Life History and Population Dynamics of Green Crabs (Carcinus maenas)
Abstract
:1. Introduction
1.1. Overview
1.2. Native European Population
1.3. Northwestern Atlantic Nonindigenous Population
1.4. Northeastern Pacific Nonindigenous Population
1.5. Other Nonindigenous Populations
1.6. Additional Introductions and Potential Invasion Sites
2. Population Genetics
3. Habitat Preferences
4. Tolerances
4.1. Temperature
4.2. Salinity
4.3. Oxygen
5. Reproduction and Development
5.1. Molting
5.2. Mating
5.3. Ovigerous Females and Egg Release
5.4. Larval Development
5.5. Life Span
6. Size
6.1. Adult Maximum Size
6.2. Size at Sexual Maturity
7. Population Characteristics
7.1. Population Density
7.2. Sex Ratios
8. Ecosystem Dynamics
8.1. Diet
8.2. Competitors
8.3. Predators
8.4. Epibionts
8.5. Pathogens and Parasites
9. Ecological Impacts
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crothers, J.H. The biology of the shore crab Carcinus maenas (L.) 1. The background—Anatomy, growth and life history. Field Stud. 1967, 1, 407–434. [Google Scholar]
- Crothers, J.H. The biology of the shore crab Carcinus maenas (L.) 2. The life of the adult crab. Field Stud. 1968, 2, 579–614. [Google Scholar]
- Broekhuysen, G.J. On development, growth and distribution of Carcinides maenas (L.). Arch. Neerlandaises de Zoologie 1936, 2, 257–400. [Google Scholar] [CrossRef]
- Edwards, R.L. Movements of individual members in a population of the shore crab Carcinus maenas (L.) in the littoral zone. J. Anim. Ecol. 1958, 27, 37–45. [Google Scholar] [CrossRef]
- Naylor, E. Seasonal changes in a population of Carcinus maenas in the littoral zone. J. Anim. Ecol. 1962, 31, 601–609. [Google Scholar] [CrossRef]
- Bruce, J.R.; Colman, J.S.; Jones, N.S. (Eds.) Marine fauna of the Isle of Man, Edn.2. In Liverpool Marine Biology Committee Memoirs on Typical British Marine Plants and Animals, No. 36; Liverpool University Press: Liverpool, UK, 1963. [Google Scholar]
- Demeusy, N. Biologie-influence des facteurs saisonniers sur la realisation de la puberte au sein dune population de Carcinus maenas L. Des cotes de la manche. Comptes rendus hebdomadaires des seances de l academie des Sci. 1963, 256, 4762–4764. [Google Scholar]
- Behrens Yamada, S. Global Invader: The European Green Crab; Oregon State University: Corvallis, OR, USA, 2001; p. 123. ISBN 1-881826-24-4. [Google Scholar]
- Klassen, G.; Locke, A. A Biological Synopsis of the European Green Crab, Carcinus maenas; Fisheries and Oceans Canada: Moncton, NB, Canada, 2007; pp. 1–75. [Google Scholar]
- Darling, J.A.; Bagley, M.J.; Roman, J.; Tepolt, C.K.; Geller, J.B. Genetic patterns across multiple introductions of the globally invasive crab genus Carcinus. Mol. Ecol. 2008, 17, 4992–5007. [Google Scholar] [CrossRef] [PubMed]
- Roman, J.; Palumbi, S.R. A global invader at home: Population structure of the green crab, Carcinus maenas, in Europe. Mol. Ecol. 2004, 13, 2891–2898. [Google Scholar] [CrossRef]
- Tepolt, C.K.; Darling, J.A.; Bagley, M.J.; Geller, J.B.; Blum, M.J.; Grosholz, E.D. European green crabs (Carcinus maenas) in the northeastern Pacific: Genetic evidence for high population connectivity and current-mediated expansion from a single introduced source population. Divers. Distrib. 2009, 15, 997–1009. [Google Scholar] [CrossRef]
- Domingues, C.P.; Creer, S.; Taylor, M.I.; Queiroga, H.; Carvalho, G.R. Genetic structure of Carcinus maenas within its native range: Larval dispersal and oceanographic variability. Mar. Ecol. Prog. Ser. 2010, 410, 111–123. [Google Scholar] [CrossRef] [Green Version]
- Darling, J.A.; Tsai, Y.H.E.; Blakeslee, A.M.; Roman, J. Are genes faster than crabs? Mitochondrial introgression exceeds larval dispersal during population expansion of the invasive crab Carcinus maenas. R. Soc. Open Sci. 2014, 1, 140202. [Google Scholar] [PubMed] [Green Version]
