First Experiences with Newborn Screening for Congenital Hypothyroidism in Ulaanbaatar, Mongolia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
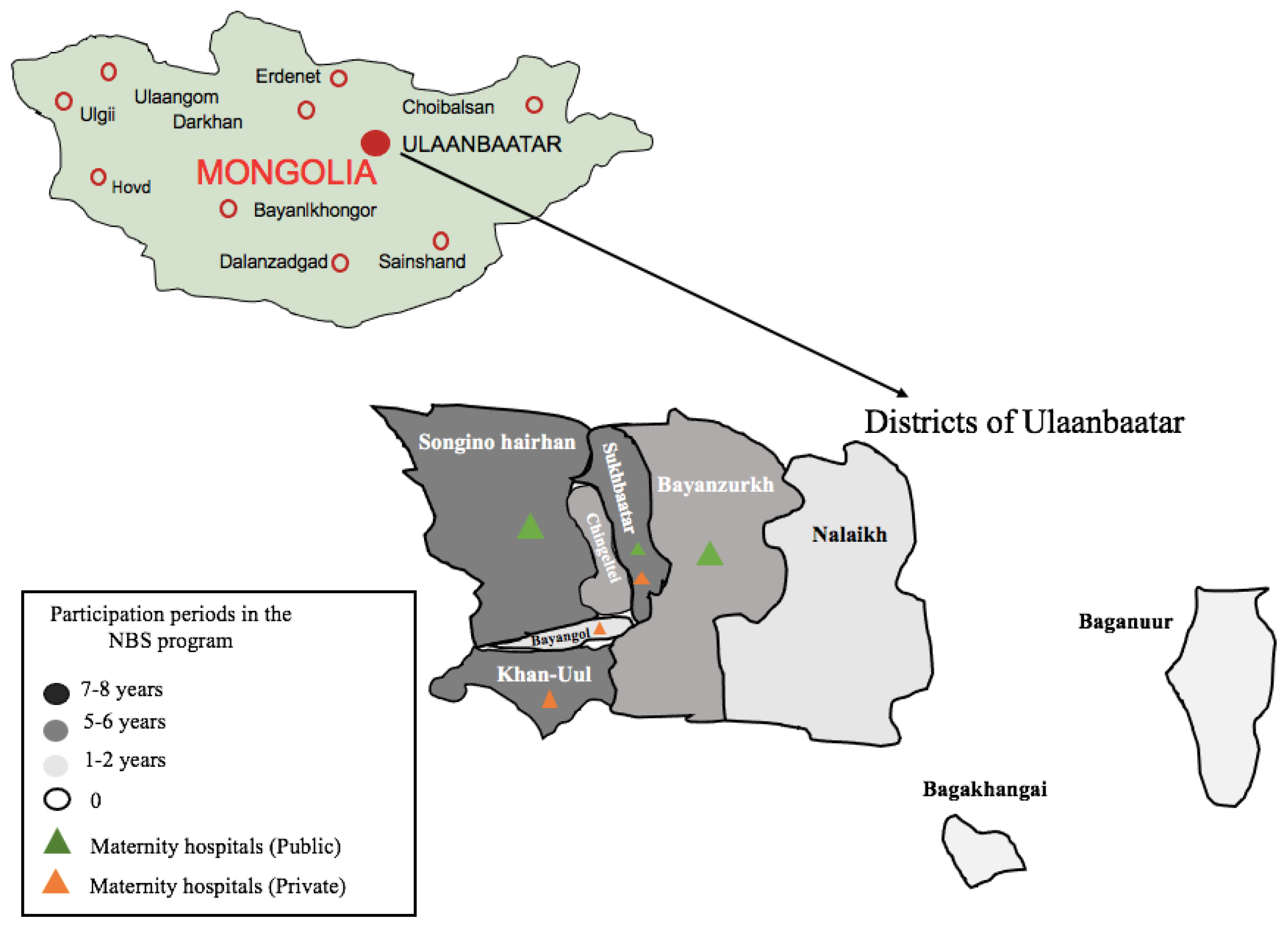
2.2. Newborn Screening for CH
2.3. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Büyükgebiz, A. Newborn screening for congenital hypothyroidism. J. Clin. Res. Pediatr. Endocrinol. 2013, 5 (Suppl. 1), 8–12. [Google Scholar] [CrossRef]
- Di Cosmo, C.; Tonacchera, M. Congenital Hypothyroidism. In Thyroid Diseases: Pathogenesis, Diagnosis and Treatment; Vitti, P., Hegedus, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lee, K.-T.; Lee, C.T.-C.; Lai, W.-T.; Huang, Y.-B. Epidemiology and Clinical Characteristics of Congenital Hypothyroidism in an Asian Population: A Nationwide Population-Based Study. J. Epidemiol. 2013, 23, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopel, J. A global perspective on newborn congenital hypothyroidism screening. Proc. Baylor Univ. Med. Center 2019, 33, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Al Jafari, M.; Jose, S.; Al Senani, A. Demographic Features and Etiology of Congenital Hypothyroidism at the National Diabetes and Endocrine Center in Oman from 2004 to 2016. Oman Med. J. 2020, 35, e171. [Google Scholar] [CrossRef] [PubMed]
- Ford, G.; LaFranchi, S.H. Screening for congenital hypothyroidism: A worldwide view of strategies. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Léger, J. Congenital hypothyroidism: A clinical update of long-term outcome in young adults. Eur. J. Endocrinol. 2015, 172, R67–R77. [Google Scholar] [CrossRef] [PubMed]
- Erdenechimeg, S. National neonatal hypothyroid screening program in Mongolia. Southeast Asian J. Trop Med. Public. Health 2003, 34 (Suppl. 3), 85–86. [Google Scholar]
- International Atomic Energy Agency. Screening of Newborns for Congenital Hypothyroidism; International Atomic Energy Agency: Vienna, Austria, 2006. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Guide for Receiving and Storing Dried Blood Spots (DBS) for Molecular Testing at Reference Laboratories; Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA, 2018.
- Anastasovska, V.; Sukarova-Angelovska, E.; Pesevska, M.; Taseva, E.; Kocova, M. Regional Variation in the Incidence of Congenital Hypothyroidism in Macedonia. Int. J. Neonat. Screen 2017, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- Deng, K.; He, C.; Zhu, J.; Liang, J.; Li, X.; Xie, X.; Yu, P.; Li, N.; Li, Q.; Wang, Y. Incidence of congenital hypothyroidism in China: Data from the national newborn screening program, 2013-2015. J. Pediatr. Endocrinol. Metab. 2018, 31, 601–608. [Google Scholar] [CrossRef]
