Spawning Induction of First-Generation (F1) Greater Amberjack Seriola dumerili in the Canary Islands, Spain Using GnRHa Delivery Systems
Abstract
:1. Introduction
2. Results
2.1. Fish Condition
2.2. Spawning and Egg Quality
2.3. Plasma Sex Steroid, Hematological and Biochemical Parameters
3. Discussion
4. Materials and Methods
4.1. Broodstock Maintenance
4.2. Fish Sampling
4.3. Spawning Induction Therapies
4.4. Evaluation of Sperm Quality
4.5. Evaluation of Egg/Larval Quality
4.6. Histological Processing
4.7. Hormone Measurements
4.8. Hematology and Blood Biochemical Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paxton, J.R.; Hoese, D.F.; Allen, G.R.; Hanley, J.E. Pisces. Petromyzontidae to Carangidae. In Zoological Catalogue of Australia; Austral. Government Publ. Serv.: Canberra, Australia, 1989; Volume 7, p. 665. [Google Scholar]
- Food and Agriculture Organization (FAO). Cultured Aquatic Species Information Programme: Seriola dumerili; FAO: Rome, Italy, 2016. [Google Scholar]
- Jover, M.; García-Gómez, A.; Tomás, A.; De la Gándara, F.; Pérez, L. Growth of Mediterranean yellowtail (Seriola dumerili) fed extruded diets containing different levels of protein and lipid. Aquaculture 1999, 179, 25–33. [Google Scholar] [CrossRef]
- Mazzola, A.; Favaloro, E.; Sará, G. Cultivation of the Mediterranean amberjack, Seriola dumerili (Risso, 1810), in submerged cages in the Western Mediterranean Sea. Aquaculture 2000, 181, 257–268. [Google Scholar] [CrossRef]
- Mylonas, C.; Katharios, P.; Grigorakis, K.; Papandroulakis, N.; Robles, R.; Corriero, A.; Pousis, C.; Zupa, P.; Fernandez-Palacios, H.; Montero, D.; et al. Advances in Greater Amberjack (Seriola dumerili) Research: The DIVERSIFY Project; Aquaculture Europe, European Aquaculture Society: Ostend, Belgium, 2016; pp. 12–19. [Google Scholar]
- Pousis, C.; Mylonas, C.C.; De Virgilio, C.; Gadaleta, G.; Santamaria, N.; Passantino, L.; Zupa, R.; Papadaki, M.; Fakriadis, I.; Ferreri, R.; et al. The observed oogenesis impairment in greater amberjack Seriola dumerili (Risso, 1810) reared in captivity is not related to an insufficient liver transcription or oocyte uptake of vitellogenin. Aquac. Res. 2018, 49, 243–252. [Google Scholar] [CrossRef]
- Zupa, P.; Fauvel, C.; Mylonas, C.C.; Pousis, C.; Santamaría, C.A.; Papadaki, M.; Fakriadis, I.; Cicirelli, V.; Mangano, S.; Passantino, L.; et al. Rearing in captivity affects spermatogenesis and sperm quality in greater amberjack, Seriola dumerili (Risso, 1810). J. Anim. Sci. 2017, 95, 4085–4100. [Google Scholar] [CrossRef] [PubMed]
- Zupa, R.; Rodríguez, C.; Mylonas, C.C.; Rosenfeld, H.; Fakriadis, I.; Papadaki, M.; Pérez, J.A.; Pousis, C.; Basilone, G.; Corriero, A. Comparative study of reproductive development in wild and captive-reared greater amberjack Seriola dumerili (Risso, 1810). PLoS ONE 2017, 12, e0169645. [Google Scholar] [CrossRef] [PubMed]
- Chavanne, H.; Janssen, K.; Hofherr, J.; Contini, F.; Haffray, P.; Komen, H.; Nielsen, E.E.; Bargelloni, L. A comprehensive survey on selective breeding programs and seed market in the European aquaculture fish industry. Aquac. Int. 2016, 24, 1287–1307. [Google Scholar] [CrossRef]
- Janssen, K.; Chavanne, H.; Berentsen, P.; Komen, H. Impact of selective breeding on European aquaculture. Aquaculture 2016, 472, 8–16. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Zohar, Y. Controlling reproduction in aquaculture. In New Technologies in Aquaculture: Improving Production Efficiency, Quality and Environmental Management; Burnell, G., Allan, G., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2009; pp. 109–142. [Google Scholar]