- Tepolt, C.K.; Palumbi, S. Transcriptome sequencing reveals both neutral and adaptive genome dynamics in a marine invader. Mol. Ecol. 2015, 24, 4145–4158. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, N.W.; DiBacco, C.; Van Wyngaarden, M.; Hamilton, L.C.; Stanley, R.R.E.; Bernier, R.; FitzGerald, J.; Matheson, K.; McKenzie, C.H.; Ravindran, P.N.; et al. RAD sequencing reveals genomewide divergence between independent invasions of the European green crab (Carcinus maenas) in the Northwest Atlantic. Ecol. Evol. 2017, 7, 2513–2524. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, N.; DiBacco, C.; Wringe, B.F.; Stanley, R.R.E.; Hamilton, L.C.; Ravindran, P.N.; Bradbury, I.R. Genomic evidence of hybridization between two independent invasions of European green crab (Carcinus maenas) in the Northwest Atlantic. Heredity 2017, 119, 154. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, N.W.; Bradbury, I.R.; Stanley, R.R.E.; Wringe, B.F.; Van Wyngaarden, M.; Lowen, J.B.; McKenzie, C.H.; Matheson, K.; Sargent, P.S.; DiBacco, C. Genomewide evidence of environmentally mediated secondary contact of European green crab (Carcinus maenas) lineages in eastern North America. Evol. Appl. 2018, 11, 869–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elner, R.W. The influence of temperature, sex, and chela size in the foraging strategy of the shore crab, Carcinus maenas. Mar. Behav. Physiol. 1980, 7, 15–24. [Google Scholar] [CrossRef]
- Carlton, J.T.; Cohen, A.N. Episodic global dispersal in shallow water marine organisms: The case history of the European shore crabs Carcinus maenas and C. aestuarii. J. Biogeogr. 2003, 30, 1809–1820. [Google Scholar] [CrossRef]
- Gomes, V. First results of tagging experiments on Carcinus maenas (L.) in the Ria de Aveiro Lagoon, Portugal. Cienc. Biol. Ecol. Syst. 1991, 11, 21–30. [Google Scholar]
- Morris-Webb, L.; Goudge, H.; Duce, C. An Introductory Review of the Biology and Population Dynamics of the Green Shore Crab, Carcinus maenas (L.), in the UK, with Specific Reference to Menai Strait; Policy Research Report No. 07/18; Countryside Council for Wales (now Natural Resources Wales): Newtown, UK, 2007. [Google Scholar]
- Lafferty, K.D.; Kuris, A.M. Biological control of marine pests. Ecology 1996, 77, 1989–2000. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; The World Conservation Union (IUCN): Aukland, New Zealand, 2000. [Google Scholar]
- Grosholz, E.D. Recent biological invasions may hasten invasional meltdowns by accelerating historical introductions. Proc. Natl. Acad. Sci. USA 2005, 102, 1088–1091. [Google Scholar] [CrossRef] [Green Version]
- Towle, D.W.; Smith, C.M. Gene discovery in Carcinus maenas and Homarus americanus via expressed sequence tags. Int. Comp. Biol. 2006, 46, 912–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darling, J.A. More than one way to invade: Lessons from genetic studies of Carcinus shore crabs. In In the Wrong Place—Alien Marine Crustaceans: Distribution, Biology and Impacts. Invading Nature—Springer Series in Invasion Ecology, Vol. 6; Galil, B.S., Clark, P.F., Carlton, J.T., Eds.; Springer: New York, NY, USA, 2011; pp. 661–685. [Google Scholar]
- Leignel, V.; Stillman, J.H.; Baringou, S.; Thabet, R.; Metais, I. Overview on the European green crab Carcinus spp. (Portunidae, Decapoda), one of the most famous marine invaders and ecotoxicological models. Environ. Sci. Pollut. Res. 2014, 21, 9129–9144. [Google Scholar] [CrossRef] [PubMed]
- Grosholz, E.D.; Ruiz, G.M. (Eds.) Management Plan for the European Green Crab Submitted to the Aquatic Nuisance Species Task Force; 2002; p. 55. [Google Scholar]
- Grosholz, E.D.; Ruiz, G.M. Spread and potential impact of the recently introduced European green crab, Carcinus maenas, in central California. Mar. Biol. 1995, 122, 239–247. [Google Scholar]
- Grosholz, E.D.; Ruiz, G.M. Predicting the impact of introduced marine species: Lessons from the multiple invasions of the European green crab Carcinus maenas. Biol. Conserv. 1996, 78, 59–66. [Google Scholar] [CrossRef]
- Audet, D.; Dais, D.S.; Miron, G.; Moriyasu, M.; Benhalima, K.; Campbell, R. Geographic expansion of a nonindigenous crab, Carcinus maenas (L.), along the Nova Scotia shore into the southeastern Gulf of St. Lawrence, Canada. J. Shellfish Res. 2003, 22, 255–262. [Google Scholar]
- Almaça, C. Sur le problème de l’origine de Carcinus maenas (L.) du littoral américain. Rev. Fac. Ciènc. Univ. Lisboa. 1963, 11, 121–136. [Google Scholar]
- Vermeij, G.J. Phenotypic evolution in a poorly dispersing snail after arrival of a predator. Nature 1982, 299, 349–350. [Google Scholar] [CrossRef]
- Chilton, C. Note on the dispersal of marine Crustacea by means of ships. Trans. Proc. R. Soc. N. Z. 1910, 43, 131–133. [Google Scholar]
- Cohen, A.N.; Carlton, J.T.; Fountain, M.C. Introduction, dispersal and potential impacts of the green crab Carcinus maenas in San Francisco Bay, California. Mar. Biol. 1995, 122, 225–237. [Google Scholar]