- Mehran, L.; Khalili, D.; Yarahmadi, S.; Amouzegar, A.; Mojarrad, M.; Ajang, N.; Azizi, F. Worldwide Recall Rate in Newborn Screening Programs for Congenital Hypothyroidism. Int. J. Endocrinol. Metab. 2017, 15, e55451. [Google Scholar] [CrossRef]
- Van Trotsenburg, P.; Stoupa, A.; Léger, J.; Rohrer, T.; Peters, C.; Fugazzola, L.; Cassio, A.; Heinrichs, C.; Beauloye, V.; Pohlenz, J.; et al. Congenital Hypothyroidism: A 2020-2021 Consensus Guidelines Update-An ENDO-European Reference Network Initiative Endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid 2021, 31, 387–419. [Google Scholar] [CrossRef]
- Padilla, C.D. Towards universal newborn screening in developing countries: Obstacles and the way forward. Ann. Acad. Med. Singap 2008, 37, 6–9. [Google Scholar]
- Therrell, B.L., Jr.; Padilla, C.D. Newborn screening in the developing countries. Curr. Opin. Pediatr. 2018, 30, 734–739. [Google Scholar] [CrossRef] [PubMed]
- McGrath, N.; Hawkes, C.P.; McDonnell, C.M.; Cody, D.; O’Connell, S.M.; Mayne, P.D.; Murphy, N.P. Incidence of Congenital Hypothyroidism Over 37 Years in Ireland. Pediatrics 2018, 142. [Google Scholar] [CrossRef] [Green Version]
- Kilberg, M.J.; Rasooly, I.R.; LaFranchi, S.H.; Bauer, A.J.; Hawkes, C.P. Newborn Screening in the US May Miss Mild Persistent Hypothyroidism. J. Pediatr. 2018, 192, 204–208. [Google Scholar] [CrossRef]
- Shaikh, A.A.; Alsofyani, A.; Shirah, B.; Noaim, K.A.; Ahmed, M.E.; Babiker, A.; Alwan, I.A. Congenital hypothyroidism in Saudi population in two major cities: A retrospective study on prevalence and therapeutic outcomes. Int. J. Health Sci. 2021, 15, 17–21. [Google Scholar]
- Olivieri, A.; Fazzini, C.; Medda, E. Multiple factors influencing the incidence of congenital hypothyroidism detected by neonatal screening. Horm Res. Paediatr. 2015, 83, 86–93. [Google Scholar] [CrossRef]