- Mylonas, C.C.; Fostier, A.; Zanuy, S. Broodstock management and hormonal manipulations of fish reproduction. Gen. Comp. Endocrinol. 2010, 165, 516–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mylonas, C.C.; Duncan, N.J.; Asturiano, J.F. Hormonal manipulations for the enhancement of sperm production in cultured fish and evaluation of sperm quality. Aquaculture 2017, 472, 21–44. [Google Scholar] [CrossRef]
- Díaz, M.V.; García, A.; Agulleiro, B. Características histológicas del ovario de la seriola Mediterránea (Seriola dumerilii, Risso) mantenida en cautividad, durante su ciclo reproductivo anual. In Proceedings of the VI Congresso National de Acuicultura; Ruiz, J.C., Abellan Martinez, E., García García, B., Ortega Ros, A., Zamora Navarro, S., Eds.; Ministerio de Agricultura, Pesca y Alimentación, Madrid: Cartagena, Spain, 1997; pp. 389–394. [Google Scholar]
- Lazzari, A.; Fusari, A.; Boglione, A.; Marino, G.; Di Francesco, M. Recent advances in reproductional and rearing aspects of Seriola dumerilii. In Cahiers Options Méditerranéennes: Mediterranean Marine Aquaculture Finfish Species Diversification; Basurco, B., Ed.; C.I.H.E.A.M.: Zaragoza, Spain, 2000; Volume 47, pp. 241–247. [Google Scholar]
- Micale, V.; Maricchiolo, G.; Genovese, L. The reproductive biology of the amberjack, Seriola dumerilii (Risso 1810). I. Oocyte development in captivity. Aquac. Res. 1999, 30, 349–355. [Google Scholar] [CrossRef]
- Pastor, E.; Grau, A.; Riera, F.; Pou, S.; Massuti, E.; Grau, A.M. Experiences in the culture of new species in the ’Estacion de Acuicultura’ of the Balearic Government (1980–1998). In Cahiers Options Méditerranéennes: Mediterranean Marine Aquaculture Finfish Species Diversification; Basurco, B., Ed.; C.I.H.E.A.M.: Zaragoza, Spain, 2000; Volume 47, pp. 371–379. [Google Scholar]
- Agulleiro, M.J.; Scott, A.P.; Duncan, N.J.; Mylonas, C.C.; Cerdá, J. Treatment of GnRHa-implanted Senegalese sole (Solea senegalensis) with 11-ketoandrostenedione stimulates spermatogenesis and increases sperm motility. Comp. Biochem. Physiol. 2007, A147, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Carazo, I.; Norambuena, F.; Oliveira, C.; Sanchez-Vazquez, F.J.; Duncan, N.J. The effect of night illumination, red and infrared light, on locomotor activity, behaviour and melatonin of Senegalese sole (Solea senegalensis) broodstock. Physiol. Behav. 2013, 118, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Duncan, N.; Sonesson, A.; Chavanne, H. Principles of finfish broodstock management in aquaculture: Control of reproduction and genetic improvement In Advances in Aquaculture Hatchery Technology; Allan, G., Burnell, G., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 23–75. [Google Scholar]
- Howell, B.R.; Conceicao, L.E.C.; Prickett, R.; Cañavate, P.; Mananos, E. The Cultivation of Soles; Report of the 4rd Workshop held at CCMAR; University of the Algarve: Faro, Portugal, 2008. [Google Scholar]
- Morais, S.; Aragão, C.; Cabrita, E.; Conceição, L.E.C.; Constenla, M.; Costas, B.; Dias, J.; Duncan, N.; Engrola, S.; Estevez, A.; et al. New developments and biological insights into the farming of Solea senegalensis reinforcing its aquaculture potential. Rev. Aquac. 2016, 8, 227–263. [Google Scholar] [CrossRef]
- Rasines, I.; Gómez, M.; Martín, I.; Rodríguez, C.; Mañanós, E.; Chereguini, O. Artificial fertilization of Senegalese sole (Solea senegalensis): Hormone therapy administration methods, timing of ovulation and viability of eggs retained in the ovarian cavity. Aquaculture 2012, 326, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Pankhurst, N.W.; Fitzgibbon, Q.P. Characteristics of spawning behaviour in cultured greenback flounder, Rhombosa tapirina. Aquaculture 2006, 253, 279–289. [Google Scholar] [CrossRef]