- Eastwood, M.M.; Donahue, M.J.; Fowler, A.E. Reconstructing past biological invasions: Niche shifts in response to invasive predators and competitors. Biol. Invasions 2007, 9, 397–407. [Google Scholar] [CrossRef]
- Jamieson, G.S. European green crab, Carcinus maenas, introductions in North America: Differences between the Atlantic and Pacific experiences. In Proceedings of the 10th International Aquatic Nuisance Species and Zebra Mussel Conference, Toronto, ON, Canada, 13–17 February 2000; pp. 307–312. [Google Scholar]
- Kelley, A.; De Rivera, C.E.; Buckley, B.A. Intraspecific variation in thermotolerance and morphology of the invasive European green crab, Carcinus maenas, on the west coast of North America. J. Exp. Mar. Biol. Ecol. 2011, 409, 70–78. [Google Scholar] [CrossRef]
- deRivera, C.E.; Ruiz, G.M.; Hines, A.H.; Jivoff, P. Biotic resistance to invasion: Native predator limits abundance and distribution of an introduced crab. Ecology 2005, 86, 3364–3376. [Google Scholar] [CrossRef]
- Smith, S.I. The stalk-eyed crustaceans of the Atlantic coast of North America north of Cape Cod. Trans. Conn. Acad. Sci. 1879, 5, 27–138. [Google Scholar]
- Rathbun, M.J. Fauna of New England. Occas. Pap. Boston Soc. Nat. Hist. 1905, 7, 1–117. [Google Scholar]
- Rathbun, M.J. The cancroid crabs of America of the families Euryalidae, Portunidae, Atelecyclidae, Cancridae and Xanthidae. Bull. USA Natl. Mus. 1930, 152, 1–609. [Google Scholar] [CrossRef]
- Glude, J.B. The effects of temperature and predators on the abundance of the soft-shell clam, Mya arenaria, in New England. Trans. Am. Fish. Soc. 1955, 84, 13–26. [Google Scholar] [CrossRef]
- Roman, J. Diluting the founder effect: Cryptic invasions expand a marine invader’s range. Proc. R. Soc. B Biol. Sci. 2006, 273, 2453–2459. [Google Scholar] [CrossRef] [Green Version]
- Blakeslee, A.M.H.; McKenzie, C.H.; Darling, J.A.; Byers, J.E.; Pringle, J.M.; Roman, J. A hitchhiker’s guide to the Maritimes: Anthropogenic transport facilitates long-distance dispersal of a marine crab to Newfoundland. Divers. Distrb. 2010, 16, 879–891. [Google Scholar] [CrossRef]
- Blakeslee, A.M.H. Northwest Atlantic population structure and gene flow in the green crab: Current understanding of a dynamic invasion front, population admixture, continued anthropogenic expansion. In Green Crab Summit Presentation; University of Maine: Orono, ME, USA, 2013; Available online: http://www.seagrant.umaine.edu/green-crab-summit (accessed on 18 July 2018).
- Kanary, L.; Musgrave, J.; Tyson, R.C.; Locke, A.; Lutscher, F. Modelling the dynamics of invasion and control of competing green crab genotypes. Theor. Ecol. 2014, 7, 391–406. [Google Scholar] [CrossRef]
- Lehnert, S.J.; Lehnert, S.J.; DiBacco, C.; Jeffery, N.W.; Blakeslee, A.M.; Isaksson, J.; Roman, J.; Wringe, B.F.; Stanley, R.R.; Matheson, K.; et al. Temporal dynamics of genetic clines of invasive European green crab (Carcinus maenas) in eastern North America. Evol. Appl. 2018, 11, 1656–1670. [Google Scholar] [CrossRef]
- Behrens Yamada, S.; Hunt, C.; Richmond, N. The Arrival of the European Green Crab, Carcinus maenas, in Oregon Estuaries. In Proceedings of the First National Conference on Marine Bioinvasions, Cambridge, MA, USA, 24–27 January 1999; Pederson, J., Ed.; MIT Press: Boston, MA, USA, 2000; pp. 24–34. [Google Scholar]
- Behrens Yamada, S.; Kosro, P.M. Linking ocean conditions to year class strength of the invasive European green crab, Carcinus maenas. Biol. Invasions 2010, 12, 1791–1804. [Google Scholar] [CrossRef]
- McGaw, I.J.; Edgell, T.C.; Kaiser, M.J. Population demographics of native and newly invasive populations of the green crab Carcinus maenas. Mar. Ecol. Prog. Ser. 2011, 430, 235–240. [Google Scholar] [CrossRef]
- Behrens Yamada, S.; Thomson, R.E.; Gillespie, G.E.; Norgard, T.C. Lifting barriers to range expansion: The European green crab Carcinus maenas (Linnaeus, 1758) enters the Salish Sea. J. Shellfish Res. 2017, 36, 201–208. [Google Scholar] [CrossRef]
- Grason, E.; Symer, K.; Martin, K. European Green Crab in Puget Sound. University of Washington Puget Sound Institute and Washington Sea Grant Crab Team. 2018. Available online: https://uw.maps.arcgis.com/apps/Cascade/index.html?appid=807fd68b00ab4f99be1cfc4b87bd5a4f (accessed on 22 July 2019).