- Rahmani, K.; Yarahmadi, S.; Etemad, K.; Mehrabi, Y.; Aghang, N.; Koosha, A.; Soori, H. Intelligence Quotient at the Age of Six years of Iranian Children with Congenital Hypothyroidism. Indian Pediatr. 2018, 55, 121–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, D.S.; Lawrence, S.; Geraghty, M.T.; Gallego, P.H.; McAssey, K.; Wherrett, D.K.; Chakraborty, P. Prediction of congenital hypothyroidism based on initial screening thyroid-stimulating-hormone. BMC Pediatr. 2016, 16, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szinnai, G. Genetics of normal and abnormal thyroid development in humans. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 133–150. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, J.X.; Yang, C.Y.; Gao, G.Q.; Zhu, W.B.; Han, B.; Zhang, L.L.; Wan, Y.Y.; Ye, X.P.; Ma, Y.R.; et al. The genetic characteristics of congenital hypothyroidism in China by comprehensive screening of 21 candidate genes. Eur. J. Endocrinol. 2018, 178, 623–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mio, C.; Grani, G.; Durante, C.; Damante, G. Molecular defects in thyroid dysgenesis. Clin. Genet. 2020, 97, 222–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, C.; Ouarezki, Y.; Jones, J.; Fitch, M.; Smith, S.; Mason, A.; Donaldson, M. Trends in Scottish newborn screening programme for congenital hypothyroidism 1980–2014: Strategies for reducing age at notification after initial and repeat sampling. Arch Dis. Child 2017, 102, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Nanu, M.; Ardeleanu, I.S.; Brezan, F.; Nanu, I.; Apostol, A.; Moldovanu, F.; Lazarescu, H.; Gheorghiu, M.L.; Kozma, A. Neonatal Screening for Congenital Hypothyroidism In Romania: Data From Medilog Medical Information Registry. Acta Endocrinol. 2019, 15, 209–214. [Google Scholar] [CrossRef]
- Rose, S.R.; Brown, R.S.; Foley, T.; Kaplowitz, P.B.; Kaye, C.I.; Sundararajan, S.; Varma, S.K. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics 2006, 117, 2290–2303. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Year | 2012 (between July and December) | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | Total/Average |
|---|---|---|---|---|---|---|---|---|---|---|
| DEMOGRAPHY | ||||||||||
| Live births | 18,607 | 41,187 | 41,786 | 41,731 | 40,687 | 38,404 | 40,703 | 41,206 | 39,779 | 344,090 |
| Screened children | 2304 | 1450 | 3095 | 686 | 2616 | 3269 | 461 | 6302 | 2819 | 23,002 |
| Coverage (%) | 12.4 | 3.5 | 7.4 | 1.6 | 6.4 | 8.5 | 1.1 | 15.3 | 7.1 | 6.7 |
| SCREENING | ||||||||||
| Positive children | 11 | 5 | 22 | 2 | 6 | 4 | 1 | 25 | 11 | 87 |
| Recall success rate (%) | 0.5 | 0.3 | 0.7 | 0.3 | 0.2 | 0.1 | 0.2 | 0.4 | 0.4 | 0.4 |
| False-positive (Second screening) | 7 | 2 | 18 | 2 | 3 | - | 1 | 15 | 10 | 58 |