- Papadaki, M.; Mazzella, D.; Santinelli, V.; Fakriadis, I.; Sigelaki, I.; Mylonas, C.C. Hermaphroditism and reproductive function of hatchery-produced sharpsnout seabream (Diplodus puntazzo) under attenuated annual thermal cycles. Aquaculture 2018, 482, 231–240. [Google Scholar] [CrossRef]
- Mañanos, E.; Duncan, N.; Mylonas, C.C. Reproduction and control of ovulation, spermiation and spawning in cultured fish . In Methods in Reproductive Aquaculture; Cabrita, E., Robles, V., Herraez, P., Eds.; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2009; pp. 3–80. [Google Scholar]
- Farquharson, K.A.; Hogg, C.J.; Grueber, C.E. A meta-analysis of birth-origin effects on reproduction in diverse captive environments. Nat. Commun. 2018, 9, 1055. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, C.C.; Zohar, Y. Use of GnRHa-delivery systems for the control of reproduction in fish. Rev. Fish Biol. Fish. 2001, 10, 463–491. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Zohar, Y. Promoting oocyte maturation, ovulation and spawning in farmed fish. In The Fish Oocyte: From Basic Studies to Biotechnological Applications; Babin, P.J., Cerdá, J., Lubzens, E., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2007; pp. 433–470. [Google Scholar]
- Zohar, Y.; Mylonas, C.C. Endocrine manipulations of spawning in cultured fish: From hormones to genes. In Reproductive Biotechnology in Finfish Aquaculture; Donaldson, E.M., Lee, C.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; pp. 99–136. [Google Scholar]
- Fernández-Palacios, H.; Schuchardt, D.; Roo, J.; Hernández-Cruz, C.M.; Izquierdo, M. Multiple GnRHa injections to induce successful spawning of wild caught greater amberjack (Seriola dumerili) matured in captivity. Aquac. Res. 2015, 46, 1748–1759. [Google Scholar] [CrossRef]
- García, A.; Díaz, M.V.; Agulleiro, B. Inducción hormonal de la puesta y desarrollo embrionario de la seriola Mediterranea (Seriola dumerilii, Risso). Monogr. Instit. Canario Cienc. Mar. 2001, 4, 561–566. [Google Scholar]
- Kozul, V.; Skaramuca, B.; Glamuzina, B.; Glavic, N.; Tutman, P. Comparative gonadogenesis and hormonal induction of spawning of cultured and wild mediterranean amberjack (Seriola dumerili, Risso 1810). Sci. Mar. 2001, 65, 215–220. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Papandroulakis, N.; Smboukis, A.; Papadaki, M.; Divanach, P. Induction of spawning of cultured greater amberjack (Seriola dumerili) using GnRHa implants. Aquaculture 2004, 237, 141–154. [Google Scholar] [CrossRef]
- Jerez, S.; Samper, M.; Santamaría, F.J.; Villamados, J.E.; Cejas, J.R.; Felipe, B.C. Natural spawning of greater amberjack (Seriola dumerili) kept in captivity in the Canary Islands. Aquaculture 2006, 252, 199–207. [Google Scholar] [CrossRef]
- Rodríguez-Barreto, D.; Jerez, S.; Cejas, J.R.; Martin, M.V.; Acosta, N.G.; Bolaños, A.; Lorenzo, A. Ovary and egg fatty acid composition of greater amberjack broodstock (Seriola dumerili) fed different dietary fatty acids profiles. Eur. J. Lipid Sci. Tech. 2014, 116, 584–595. [Google Scholar] [CrossRef]
- Kawabe, K.; Kimura, J.; Ando, K.; Kakiuchi, K. Natural spawning from 2-year-old reared amberjack, Seriola dumerili in Chichijima Ogasawara Islands, southern Japan. Aquac. Sci. 1998, 46, 31–36. [Google Scholar]
- Setiawan, A.N.; Muncaster, S.; Pether, S.; King, A.; Irvine, G.W.; Lokman, P.M.; Symonds, J.E. The effects of gonadotropin-releasing hormone analog on yellowtail kingfish Seriola lalandi (Valenciennes, 1833) spawning and egg quality. Aquac. Rep. 2016, 4, 1–9. [Google Scholar] [CrossRef]
- Marino, G.; Mandich, A.; Massari, A.; Andaloro, F.; Porrello, S. Aspects of reproductive biology of the Mediterranean amberjack (Seriola dumerilii Risso) during the spawning period. J. Appl. Ichthyol. 1995, 11, 9–24. [Google Scholar] [CrossRef]