- Bagley, M.J. Microsatellite DNA analysis of native and invading populations of European green crabs. In Proceedings of the First National Conference on Marine Bioinvasions, Cambridge, MA, USA, 24–27 January 1999; Pederson, J., Ed.; MIT Press: Boston, MA, USA, 2000; pp. 241–243. [Google Scholar]
- Carlton, J.T. Man’s role in changing the face of the ocean: Biological invasions and implications for conservation of near-shore environments. Conserv. Biol. 1989, 3, 265–273. [Google Scholar] [CrossRef]
- Carlton, J.T. Introduced marine and estuarine mollusks of North America: An end-of-the-20th-century perspective. J. Shellfish Res. 1992, 11, 489–505. [Google Scholar]
- Yamada, S.B.; Peterson, W.T.; Kosro, P.M. Biological and physical ocean indicators predict the success of an invasive crab, Carcinus maenas, in the northern California Current. Mar. Ecol. Prog. Ser. 2015, 537, 175–189. [Google Scholar] [CrossRef]
- Gillespie, G.E.; Norgard, T.C.; Anderson, E.D.; Haggarty, D.R.; Phillips, A.C. Distribution and biological characteristics of European green crab, Carcinus maenas, in British Columbia. 2006–2013. In Canadian Technical Report of Fisheries and Aquatic Science 3120; Fisheries and Oceans Canada, Science Branch, Pacific Region, Pacific Biological Station: Nanaimo, BC, Canada, 2015. [Google Scholar]
- Jamieson, G.S.; Grosholz, E.D.; Armstrong, D.A.; Elner, R.W. Potential ecological implication for the introduction of the European green crab, Carcinus maenas (Linnaeus), to British Columbia, Canada, and Washington, USA. J. Nat. Hist. 1998, 32, 1587–1598. [Google Scholar] [CrossRef]
- Mach, M.E.; Chan, K.M. Trading Green Backs for Green Crabs: Evaluating the Commercial Shellfish Harvest at Risk to European Green Crab Invasion. F1000Res 2013, 2. [Google Scholar] [CrossRef]
- Colnar, A.M.; Landis, W.G. Conceptual model development for invasive species and a regional risk assessment case study: The European green crab, Carcinus maenas, at Cherry Point, Washington, USA. Hum. Ecol. Risk Assess. 2007, 13, 120–155. [Google Scholar] [CrossRef]
- Darling, J.A. Interspecific hybridization and mitochondrial introgression in invasive Carcinus shore crabs. PLoS ONE 2011, 6, e17828. [Google Scholar] [CrossRef] [Green Version]
- Joska, M.A.P.; Branch, G.M. The European shore-crab—Another alien invader? Afr. Wildl. 1986, 40, 63–65. [Google Scholar]
- Le Roux, P.J.; Branch, G.M.; Joska, A.P. On the distribution, diet and possible impact of the invasive European shore crab Carcinus maenas (L.) along the South African coast. S. Afr. J. Mar. Sci. 1990, 9, 85–93. [Google Scholar] [CrossRef]
- Griffiths, C.L.; Hockey, P.A.R.; van Erkom Schuink, C.; Le Roux, P.J. Marine invasive aliens on South African shores: Implications for community structure and trophic functioning. S. Afr. J. Mar. Sci. 1992, 12, 713–722. [Google Scholar] [CrossRef]
- Robinson, T.B.; Griffiths, C.L.; McQuaid, C.D.; Rius, M. Marine alien species of South Africa—Status and impacts. Afr. J. Mar. Sci. 2005, 27, 297–306. [Google Scholar] [CrossRef]
- Griffiths, C.; Robinson, T.; Mead, A. The alien and cryptogenic marine crustaceans of South Africa. In In the Wrong Place—Alien Marine Crustaceans: Distribution, Biology and Impacts. Invading Nature—Springer Series in Invasion Ecology Vol. 6; Galil, B.S., Clark, P.F., Carlton, J.T., Eds.; Springer: New York, NY, USA, 2011; pp. 269–282. [Google Scholar]
- Fulton, S.W.; Grant, F.E. Note on the occurrence of the European crab, Carcinus maenas, Leach, in Port Phillip. Vic. Nat. 1900, 17, 147–148. [Google Scholar]
- Fulton, S.W.; Grant, F.E. Some little known decapod crustacean with a description of a new species. Proc. R. Soc. Vic. 1902, 14, 55–64. [Google Scholar]
- Zeidler, W. Note on the occurrence of the European shore crab Carcinus maenas (Linn., 1758) in Australia. S. Aust. Nat. 1978, 53, 11–12. [Google Scholar]
- Rosenzweig, P.A. A range extension for the European shore crab Carcinus maenas (Linn., 1758) in South Australia. S. Aust. Nat. 1984, 59, 18–19. [Google Scholar]
- Gardner, N.C.; Kwa; Paturusi, A. First recording of the European shore crab Carcinus maenas in Tasmania. Tasman. Nat. J. 1994, 116, 26–28. [Google Scholar]
- Furlani, D.M. A Guide to the Introduced Marine Species in Australian Waters. In Centre for Research on Introduced Marine Pests; Report No. 5.; CSIRO Division of Fisheries; Centre for Research on Introduced Marine Pests, CSIRO Publishing: Victoria, Australia, 1996. [Google Scholar]
