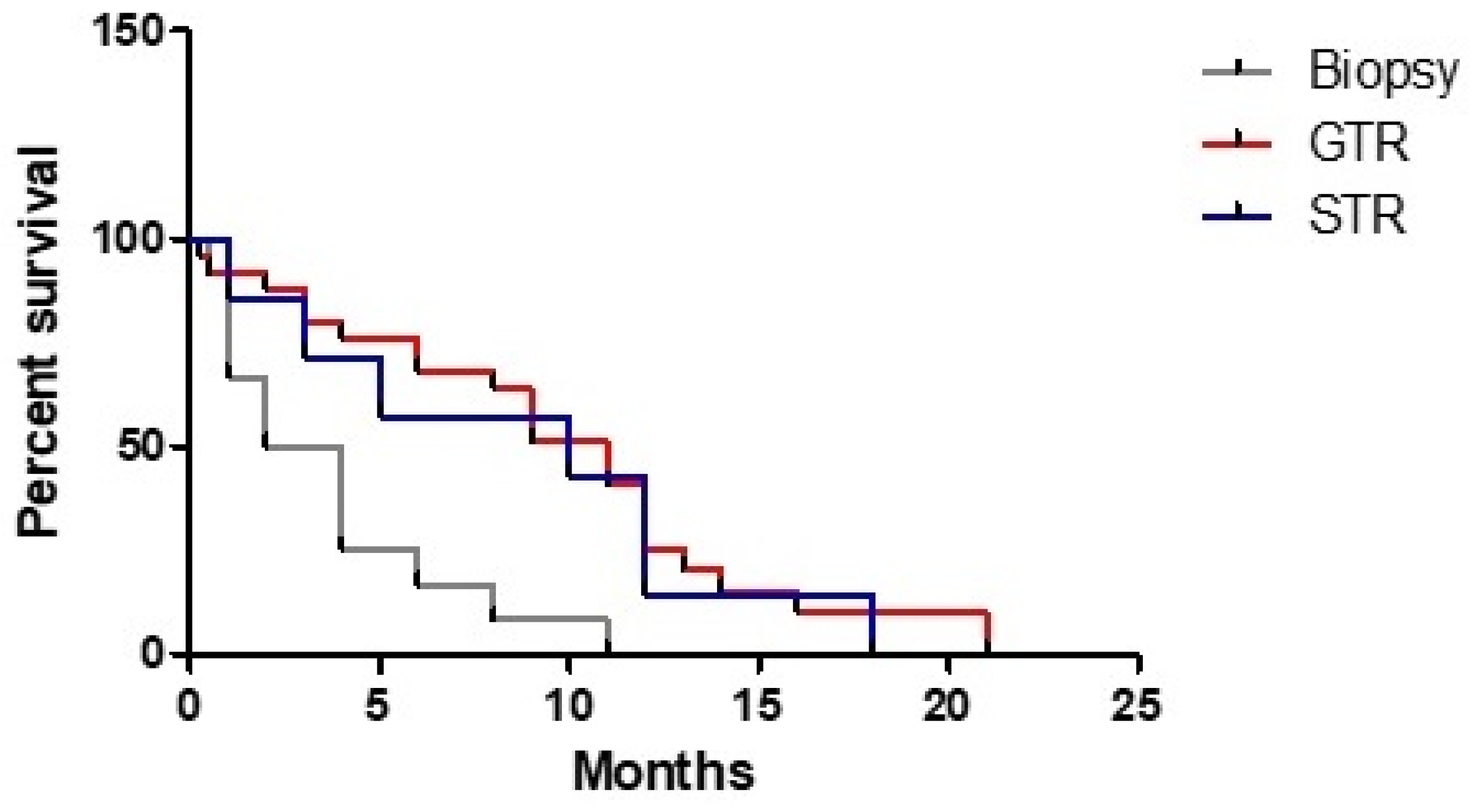
3. Results
3.1. Clinical Study
3.2. Systematic Review of Literature
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stark, A.M.; van de Bergh, J.; Hedderich, J.; Mehdorn, H.M.; Nabavi, A. Glioblastoma: Clinical characteristics, prognostic factors and survival in 492 patients. Clin. Neurol. Neurosurg. 2012, 114, 840–845. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Komori, T. The 2016 WHO Classification of Tumours of the Central Nervous System: The Major Points of Revision. Neurol. Med.-Chir. 2017, 57, 301–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesseling, P.; Capper, D. WHO 2016 Classification of gliomas. Neuropathol. Appl. Neurobiol. 2018, 44, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Batzdorf, U.; Malamud, N. The Problem of Multicentric Gliomas. J. Neurosurg. 1963, 20, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Bloch, O.; Han, S.J.; Cha, S.; Sun, M.Z.; Aghi, M.K.; McDermott, M.W.; Berger, M.S.; Parsa, A.T. Impact of extent of resection for recurrent glioblastoma on overall survival: Clinical article. J. Neurosurg. 2012, 117, 1032–1038. [Google Scholar] [CrossRef]
- Sezer, S.; van Amerongen, M.J.; Delye, H.H.K.; Ter Laan, M. Accuracy of the neurosurgeons estimation of extent of resection in glioblastoma. Acta Neurochir. 2020, 162, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Barnard, R.O.; Geddes, J.F. The incidence of multifocal cerebral gliomas. A histologic study of large hemisphere sections. Cancer 1987, 60, 1519–1531. [Google Scholar] [CrossRef]
- Showalter, T.N.; Andrel, J.; Andrews, D.W.; Curran, W.J.; Jr Daskalakis, C.; Werner-Wasik, M. Multifocal glioblastoma multiforme: Prognostic factors and patterns of progression. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 820–824. [Google Scholar] [CrossRef]
- Kyritsis, A.P.; Levin, V.A.; Yung, W.K.; Leeds, N.E. Imaging patterns of multifocal gliomas. Eur. J. Radiol. 1993, 16, 163–170. [Google Scholar] [CrossRef]