- Fakriadis, I.; Lisi, F.; Sigelaki, I.; Papadaki, M.; Raftopoulos, A.; Mylonas, C.C. Spawning kinetics of greater amberjack Seriola dumerili in response to multiple GnRHa injections or implants. In Aquaculture Europe 2017 EAS; European Aquaculture Society: Dubrovnik, Croatia, 2017; p. 348. [Google Scholar]
- Mylonas, C.C.; Fakriadis, I.; Papandroulakis, N.; Raftopoulos, A.; Iakovopoulos, G.; Papadaki, M.; Sigelaki, I. Broodstock management and spawning induction of greater amberjack Seriola dumerili reared in tanks and sea cages in Greece. In Aquaculture Europe 2017 EAS; European Aquaculture Society: Dubrovnik, Croatia, 2017; p. 791. [Google Scholar]
- Mylonas, C.C.; Bridges, C.; Gordin, H.; Rios, A.B.; Garcia, A.; de la Gandara, F.; Fauvel, C.; Suquet, M.; Medina, A.; Papadaki, M.; et al. Preparation and administration of gonadotropin-releasing hormone agonist (GnRHa) implants for the artificial control of reproductive maturation in captive-reared Atlantic bluefin tuna (Thunnus thynnus thynnus). Rev. Fish. Sci. 2007, 15, 183–210. [Google Scholar] [CrossRef]
- Zupa, P.; Fauvel, C.; Mylonas, C.C.; Santamaria, N.; Valentini, L.; Pousis, C.; Papadaki, M.; Suquet, M.; De la Gándara, F.; Bello, G.; et al. Comparative analysis of male germ cell proliferation and apoptosis in wild and captive Atlantic bluefin tuna (Thunnus thynnus). J. Appl. Ichthyol. 2013, 29, 71–81. [Google Scholar] [CrossRef]
- Zohar, Y.; Mylonas, C.C. Endocrine manipulations of spawning in cultured fish: From hormones to genes. Aquaculture 2001, 197, 99–136. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Zohar, Y.; Woods, L.C.; Thomas, P.; Schulz, R.W. Hormone profiles of captive striped bass Morone saxatilis during spermiation, and long-term enhancement of milt production. J. World Aquac. Soc. 1998, 29, 379–392. [Google Scholar] [CrossRef]
- Vermeirssen, E.L.M.; Scott, A.P.; Mylonas, C.C.; Zohar, Y. Gonadotrophin-releasing hormone agonist stimulates milt fluidity and plasma concentrations of 17,20β-dihydroxylated and 5β-reduced, 3a-hydroxylated C21 steroids in male plaice (Pleuronectes platessa). Gen. Comp. Endocrinol. 1998, 112, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Clearwater, S.J.; Crim, L.W. Gonadotropin releasing hormone-analogue treatment increases sperm motility, seminal plasma pH and sperm production in yellowtail flounder Pleuronectes ferrugineus. Fish Physiol. and Biochem. 1998, 19, 349–357. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Gissis, A.; Magnus, Y.; Zohar, Y. Hormonal changes in male white bass (Morone chrysops) and evaluation of milt quality after treatment with a sustained-release GnRHa delivery system. Aquaculture 1997, 153, 301–313. [Google Scholar] [CrossRef]
- Rainis, S.; Mylonas, C.C.; Kyriakou, Y.; Divanach, P. Enhancement of spermiation in European sea bass (Dicentrarchus labrax) at the end of the reproductive season using GnRHa implants. Aquaculture 2003, 219, 873–890. [Google Scholar] [CrossRef]
- Sorbera, L.A.; Mylonas, C.C.; Zanuy, S.; Carillo, M.; Zohar, Y. Sustained administration of GnRHa increases milt volume without altering sperm counts in the sea bass. J. Exp. Zool. 1996, 276, 361–368. [Google Scholar] [CrossRef]
- Goren, A.; Gustafson, H.; Doering, D. Field trials demonstrate the efficacy and commercial benefit of a GnRHa implant to control ovulation and spermiation in salmonids. In Reproductive Physiology of Fish; Goetz, F.W., Thomas, P., Eds.; Fish Symposium 95: Austin, TX, USA, 1995. [Google Scholar]