- Proctor, C.; Thresher, R. The invasive history and abundance of C. maenas in Australia. In Proceedings of the First International Workshop on the Demography, Impacts and Management of Introduced Populations of the European crab, Carcinus maenas, Hobart, Australia, 20–21 March 1997; CRIMP Technical Report 11. Thresher, R.E., Ed.; CSIRO: Hobart, TAS, Australia, 1997; pp. 31–33. [Google Scholar]
- Thresher, R.; Proctor, C.; Ruiz, G.; Gurney, R.; MacKinnon, C. Invasion dynamics of the European shore crab, Carcinus maenas, in Australia. Mar. Biol. 2003, 142, 867–876. [Google Scholar] [CrossRef]
- Sinclair, M.A. Interactions between native grapsid crabs and Carcinus maenas in Victoria. In Proceedings of the First International Workshop on the Demography, Impacts and Management of Introduced Populations of the European Crab, Carcinus maenas, Hobart, Australia, 20–21 March 1997; Thresher, R.E., Ed.; Technical Report 11. Centre for Research on Introduced Marine Pests: Hobart, TAS, Australia, 1997; pp. 35–41. [Google Scholar]
- Ahyong, S.T.; Wilkens, S.L. Aliens in the Antipodes: Non-indigenous marine crustaceans of New Zealand and Australia. In In the Wrong Place-Alien Marine Crustaceans: Distribution, Biology and Impacts; Springer: Berlin/Heidelberg, Germany, 2011; pp. 451–485. [Google Scholar]
- Ross, D.; Johnson, C.; Hewitt, C.; Ruiz, G.M. Interaction and impacts of two introduced species on a soft-sediment marine assemblage in SE Tasmania. Mar. Biol. 2004, 144, 747–756. [Google Scholar] [CrossRef]
- Ahyong, S.T. Range extension of two invasive crab species in eastern Australia: Carcinus maenas (Linnaeus) and Pyromaia tuberculata (Lockington). Mar. Pollut. Bull. 2005, 50, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, P.; Vander-Velde, J.; Keable, S. Baseline survey of the benthic macrofauna of Twofold Bay, N.S.W., with a discussion of the marine species introduced to the bay. Proc. Linn. Soc. NSW 1989, 110, 339–367. [Google Scholar]
- Vinuesa, J.H. Molt and reproduction of the European green crab Carcinus maenas (Decapoda: Portunidae) in Patagonia, Argentina. Revista de Biología Trop. 2007, 55 (Suppl. 1), 49–54. [Google Scholar] [CrossRef]
- Vinuesa, J.H. Distribucion de crustaceos decapodos y estomatopodos del golfo San Jorge, Argentina. Revista de biología marina y oceanografía 2005, 40, 7–21. [Google Scholar] [CrossRef]
- Furota, T.S.; Watanabe, T.; Watanabe, S.; Akiyama, S.; Kinasita, K. Life history of the Mediterranean green crab, Carcinus aestuarii Nardo, in Tokyo Bay, Japan. Crustac. Res. 1999, 28, 5–15. [Google Scholar] [CrossRef] [Green Version]
- deRivera, C.E.; Gray Hitchcock, N.; Teck, S.J.; Steves, B.P.; Hines, A.H.; Ruiz, G.M. Larval development rate predicts range expansion of an introduced crab. Mar. Biol. 2006, 10, 1275–1288. [Google Scholar] [CrossRef]
- Compton, T.J.; Leathwick, J.R.; Inglis, G.J. Thermogeography predicts the potential global range of the invasive European green crab (Carcinus maenas). Divers. Distrib. 2010, 16, 243–255. [Google Scholar] [CrossRef]
- Carlton, J.T. The global dispersal of marine and estuarine crustaceans. In In the Wrong Place—Alien Marine Crustaceans: Distribution, Biology and Impacts; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–23. [Google Scholar]
- Bulnheim, H.P.; Bahns, S. Genetic variation and divergence in the genus Carcinus (Crustacea, Decapoda). Internationale Revue der gesamten Hydrobiologie und Hydrographie 1996, 81, 611–619. [Google Scholar] [CrossRef]
- Geller, J.; Walton, E.D.; Grosholz, E.D.; Ruiz, G.M. Cryptic invasions of the crab Carcinus detected by molecular phylogeography. Mol. Ecol. 1997, 6, 901–906. [Google Scholar] [CrossRef]
- Pringle, J.M.; Blakeslee, A.M.; Byers, J.E.; Roman, J. Asymmetric dispersal allows an upstream region to control population structure throughout a species’ range. Proc. Natl. Acad. Sci. USA 2011, 108, 15288–15293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascoal, S.; Creer, S.; Taylor, M.I.; Queiroga, H.; Carvalho, G.; Mendo, S. Development and application of microsatellites in Carcinus maenas: Genetic differentiation between northern and central Portuguese populations. PLoS ONE 2009, 4, e7268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tepolt, C.K.; Bagley, M.J.; Geller, J.B.; Blum, M.J. Characterization of microsatellite loci in the European green crab (Carcinus maenas). Mol. Ecol. Notes 2006, 6, 343–345. [Google Scholar] [CrossRef]
- Cosham, J.; Beazley, K.F.; McCarthy, C. Environmental factors influencing local distributions of European green crab (Carcinus maenas) for modeling and management applications. Environ. Rev. 2016, 24, 244–252. [Google Scholar] [CrossRef] [Green Version]
- Hampton, S.L.; Griffiths, C.L. Why Carcinus maenas cannot get a grip on South Africa’s wave-exposed coastline. Afr. J. Mar. Sci. 2007, 29, 123–126. [Google Scholar] [CrossRef]