| False-positive (%) | 63.6 | 40 | 81.8 | 100 | 50 | - | 100 | 60 | 91 | 66.7 |
| Inadequate samples 1 | 121 | 63 | 119 | 22 | 84 | 97 | 9 | 122 | 44 | 681 |
| Inadequate samples (%) | 5.3 | 4.3 | 3.8 | 3.2 | 3.2 | 3.0 | 2.0 | 1.9 | 1.6 | 3.0 |
| CONFIRMATION | ||||||||||
| Confirmed children with CH 2 | 4 | 3 | 4 | - | 3 | 4 | - | 10 | 1 | 29 |
| Recall success rate (%) 3 | 36.4 | 60 | 18.2 | - | 50.0 | 100 | - | 40 | 9.1 | 33.3 |
| Children with permanent CH | 1 | - | 1 | - | 1 | 2 | - | 5 | 1 | 11 |
| Children with transient CH | 2 | 2 | 2 | - | 1 | 2 | - | 4 | - | 13 |
| False-positive (Confirmation for CH) | 1 | 1 | 1 | - | 1 | - | - | 1 | - | 5 |
| False positive (%) | 25 | 33.3 | 25 | - | 33.3 | - | - | 10 | - | 17.2 |
| Patient | Sex | Clinical Manifestations | Thyroid Ultrasound |
|---|---|---|---|
| 1 | F | Cold or mottled skin | Hypoplasia |
| 2 | F | Asymptomatic | Hypoplasia |
| 3 | F | Asymptomatic | Cysts with hypoplasia |
| 4 | M | Asymptomatic | Hypoplasia |
| 5 | F | Asymptomatic | Hypoplasia |
| 6 | F | Asymptomatic | Normal |
| 7 | F | Umbilical Hernia | Cysts with hypoplasia |
| 8 | M | Asymptomatic | Normal |
| 9 | M | Asymptomatic | Hypoplasia |
| 10 | F | Asymptomatic | Hypoplasia |
| 11 | M | Umbilical Hernia | Hypoplasia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsevgee, A.; Batjargal, K.; Munkhchuluun, T.; Khurelbaatar, N.; Nansal, G.; Bulgan, O.-E.; Nyamjav, S.; Zagd, G.; Ganbaatar, E. First Experiences with Newborn Screening for Congenital Hypothyroidism in Ulaanbaatar, Mongolia. Int. J. Neonatal Screen. 2021, 7, 29. https://0-doi-org.brum.beds.ac.uk/10.3390/ijns7020029
Tsevgee A, Batjargal K, Munkhchuluun T, Khurelbaatar N, Nansal G, Bulgan O-E, Nyamjav S, Zagd G, Ganbaatar E. First Experiences with Newborn Screening for Congenital Hypothyroidism in Ulaanbaatar, Mongolia. International Journal of Neonatal Screening. 2021; 7(2):29. https://0-doi-org.brum.beds.ac.uk/10.3390/ijns7020029
Chicago/Turabian StyleTsevgee, Altantuya, Khishigjargal Batjargal, Tsolmon Munkhchuluun, Naranchimeg Khurelbaatar, Gerelmaa Nansal, Oyun-Erdene Bulgan, Sumberzul Nyamjav, Gerelmaa Zagd, and Erdenetuya Ganbaatar. 2021. "First Experiences with Newborn Screening for Congenital Hypothyroidism in Ulaanbaatar, Mongolia" International Journal of Neonatal Screening 7, no. 2: 29. https://0-doi-org.brum.beds.ac.uk/10.3390/ijns7020029