- Mazorra de Quero, C.; Shields, R.J.; Scott, A.P.; Mylonas, C.C.; Zohar, Y.; Bromage, N. Effects of late season GnRHa implants on male Atlantic halibut Hippoglossus hippoglossus spermiation. In Aqua 2000; Flos, R., Cresswell, L., Eds.; European Aquaculture Society: Nice, France, 2000; p. 455. [Google Scholar]
- Vermeirssen, E.L.M.; Shields, R.J.; Mazorra de Quero, C.; Scott, A.P. Gonadotrophin-releasing hormone agonist raises plasma concentrations of progestogens and enhances milt fluidity in male Atlantic halibut (Hippoglossus hippoglossus). Fish Physiol. Biochem. 2000, 22, 77–87. [Google Scholar] [CrossRef]
- Greenwood, L.N.; Scott, A.P.; Vermeirssen, E.L.M.; Mylonas, C.C.; Pavlides, M. Plasma steroids in mature common dentex (Dentex dentex) stimulated with a gonadotropin-releasing hormone agonist. Gen. Comp. Endocrinol. 2001, 123, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Berlinsky, D.L.; William, K.; Hodson, R.G.; Sullivan, C.V. Hormone induced spawning of summer flounder Paralichthys dentatus. J. World Aquac. Soc. 1997, 28, 79–86. [Google Scholar] [CrossRef]
- Berlinsky, D.L.; King, W.V.; Smith, T.I.J.; Hamilton, R.D., II; Holloway, J., Jr.; Sullivan, C.V. Induced ovulation of Southern flounder Paralichthys lethostigma using gonadotropin releasing hormone analogue implants. J. World Aquac. Soc. 1996, 27, 143–152. [Google Scholar] [CrossRef]
- Grau, A.; Crespo, S.; Sarasquete, C.; González de Canales, M.L. The digestive tract of the amberjack Seriola dummerili, Risso: A light and scanning electron microscope study. J. Fish Biol. 1992, 41, 287–303. [Google Scholar] [CrossRef]
- Lazzari, A. Some notes to the aquaculture development of the new Mediterranean species: The yellowtail (Seriola dumerilii) case and strategy to come. In Aquaculture Europe 91; De Pauw, N., Joyce, J., Eds.; European Aquaculture Society, Special Publication No. 14: Dublin, Ireland, 1991; pp. 183–184. [Google Scholar]
- Kawabe, K.; Kato, K.; Kimura, J.; Okamura, Y.; Ando, K.; Saito, M.; Yoshida, K. Rearing of broodstock fish and egg-taking from amberjack Seriola dumerili in Chichi-jima, Ogasawara Islands, southern Japan. Aquac. Sci. 1996, 44, 151–157. [Google Scholar]
- Fernández-Palacios, H.; Schuchardt, D.; Roo, J.; Hernández-Cruz, C.; Izquierdo, M. Spawn quality and GnRHa induction efficiency in longfin yellowtail (Seriola rivoliana) broodstock kept in captivity. Aquaculture 2015, 435, 167–172. [Google Scholar] [CrossRef]
- Roo, J.; Fernández-Palacios, H.; Hernández-Cruz, C.; Mesa-Rodriguez, A.; Schuchardt, D.; Izquierdo, M. First results of spawning and larval rearing of longfin yelowtail Seriola rivoliana as a fast-growing candidate for European marine finfish aquaculture diversification. Aquac. Res. 2014, 45, 689–700. [Google Scholar] [CrossRef]
- Carolsfeld, J.; Sherwood, N.M.; Kreiberg, H.; Sower, S.A. Induced sexual maturation of herring using GnRHa ‘quick-release’ cholesterol pellets. Aquaculture 1988, 70, 169–181. [Google Scholar] [CrossRef]
- García-Gómez, A.; De la Gándara, F. Observations on the embryonic and larval development of Mediterranean yellowtail (Seriola dumerili). In Seafarming Today and Tomorrow; Basurco, B., Saroglia, M., Eds.; European Aquaculture Society: Oostende, Belgium, 2003; pp. 246–247. [Google Scholar]
- Bobe, J.; Labbé, C. Egg and sperm quality in fish. Gen. Comp. Endocrinol. 2010, 165, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Agulleiro, M.J.; Anguis, V.; Cañavate, J.P.; Martínez-Rodríguez, G.; Mylonas, C.C.; Cerdá, J. Induction of spawning of captive-reared Senegal sole (Solea senegalensis) using different administration methods for gonadotropin-releasing hormone agonist. Aquaculture 2006, 257, 511–524. [Google Scholar] [CrossRef]