- Breteler, W.C.M. Settlement, growth and production of the shore crab, Carcinus maenas, on tidal flats in the Dutch Wadden Sea. Neth. J. Sea Res. 1976, 10, 354–376. [Google Scholar] [CrossRef]
- Eriksson, S.; Evans, S.; Tallmark, B. On the coexistence of 0-group Carcinus maenas (L.) from a shallow sandy bottom in Gullmar Fjord, Sweden. Zoon 1975, 3, 65–70. [Google Scholar]
- Eriksson, S.; Edlund, A.-M. On the ecological energetics of 0-group Carcinus maenas (L.) from a shallow sandy bottom in Gullmar Fjord, Sweden. J. Exp. Mar. Biol. Ecol. 1977, 30, 233–248. [Google Scholar] [CrossRef]
- Pihl, L.; Rosenberg, R. Production, abundance, and biomass of mobile epibenthic marine fauna in shallow waters, western Sweden. J. Exp. Mar. Biol. Ecol. 1982, 57, 273–301. [Google Scholar] [CrossRef]
- Ropes, J.W. The food habits of five crab species at Pettaquamscutt River, Rhode Island. Fish. Bull. 1989, 87, 197–204. [Google Scholar]
- Zeng, C.; Naylor, E.; Abelló, P. Endogenous control of timing of metamorphosis in megalopae of the shore crab Carcinus maenas. Mar. Biol. 1997, 128, 299–305. [Google Scholar] [CrossRef]
- Moksnes, P.-O.; Pihl, L.; van Montirans, J. Predation on postlarvae and juveniles of the shore crab Carcinus maenas: Importance of shelter, size and cannibalism. Mar. Ecol. Prog. Ser. 1998, 166, 211–225. [Google Scholar] [CrossRef] [Green Version]
- Hedvall, O.; Moksnes, P.-O.; Pihl, L. Active habitat selection by megalopae and juvenile shore crabs Carcinus maenas: A laboratory study in an annular flume. Hydrobiologia 1998, 375, 89–100. [Google Scholar] [CrossRef]
- Amaral, V.; Paula, J. Carcinus maenas (Crustacea: Brachyura): Influence of artificial substrate type and patchiness on estimation of megalopae settlement. J. Exp. Mar. Biol. Ecol. 2007, 346, 21–27. [Google Scholar] [CrossRef]
- Almeida, M.J.; Flores, A.A.V.; Queiroga, H. Effect of crab size and habitat type on the locomotory activity of juvenile shore crabs, Carcinus maenas. Estuar. Coast. Shelf Sci. 2008, 80, 509–516. [Google Scholar] [CrossRef]
- Almeida, M.J.; Gonzalez-Gordillo, J.I.; Flores, A.A.V.; Queiroga, H. Cannibalism, post-settlement growth rate and size refuge in a recruitment-limited population of the shore crab Carcinus maenas. J. Exp. Mar. Biol. Ecol. 2011, 410, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Gehrels, H.; Knysh, K.M.; Boudreau, M.; Thériault, M.H.; Courtenay, S.C.; Cox, R.; Quijón, P.A. Hide and seek: Habitat-mediated interactions between European green crabs and native mud crabs in Atlantic Canada. Mar. Biol. 2016, 163, 152. [Google Scholar] [CrossRef]
- Todd, P.A.; Briers, R.A.; Ladle, R.J.; Middleton, F. Phenotype-environment matching in the shore crab (Carcinus maenas). Mar. Biol. 2006, 148, 1357–1367. [Google Scholar] [CrossRef]
- Price, N.; Green, S.; Troscianko, J.; Tregenza, T.; Stevens, M. Background matching and disruptive coloration as habitat-specific strategies for camouflage. Sci. Rep. 2019, 9, 7840. [Google Scholar] [CrossRef]
- Thiel, M.; Dernedde, T. Recruitment of shore crabs Carcinus maenas on tidal flats: Mussel clumps as an important refuge for juveniles. Helgoländer Meeresunters. 1994, 48, 321–332. [Google Scholar] [CrossRef] [Green Version]
- Amaral, V.; Cabral, H.N.; Jenkins, S.; Hawkins, S.; Paula, J. Comparing quality of estuarine and nearshore intertidal habitats for Carcinus maenas. Estuar. Coast. Shelf Sci. 2009, 83, 219–226. [Google Scholar] [CrossRef]
- Bessa, F.; Baeta, A.; Martinho, F.; Marques, S.; Pardal, M.A. Seasonal and temporal variations in population dynamics of the Carcinus maenas (L.): The effect of an extreme drought event in a southern European estuary. J. Mar. Biol. Assoc. UK 2010, 90, 867–876. [Google Scholar] [CrossRef]
- Ropes, J.W. The feeding habits of the green crab, Carcinus maenas (L.). Fish. Bull. 1968, 67, 183–203. [Google Scholar]
- Young, A.M.; Elliott, J.A.; Incatasciato, J.M.; Taylor, M.L. Seasonal catch, size, color, and assessment of trapping variables for the European green crab Carcinus maenas (Linnaeus, 1758) (Brachyura: Portunoidea: Carcinidae), a nonindigenous species in Massachusetts, USA. J. Crustac. Biol. 2017, 37, 556–570. [Google Scholar] [CrossRef]
- Jensen, G.C.; McDonald, P.S.; Armstrong, D.A. East meets west: Competitive interactions between green crab Carcinus maenas, and native and introduced shore crab Hemigrapsus spp. Mar. Ecol. Prog. Ser. 2002, 225, 251–262. [Google Scholar] [CrossRef]
- Ameyaw-Akumfi, C.; Naylor, E. Spontaneous and induced components of salinity preference behavior in Carcinus maenas. Mar. Ecol. Prog. Ser. 1987, 37, 153–158. [Google Scholar] [CrossRef]