- Hassaneen, W.; Levine, N.B.; Suki, D.; Salaskar, A.L.; de Moura Lima, A.; McCutcheon, I.E.; Prabhu, S.S.; Lang, F.F.; DeMonte, F.; Rao, G.; et al. Multiple craniotomies in the management of multifocal and multicentric glioblastoma. Clin. Artic. J. Neurosurg. 2011, 114, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Karlowee, V.; Amatya, V.J.; Hirano, H.; Takayasu, T.; Nosaka, R.; Kolakshyapati, M.; Yoshihiro, M.; Takeshima, Y.; Sugiyama, K.; Arita, K.; et al. Multicentric Glioma Develops via a Mutant IDH1-Independent Pathway: Immunohistochemical Study of Multicentric Glioma. Pathobiology 2017, 84, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, V.; Urbanski, L.M.; Bi, W.L.; Thistle, K.; Miller, M.B.; Ramkissoon, S.; Reardon, D.A.; Dunn, I.F. Multicentric Low-Grade Gliomas. World Neurosurg. 2015, 84, 1045–1050. [Google Scholar] [CrossRef]
- Wang, T.; Niu, X.; Gao, T.; Zhao, L.; Li, J.; Gan, Y.; Liu, Y.; Mao, Q. Prognostic Factors for Survival Outcome of High-Grade Multicentric Glioma. World Neurosurg. 2018, 112, e269–e277. [Google Scholar] [CrossRef]
- Alifieris, C.; Trafalis, D.T. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Farah, P.; Ondracek, A.; Chen, Y.; Wolinsky, Y.; Stroup, N.E.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro-Oncology 2013, 15 (Suppl. 2), ii1–ii56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvati, M.; Caroli, E.; Orlando, E.R.; Frati, A.; Artizzu, S.; Ferrante, L. Multicentric glioma: Our experience in 25 patients and critical review of the literature. Neurosurg. Rev. 2003, 26, 275–279. [Google Scholar] [CrossRef]
- Fernandez-Torre, J.L.; Hernandez-Hernandez, M.; Martino, J.; Hinojo, C. Subclinical focal seizures as a sign of progression in gliomas. Epileptic Disord. 2014, 16, 546–553. [Google Scholar] [CrossRef]
- Parsa, A.T.; Wachhorst, S.; Lamborn, K.R.; Prados, M.D.; McDermott, M.W.; Berger, M.S.; Chang, S.M. Prognostic significance of intracranial dissemination of glioblastoma multiforme in adults. J. Neurosurg. 2005, 102, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Karsy, M.; Yoon, N.; Boettcher, L.; Jensen, R.; Shah, L.; MacDonald, J.; Menacho, S.T. Surgical treatment of glioblastoma in the elderly: The impact of complications. J. Neuro-Oncol. 2018, 138, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Baldock, A.L.; Yagle, K.; Born, D.E.; Ahn, S.; Trister, A.D.; Neal, M.; Neal, M.; Johnston, S.K.; Bridge, C.A.; Basanta, D.; et al. Invasion and proliferation kinetics in enhancing gliomas predict IDH1 mutation status. Neuro-Oncology 2014, 16, 779–786. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, J.A.; Lai, A.; Nghiemphu, P.L.; Kim, H.J.; Phillips, H.S.; Kharbanda, S.; Moftakhar, P.; Lalaezari, S.; Yong, W.; Ellingson, B.M.; et al. Relationship between tumor enhancement, edema, IDH1 mutational status, MGMT promoter methylation, and survival in glioblastoma. AJNR Am. J. Neuroradiol. 2012, 33, 1349–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Liu, Y.; Li, W.; Wang, X.; Sawaya, R.; Lang, F.F.; Yung, W.K.A.; Chen, K.; Fuller, G.N.; Zhang, W.; et al. Genetic, epigenetic, and molecular landscapes of multifocal and multicentric glioblastoma. Acta Neuropathol. 2015, 130, 587–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forghani, R. Precision Digital Oncology: Emerging Role of Radiomics based Biomarkers and Artificial Intelligence. Radiol. Imaging Cancer 2020, 2, e190047. [Google Scholar] [CrossRef] [PubMed]

| # | Age | Sex | M/S | Seiz | Cogn/Aph | H | preKPS | Localiz | Epend |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | M | x | 100 | Supra | Yes | |||
| 2 | 58 | M | x | x | 90 | Supra | Yes | ||
| 3 | 59 | F | x | 100 | Supra | No | |||
| 4 | 69 | F | x | 100 | Supra | No | |||
| 5 | 58 | M | x | 90 | Supra | Yes | |||
| 6 | 59 | F | x | x | 50 | Supra | Yes | ||
| 7 | 78 | F | x | x | 60 | Supra | No | ||
| 8 | 69 | F | x | x | 100 | Supra | Yes | ||
| 9 | 44 | M | x | 100 | Supra | No | |||
| 10 | 68 | M | x | 90 | Supra/Infra | Yes | |||
| 11 | 62 | M | x | 100 | Supra | Yes | |||
| 12 | 59 | M | x | 100 | Supra | No | |||
| 13 | 83 | M | x | 90 | Supra | No | |||
| 14 | 80 | M | x | x | 60 | Supra | Yes | ||
| 15 | 74 | M | x | 60 | Supra | No | |||
| 16 | 77 | M | x | x | 100 | Supra/Infra | Yes |
| # | EOR | postKPS | Che1 | RT | Che2 | KPS3 | KPS6 | KPS12 | KPS18 | EOR2 | OS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | GTR | 100 | Yes | Yes | 70 | 70 | 70 | 70 | 20 | ||
| 2 | GTR | 100 | Yes | Yes | 20 | 0 | 0 | 6 | |||
| 3 | GTR | 100 | Yes | Yes | 100 | 80 | 50 | 13 | |||
| 4 | GTR | 40 | No | No | 30 | 0 | 0 | 4 | |||
| 5 | Biopsy | 90 | No | Yes | 90 | 40 | 0 | 11 | |||
| 6 | GTR | 40 | No | No | 0 | 0 | 0 | 2 | |||
| 7 | Biopsy | 60 | Yes | Yes | 60 | 0 | 0 | 3 | |||
| 8 | Biopsy | 100 | 70 | 0 | 0 | 5 | |||||
| 9 | GTR | 100 | Yes | Yes | 100 | 100 | 0 | GTR | 11 | ||
| 10 | GTR | 100 | Yes | Yes | 70 | 60 | 0 | 12 | |||
| 11 | GTR | 100 | Yes | Yes | 100 | 100 | 100 | 100 | 23 | ||