- Bonnet, E.; Fostier, A.; Bobe, J. Microarray-based analysis of fish egg quality after natural or controlled ovulation. BMC Genomics 2007, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Forniés, M.A.; Mañanos, E.; Laureau, S.; do Santos, A.; Carrillo, M.; Mylonas, C.C.; Zohar, Y.; Zanuy, S. Different delivery systems for the optimization of the induction of the spawn in sea bass (Dicentrarchus labrax). In Aqua; Flos, R., Creswell, L., Eds.; European Aquaculture Society: Nice, France, 2000; p. 217. [Google Scholar]
- Garber, A.F.; Fordham, S.E.; Symonds, J.E.; Trippel, E.A.; Berlinsky, D.L. Hormonal induction of ovulation and spermiation in Atlantic cod (Gadus morhua). Aquaculture 2009, 296, 179–183. [Google Scholar] [CrossRef]
- Mugnier, C.; Gaignon, J.L.; Lebegue, E.; Fostier, A.; Breton, B. Induction and synchronisation of spawning in cultivated turbot (Scophthalmus maximus L.) broodstock by implantation of sustained-release GnRH-a pellet. Aquaculture 2000, 181, 241–255. [Google Scholar] [CrossRef]
- Hamasaki, K.; Tsuruoka, K.; Teruya, K.; Hashimoto, H.; Hamada, K.; Hotta, T.; Mushiake, K. Feeding habits of hatchery-reared larvae of greater amberjack Seriola dumerili. Aquaculture 2009, 288, 216–225. [Google Scholar] [CrossRef]
- Tachihara, K.; Ebisu, R.; Tukashima, Y. Spawning, eggs, larvae and juveniles of the purplish amberjack Seriola dumerili. Nippon Suisan Gakkaishi 1993, 59, 1479–1488. [Google Scholar] [CrossRef]
- Blaxter, J.H.S. Pattern and variety in development. In Fish. Physiology; Hoar, W.S., Randall, D.J., Eds.; Academic Press Inc.: New York, NY, USA, 1988; pp. 1–58. [Google Scholar]
- Mihelakakis, A.; Yoshimatsu, T.; Kitajima, C. Change in egg size of Japanese flounder during one spawning season. J. Fac. Agric. Kyushu Univ. 1995, 40, 53–59. [Google Scholar]
- Mihelakakis, A.; Yoshimatsu, T.; Tsolkas, C. Spawning in captivity and early life history of cultured red porgy, Pagrus pagrus. Aquaculture 2001, 199, 333–352. [Google Scholar] [CrossRef]
- Mandich, A.; Massari, A.; Bottero, S.; Pizzicori, P.; Goos, H.; Marino, G. Plasma sex steroid and vitellogenin profiles during gonad development in wild Mediterranean amberjack (Seriola dumerilii, Risso). Mar. Biol. 2004, 144, 127–138. [Google Scholar]
- Poortenaar, C.W.; Hooker, S.H.; Sharp, N. Assessment of yellowtail kingfish (Seriola lalandi lalandi) reproductive physiology, as a basis for aquaculture development. Aquaculture 2001, 201, 271–286. [Google Scholar] [CrossRef]
- Kagawa, H.; Young, G.; Adachi, S.; Nagahama, Y. Estradiol-17β production in amago salmon (Ocorhynchus rhodurus) ovarian follicles: Role of the thecal and granulosa cells. Gen. Comp. Endocrinol. 1982, 47, 440–448. [Google Scholar] [CrossRef]
- Rinchard, J.; Kestemont, P.; Kuhn, E.R.; Fostier, A. Seasonal changes in plasma levels of steroid hormones in an asynchronous fish the gudgeon Gobio gobio L. (Teleostei, Cyprinidae). Gen. Comp. Endocrinol. 1993, 92, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.P.; Sumpter, J.P.; Stacey, N. The role of the maturation-inducing steroid, 17,20β-dihydroxypregn-4-en-3-one, in male fishes: A review. J. Fish Biol. 2010, 76, 183–224. [Google Scholar] [CrossRef] [PubMed]
- Uyan, O.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Uyan, S.; Ren, T.; Hernandez, L.H.H. The influence of dietary phospholipid level on the performances of juvenile amberjack, Seriola dumerili, fed non-fishmeal diets. Aquac. Nutr. 2009, 15, 550–557. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Ishikawa, M.; Yokoyama, S. Effects of partial substitution of fish meal by soybean meal with or without heat-killed Lactobacillus plantarum (LP20) on growth performance, digestibility, and immune response of amberjack, Seriola dumerili juveniles. BioMed Res. Int. 2015, 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Sony, N.M.; Fujieda, T. Nucleoside by product dietary supplementation influences blood chemistry, immune response, oxidative stress resistance and intestinal morphology of juvenile amberjack, Seriola dumerili. Aquac. Nutr. 2017, 23, 1390–1400. [Google Scholar] [CrossRef]