- Singh, R. Natural Diet of, and Shelter-Use by, the Green Crab, Carcinus maenas (L.) in Nova Scotia. Master’s Thesis, University of New Brunswick, Fredericton, NB, Canada, 1991. [Google Scholar]
- Yonge, C.M. The Sea Shore; Collins: London, UK, 1949. [Google Scholar]
- Dare, P.J.; Edwards, D.B. Underwater television observations on the intertidal movements of shore crabs, Carcinus maenas, across a mudflat. J. Mar. Biol. Assoc. UK 1981, 61, 107–116. [Google Scholar] [CrossRef]
- Green, J. The Biology of Estuarine Animals; Sidgwick and Jackson: London, UK, 1968. [Google Scholar]
- Ingle, R.W. British Crabs; Oxford University Press and British Museum: London, UK, 1980. [Google Scholar]
- Ingle, R.W. Shallow-Water Crabs Synopsis of the British Fauna; Cambridge University Press and the Estuarine and Brackish-water Science Association: Cambridge, UK, 1983. [Google Scholar]
- Elner, R.W. Diet of green crab Carcinus maenas (L.) from Port Hebert, southwestern Nova Scotia. J. Shellfish Res. 1981, 1, 89–94. [Google Scholar]
- Clark, P.F. North-East Atlantic Crabs: An Atlas of Distribution; Marine Conservation Society: Ross-on-Wye, UK, 1986. [Google Scholar]
- Novak, M. Diurnal activity in a group of Gulf of Maine decapods. Crustaceana 2004, 77, 603–620. [Google Scholar] [CrossRef] [Green Version]
- Himes, A.R.; Balschi, W.S.; Pelletier, G.; Frederich, M. Color phase—Specific ion regulation of the European green crab Carcinus maenas in an oscillating salinity environment. J. Shellfish Res. 2017, 36, 465–479. [Google Scholar] [CrossRef]
- MacDonald, A.J.; Kienzle, H.M.; Drolet, D.; Hamilton, D.J. Distribution and habitat use of the invasive Carcinus maenas L. (European green crab) and the native Cancer irroratus (Say) (rock crab) in intertidal zones in the Upper Bay of Fundy, Canada. Northeast. Nat. 2018, 25, 161–180. [Google Scholar] [CrossRef]
- Quinn, B.K. Dramatic decline and limited recovery of a green crab (Carcinus maenas) population in the Minas Basin, Canada after the summer of 2013. PeerJ 2018, 6, e5566. [Google Scholar] [CrossRef] [PubMed]
- Edgell, C.; Hollander, J. The evolutionary ecology of European green crab, Carcinus maenas, in North America. In In the Wrong Place—Alien Marine Crustaceans: Distribution, Biology and Impacts. Invading Nature – Springer Series in Invasion Ecology, Vol. 6; Galil, B.S., Clark, P.F., Carlton, J.T., Eds.; Springer: New York, NY, USA, 2011; pp. 641–659. [Google Scholar]
- Tepolt, C.K.; Somero, G.N. Master of all trades: Thermal acclimation and adaptation of cardiac function in a broadly distributed marine invasive species, the European green crab, Carcinus maenas. J. Exp. Biol. 2014, 217, 1129–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahsanullah, M.; Newell, R.C. The effects of humidity and temperature on water loss in Carcinus maenas (L) and Portunus marmoreus (Leach). Comp. Biochem. Physiol. 1977, 56, 593–601. [Google Scholar] [CrossRef]
- Madeira, D.; Narciso, L.; Cabral, H.N.; Vinagre, C.; Diniz, M.S. HSP70 production patterns in coastal and estuarine organisms facing increasing temperatures. J. Sea Res. 2012, 73, 137–147. [Google Scholar] [CrossRef]
- Cuculescu, M.; Hyde, D.; Bowler, K. Thermal tolerance of two species of marine crab, Cancer pagurus and Carcinus maenas. J. Therm. Biol. 1988, 23, 107–110. [Google Scholar] [CrossRef]
- Crisp, D.J. (Ed.) The effects of the severe winter of 1962-63 on marine life in Britain. J. Anim. Ecol. 1964, 33, 165–210. [Google Scholar] [CrossRef]
- Beukema, J.J. The abundance of shore crabs Carcinus maenas (L.) on a tidal flat in the Wadden Sea after cold and mild winters. J. Exp. Mar. Biol. Ecol. 1991, 153, 97–113. [Google Scholar] [CrossRef]
- Dexter, R.W. The marine communities of a tidal inlet at Cape Ann, Massachusetts: A study in bio-ecology. Ecol. Monogr. 1947, 17, 261–294. [Google Scholar] [CrossRef]
- Kienzle, H. Population Dynamics and Interactions between Invasive Green Crabs (Carcinus maenas) and Native Rock Crabs (Cancer irroratus) in Intertidal Zones in the Upper Bay of Fundy. Bachelor’s Thesis, Mount Allison University, Sackville, NB, Canada, 2015; p. 41. [Google Scholar]
- Kelley, A.; De Riviera, C.E.; Buckley, B.A. Cold tolerance of the invasive Carcinus maenas in the east Pacific: Molecular mechanisms and implications for range expansion in a changing climate. Biol. Invasions 2013, 15, 2299–2309. [Google Scholar] [CrossRef]
- Naylor, E. Biological effects of heat effluent in the docks at Swansea. Proc. Zool. Soc. Lond. 1965, 144, 253–268. [Google Scholar] [CrossRef]