| 12 | GTR | 100 | Yes | Yes | 100 | 100 | 100 | 100 | GTR | 24 | |
| 13 | GTR | 100 | No | Yes | 100 | 40 | 0 | 6 | |||
| 14 | Biopsy | 40 | No | No | 0 | 0 | 0 | 2 | |||
| 15 | Biopsy | 70 | Yes | Yes | Yes | 70 | 50 | 0 | 8 | ||
| 16 | STR | 50 | No | No | 0 | 0 | 0 | 1 |
| # | Diagnosis | IDH1 | ATRX | Ki67 | TP53 | EGFR | 1p19q |
|---|---|---|---|---|---|---|---|
| 1 | GBM | WT | neg | 10 | 5 | No | No |
| 2 | GBM | WT | pos | 60 | 10 | Yes | No |
| 3 | GBM | WT | pos | 20 | 5 | Yes | No |
| 4 | GBM | WT | neg | 60 | 60 | Yes | No |
| 5 | GBM | WT | pos | 35 | 20 | Yes | No |
| 6 | GBM | WT | pos | 70 | 20–30 | Yes | No |
| 7 | GBM | WT | pos | 40 | 30–35 | Yes | No |
| 8 | GBM | WT | pos | 50 | 70 | Yes | No |
| 9 | GBM | WT | pos | 10 | 0 | Yes | No |
| 10 | GBM | WT | pos | 50 | 20 | Yes | No |
| 11 | GBM | WT | pos | 10 | 5 | Yes | No |
| 12 | GBM | WT | neg | 40 | 30 | Yes | No |
| 13 | Olig III | WT | pos | 25 | 8 | Yes | Yes |
| 14 | AA | WT | pos | 10 | 0 | Yes | No |
| 15 | GBM | WT | pos | 35 | 50 | Yes | No |
| 16 | GBM | mutated | pos | 30 | 5 | Yes | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrini, F.; Mazzeo, L.A.; Rossi, G.; Verlotta, M.; Del Maestro, M.; Rampini, A.D.; Pesce, A.; Viganò, M.; Luzzi, S.; Galzio, R.J.; et al. Is It Worth Considering Multicentric High-Grade Glioma a Surgical Disease? Analysis of Our Clinical Experience and Literature Review. Tomography 2021, 7, 523-532. https://0-doi-org.brum.beds.ac.uk/10.3390/tomography7040045
Guerrini F, Mazzeo LA, Rossi G, Verlotta M, Del Maestro M, Rampini AD, Pesce A, Viganò M, Luzzi S, Galzio RJ, et al. Is It Worth Considering Multicentric High-Grade Glioma a Surgical Disease? Analysis of Our Clinical Experience and Literature Review. Tomography. 2021; 7(4):523-532. https://0-doi-org.brum.beds.ac.uk/10.3390/tomography7040045
Chicago/Turabian StyleGuerrini, Francesco, Lucio Aniello Mazzeo, Giorgio Rossi, Mariarosaria Verlotta, Mattia Del Maestro, Angela Dele Rampini, Alessandro Pesce, Marco Viganò, Sabino Luzzi, Renato Juan Galzio, and et al. 2021. "Is It Worth Considering Multicentric High-Grade Glioma a Surgical Disease? Analysis of Our Clinical Experience and Literature Review" Tomography 7, no. 4: 523-532. https://0-doi-org.brum.beds.ac.uk/10.3390/tomography7040045