- Panini, E.; Mylonas, C.C.; Zanuy, S.; Carrillo, M.; Ramos, J.; Bruce, M. Incubation of embryos and larvae of marine fish using microtiter plates. Aquac. Int. 2001, 9, 189–196. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Hinshaw, J.M.; Sullivan, C.V. GnRHa-induced ovulation of brown trout (Salmo trutta) and its effects on egg quality. Aquaculture 1992, 106, 379–392. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Papadaki, M.; Pavlidis, M.; Divanach, P. Evaluation of egg production and quality in the Mediterranean red porgy (Pagrus pagrus) during two consecutive spawning seasons. Aquaculture 2004, 232, 637–649. [Google Scholar] [CrossRef]
- Bennett, H.S.; Wyrick, A.D.; Lee, S.W.; McNeil, J.H. Science and art in preparing tissues embedded in plastic for light microscopy, with special reference to glycol methacrylate, glass knives and simple stains. Stain Tech. 1976, 51, 71–97. [Google Scholar] [CrossRef]
- Rodríguez, L.; Begtashi, I.; Zanuy, S.; Carrillo, M. Development and validation of an enzyme immunoassay for testosterone: Effects of photoperiod on plasma testosterone levels and gonadal development in male sea bass (Dicentrarchus labrax, L.) at puberty. Fish Physiol. Biochem. 2000, 23, 141–150. [Google Scholar] [CrossRef]
- Nash, J.P.; Davail-Cuisset, B.; Bhattacharyya, S.; Suter, H.C.; Le Menn, F.; Kime, D.E. An enzyme linked immunosorbent assay (ELISA) for testosterone, estradiol, and 17,20b-dihydroxy-4-pregnen-3-one using acetylcholinesterase as tracer: Application to measurement of diel patterns in rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2000, 22, 355–363. [Google Scholar] [CrossRef]
- Cuisset, B.; Pradelles, P.; Kime, D.E.; Kühn, E.R.; Babin, P.; Davail, S.; Le Menn, F. Enzyme immunoassay for 11-ketotestosterone using acetylcholinesterase as label: Application to the measurement of 11-ketotestosterone in plasma of Siberian sturgeon. Comp. Biochem. Physiol. 1994, 108C, 229–241. [Google Scholar]

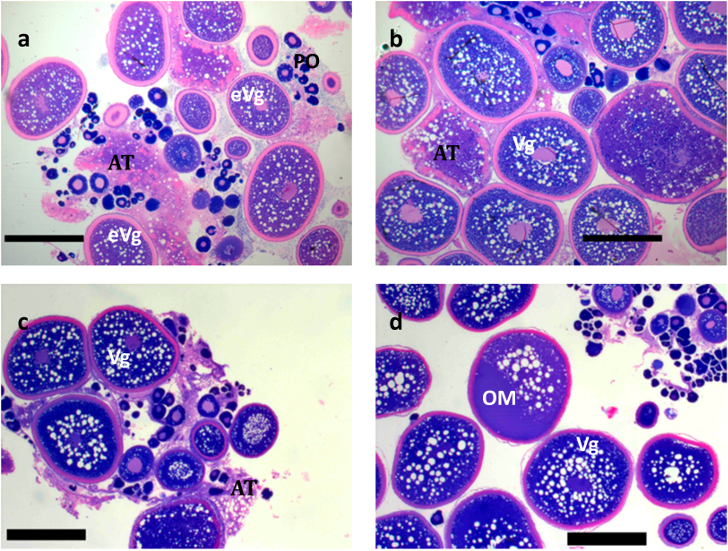
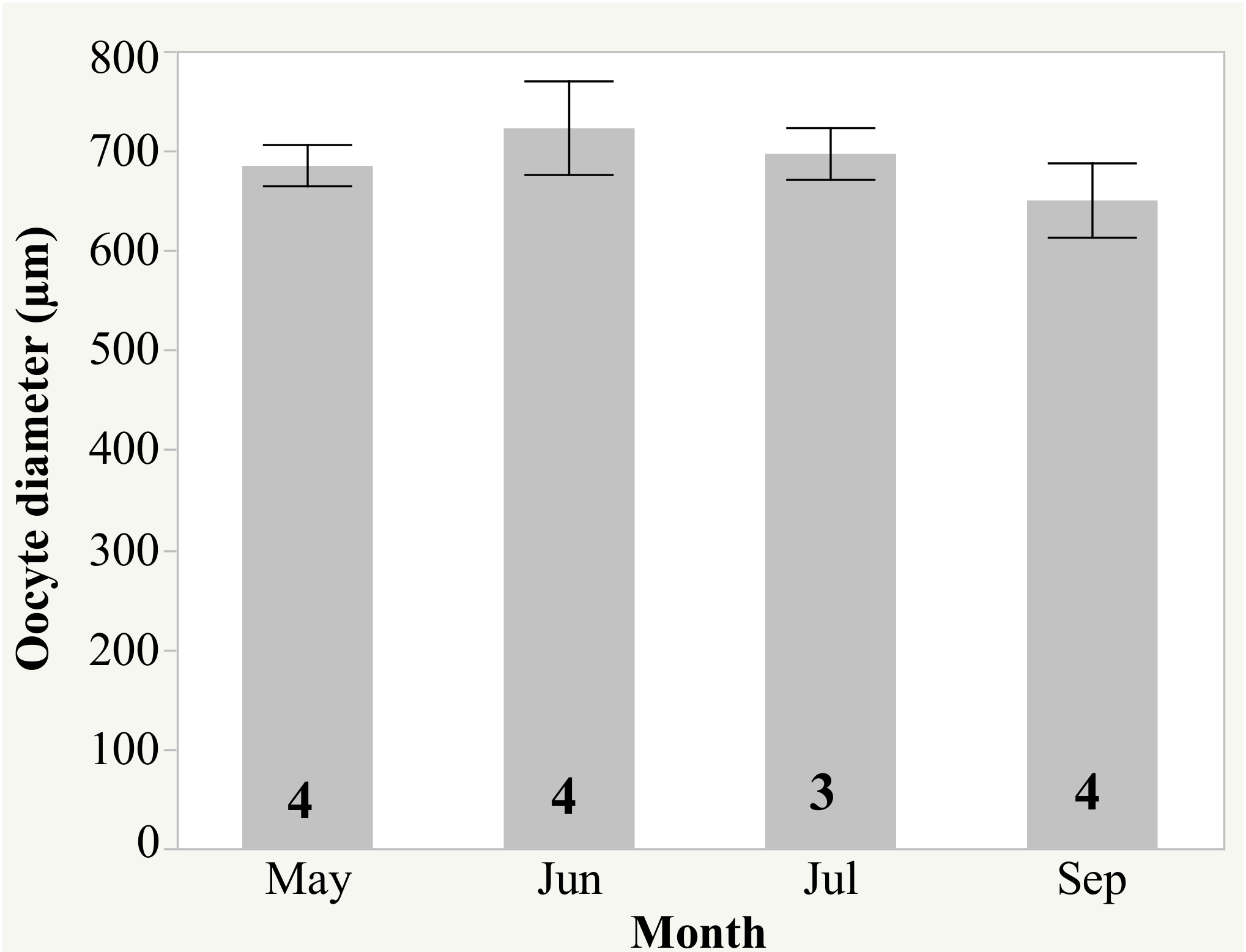

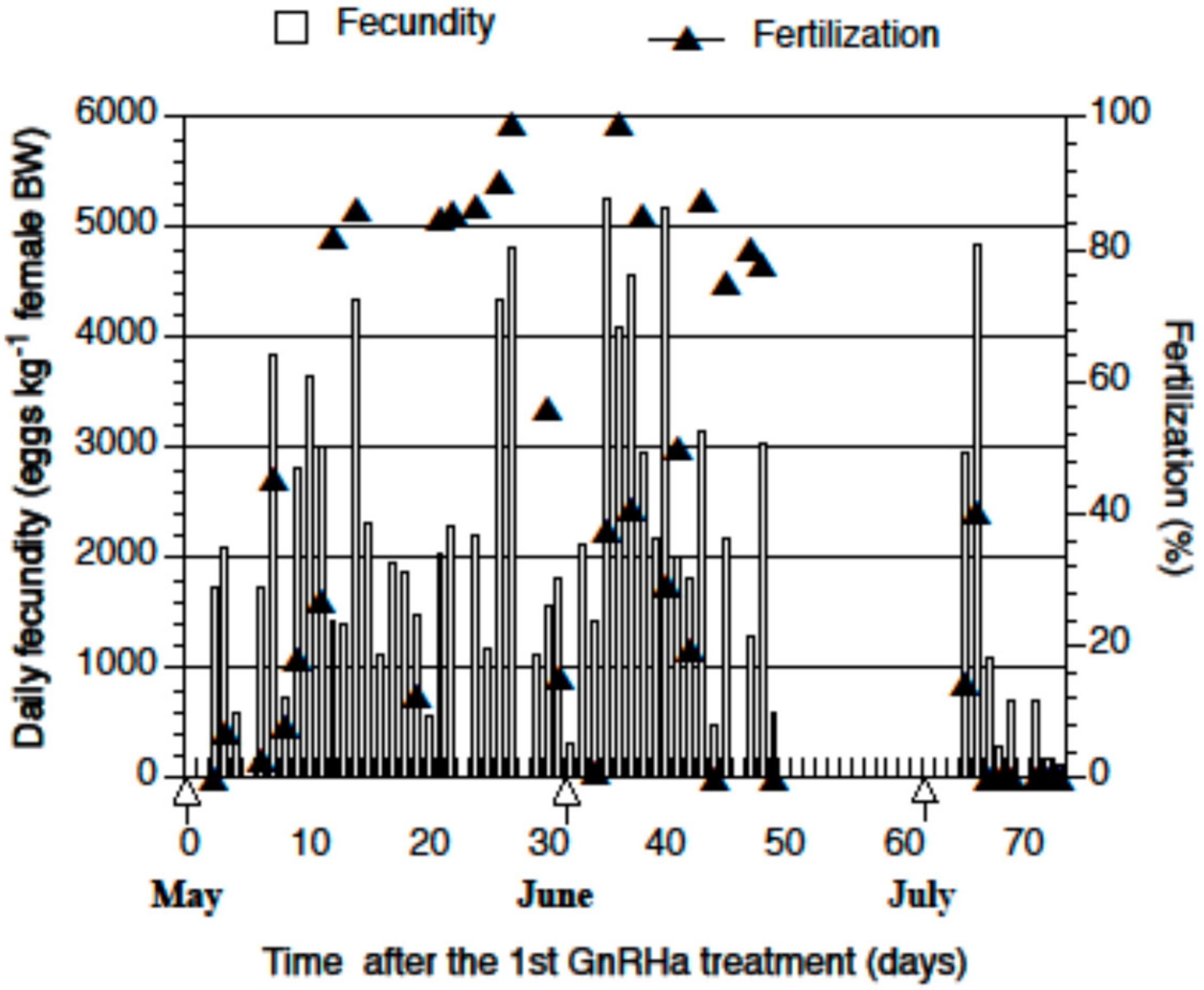





| Month | Sex | n | Weight (kg) (Mean ± SD) | Weight Change (%) (Mean ± SD) |
|---|---|---|---|---|
| May | Females | 7 | 23.3 ± 10.8 | |
| Males | 7 | 14.9 ± 5.0 | ||
| July | Females | 7 | 21.6 ± 10.2 | −7.7 ± 5.0 |
| Males | 7 | 13.3 ± 4.5 | −10.5 ± 4.1 | |
| September | Females | 6 | 23.9 ± 10.0 | −1.4 ± 9.3 |
| Males | 5 | 15.3 ± 4.7 | 1.2 ± 10.6 |
| Sex | Females | Males | |||||
|---|---|---|---|---|---|---|---|
| Sampling (Month) | Treatment | N | Dose (µg kg−1) | N | Dose (µg kg−1) | ||
| Biopsied | Treated | Biopsied | Treated | ||||
| May | First | 7 | 4 (29.1 ± 5.1) | 53.9 ± 10.9 | 7 | 7 (14.9 ± 1.9) | 67.9 ± 20.3 |
| June | Second * | 7 | 7 (23.3 ± 4.1) | 54.4 ± 8.5 | 7 | 5 (16.9 ± 1.9) | 38.5 ± 4.1 |
| July | Third | 7 | 6 (23.9 ± 3.6) | 52.7 ± 4.6 | 7 | 6 (13.9 ± 1.9) | 39.9 ± 14.5 |
| September | 6 | 5 | |||||
| Treatment | Spawns (n) | Eggs Spawn−1 kg−1 | Total Eggs kg−1 (×1000 Eggs) | Total Eggs (×106 Eggs) |
|---|---|---|---|---|
| 1 | 29 | 2087 ± 218 | 60.54 | 7.05 |
| 2 | 15 | 2828 ± 420 | 42.42 | 6.55 |
| 3 | 8 | 1895 ± 827 | 15.16 | 1.35 |
| Females | May | June | July | September | ||||
|---|---|---|---|---|---|---|---|---|
| Erythrocytes | 341.87 ± 29.00 | a | 283.84 ± 34.71 | ab | 171.87 ± 30.35 | bc | 99.25 ± 17.1 | c |
| Leucocytes | 93.5 ± 21.65 | 53.14 ± 11.65 | 62.54 ± 9.48 | 82.2 ± 12.01 | ||||
| Hematocrit | 51.00 ± 2.66 | 46.14 ± 4.85 | 41.83 ± 5.52 | 39.00 ± 4.96 | ||||
| Triglycerides | 161.25 ± 25.48 | 204.23 ± 58.28 | 188.05 ± 26.07 | 193.95 ± 36.23 | ||||
| Cholesterol | 264.15 ± 26.02 | 275.28 ± 26.59 | 278.46 ± 43.10 | 229.89 ± 60.84 | ||||
| Protein | 46.39 ± 4.58 | ab | 46.98 ± 6.00 | ab | 52.54 ± 5.66 | a | 26.65 ± 2.14 | b |
| Glucose | 98.48 ± 14.77 | 83.68 ± 11.24 | 95.98 ± 13.40 | 127.54 ± 31.97 | ||||
| ALT/GPT | 13.05 ± 0.95 | 17.78 ± 5.03 | 13.33 ± 2.64 | 23.03 ± 9.04 | ||||
| AST/GOT | 35.00 ± 7.93 | 35.83 ± 9.63 | 23.79 ± 6.60 | 14.30 ± 2.49 | ||||
| Alkaline phosphatase | 61.04 ± 7.88 | c | 78.93 ± 11.16 | bc | 104.51 ± 5.17 | b | 142.12 ± 6.23 | a |
| Cholinesterase | 186.44 ± 10.97 | 153.54 ± 21.93 | 296.12 ± 65.25 | 235.80 ± 16.45 | ||||
| Amylase | 12.44 ± 2.04 | a | 14.81 ± 2.74 | a | 8.34 ± 0.81 | ab | 1.83 ± 0.73 | b |
| Cortisol | 44.00 ± 27.82 | 24.46 ± 18.55 | 31.34 ± 12.31 | 13.56 ± 7.21 | ||||
| Lactate | 35.61 ± 4.38 | 33.88 ± 1.90 | 41.53 ± 7.61 | 40.35 ± 5.42 | ||||
| Sodium | 423.28 ± 9.45 | 416.34 ± 11.67 | 496.45 ± 72.57 | 376.26 ± 5.26 | ||||
| Potassium | 19.52 ± 3.98 | 16.28 ± 1.68 | 24.41 ± 3.97 | 15.40 ± 1.08 | ||||
| Males | May | June | July | September | ||||
|---|---|---|---|---|---|---|---|---|
| Erythrocytes | 426.25 ± 15.93 | a | 256.25 ± 19.44 | b | 197.75 ± 36.79 | bc | 140.50 ± 17.45 | c |
| Leucocytes | 92.79 ± 16.40 | 71.33 ± 13.90 | 50.63 ± 10.89 | 58.25 ± 9.35 | ||||
| Hematocrit | 43.00 ± 4.00 | 56.00 ± 5.00 | 42.00 ± 9.04 | 21.00 ± 11.00 | ||||
| Triglycerides | 98.01 ± 5.27 | 129.04 ± 46.27 | 236.38 ± 49.35 | 169.38 ± 40.80 | ||||
| Cholesterol | 209.20 ± 15.76 | ab | 285.51 ± 17.84 | a | 265.11 ± 35.76 | a | 151.47 ± 17.45 | b |
| Protein | 35.98 ± 3.56 | 40.75 ± 2.87 | 49.08 ± 6.06 | 30.80 ± 5.54 | ||||
| Glucose | 91.32 ± 8.94 | 55.53 ± 16.27 | 99.10 ± 14.65 | 101.85 ± 21.66 | ||||
| ALT/GPT | 12.78 ± 2.15 | 11.67 ± 1.32 | 14.44 ± 3.31 | 19.63 ± 0.19 | ||||
| AST/GOT | 12.92 ± 3.79 | 15.00 ± 3.82 | 40.08 ± 11.32 | 13.33 ± 1.11 | ||||
| Alcaline phosphatase | 65.18 ± 5.25 | b | 105.95 ± 7.83 | ab | 103.06 ± 9.45 | ab | 147.62 ± 24.26 | a |
| Cholinesterase | 197.41 ± 10.97 | 219.35 ± 0.00 | 179.86 ± 10.75 | 257.73 ± 25.91 | ||||
| Amylase | 9.88 ± 0.78 | b | 15.36 ± 4.39 | a | 7.90 ± 0.41 | b | 1.98 ± 0.41 | c |
| Cortisol | 6.61 ± 0.69 | 17.86 ± 8.44 | 35.28 ± 15.39 | 4.90 ± 1.92 | ||||
| Lactate | 42.79 ± 5.21 | 45.41 ± 2.94 | 40.95 ± 1.70 | 35.97 ± 10.08 | ||||
| Sodium | 441.71 ± 9.17 | a | 414.56 ± 14.23 | ab | 531.77 ± 46.09 | ab | 382.95 ± 1.73 | b |
| Potassium | 24.62 ± 3.56 | 15.69 ± 1.39 | 18.69 ± 3.05 | 14.99 ± 2.03 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerez, S.; Fakriadis, I.; Papadaki, M.; Martín, M.V.; Cejas, J.R.; Mylonas, C.C. Spawning Induction of First-Generation (F1) Greater Amberjack Seriola dumerili in the Canary Islands, Spain Using GnRHa Delivery Systems. Fishes 2018, 3, 35. https://0-doi-org.brum.beds.ac.uk/10.3390/fishes3030035
Jerez S, Fakriadis I, Papadaki M, Martín MV, Cejas JR, Mylonas CC. Spawning Induction of First-Generation (F1) Greater Amberjack Seriola dumerili in the Canary Islands, Spain Using GnRHa Delivery Systems. Fishes. 2018; 3(3):35. https://0-doi-org.brum.beds.ac.uk/10.3390/fishes3030035
Chicago/Turabian StyleJerez, Salvador, Ioannis Fakriadis, Maria Papadaki, M. Virginia Martín, Juana Rosa Cejas, and Constantinos C. Mylonas. 2018. "Spawning Induction of First-Generation (F1) Greater Amberjack Seriola dumerili in the Canary Islands, Spain Using GnRHa Delivery Systems" Fishes 3, no. 3: 35. https://0-doi-org.brum.beds.ac.uk/10.3390/fishes3030035