- Berrill, M. The life cycle of the green crab Carcinus maenas at the northern end of its range. J. Crustac. Biol. 1982, 2, 31–39. [Google Scholar] [CrossRef]
- McGaw, I.J.; Naylor, E. Distribution and rhythmic locomotor patterns of estuarine and open shore populations of Carcinus maenas. J. Mar. Biol. Assoc. UK 1992, 72, 599–609. [Google Scholar] [CrossRef]
- Atkinson, R.J.A.; Parsons, A.J. Seasonal patterns of migration and locomotion rhythmicity in populations of Carcinus. Neth. J. Sea Res. 1973, 7, 81–93. [Google Scholar] [CrossRef]
- Dries, M.; Adelung, D. Die schlei, ein modell für die verbreitung der strandkrabbe Carcinus maenas. Helgoländer Meeresunter. 1982, 35, 65. [Google Scholar] [CrossRef] [Green Version]
- Hunter, E.; Naylor, E. Intertidal migration by the shore crab Carcinus maenas. Mar. Ecol. Prog. Ser. 1993, 101, 131–138. [Google Scholar] [CrossRef]
- Warman, C.G.; Reid, D.G.; Naylor, E. Variation in the tidal migratory behavior and rhythmic light-responsiveness in the shore crab, Carcinus maenas. J. Mar. Biol. Assoc. UK 1993, 73, 355–364. [Google Scholar] [CrossRef]
- Aagaard, A.; Warman, C.G.; Depledge, M.H. Tidal and seasonal changes in the temporal and spatial distribution of foraging Carcinus maenas in the weakly tidal littoral zone of Kerteminde Fjord, Denmark. Mar. Ecol. Prog. Ser. 1995, 122, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Reid, D.; Abelló, P.; Kaiser, M.; Warman, C. Carapace colour, inter-moult duration and the behavioural and physiological ecology of the shore crab Carcinus maenas. Estuar. Coast. Shelf Sci. 1997, 44, 203–211. [Google Scholar] [CrossRef]
- Audet, D.; Miron, G.; Morivasu, M. Biological characteristics of a newly established green crab population in the southern Gulf of St. Lawrence, Canada. J. Shellfish Res. 2008, 27, 427–441. [Google Scholar] [CrossRef]
- Dawirs, R.R.; Pueschel, C.; Schorn, F. Temperature and growth in Carcinus maenas L. (Decapoda: Portunidae) larvae reared in the laboratory from hatching through metamorphosis. J. Exp. Mar. Biol. Ecol. 1986, 100, 47–74. [Google Scholar] [CrossRef]
- McKnight, A.; Mathews, L.M.; Avery, R.; Lee, K.T. Distribution is correlated with color phase in green crabs, Carcinus maenas (Linnaeus, 1758) in southern New England. Crustaceana 2000, 73, 763–768. [Google Scholar]
- Baeta, A.; Cabral, H.N.; Neto, J.M.; Marques, J.C. Biology, population dynamics and secondary production of the green crab Carcinus maenas (L.) in a temperate estuary. Estuar. Coast. Shelf Sci. 2005, 65, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Dawirs, R.R. Temperature and larval development of Carcinus maenas (Decapoda) in the laboratory; predictions of larval dynamics in the sea. Mar. Ecol. Prog. Ser. 1985, 24, 297–302. [Google Scholar] [CrossRef]
- Nagaraj, M. Combined effects of temperature and salinity on the zoeal development of the green crab, Carcinus maenas (Linnaeus, 1758) (Decapoda: Portunidae). Sci. Mar. 1993, 57, 1–8. [Google Scholar]
- Henry, R.P.; Garrelts, E.E.; McCarty, M.M.; Towle, D.W. Salinity adaptations in the euryhaline green crab, Carcinus maenas. Bull. Mt. Desert Isl. Biol. Lab. 1999, 38, 55. [Google Scholar]
- Perkins, E.J.; Gribbon, E.; Murray, R.B. Some aspects of the biology of Carcinus maenas (L.) II. survival at low salinity. Trans. Dumfries. Galloway Nat. Hist. Antiqu. Soc. 1969, 46, 27–28. [Google Scholar]
- Taylor, A.C. The respiratory responses of Carcinus maenas (L.) to changes in environmental salinity. J. Exp. Mar. Biol. Ecol. 1977, 29, 197–210. [Google Scholar] [CrossRef]
- McGaw, I.J. Behavioural Responses of the Shore Crab Carcinus maenas to Salinity Variation. Ph.D. Thesis, University of Wales, Bangor, UK, 1991. [Google Scholar]
- McGaw, I.; Naylor, E. Salinity preference of the shore crab Carcinus maenas in relation to coloration during intermoult and to prior acclimation. J. Exp. Mar. Biol. Ecol. 1992, 155, 145–159. [Google Scholar] [CrossRef]
- Reid, D.; Abelló, P.; McGaw, I.J.; Naylor, E. Differential tolerances of desiccation and hypo-osmotic stress in sub- and inter-tidal Carcinus maenas. In Phenotypic Responses and Individuality in Aquatic Ectotherms; Aldrich, J.C., Ed.; Japaga Publishers: Wicklow, Ireland, 1989; pp. 89–96. [Google Scholar]
- Lee, K.T.; McKnight, A.; Kellogg, K.; Juanes, F. Salinity tolerance in color phases of female green crabs Carcinus maenas (Linnaeus, 1758). Crustaceana 2003, 76, 247–253. [Google Scholar]
- Styrishave, B.; Andersen, O. Seasonal variations in hepatopancreas fatty acid profiles of two colour forms of shore crabs, Carcinus maenas. Mar. Biol. 2000, 137, 415–422. [Google Scholar] [CrossRef]
- Thomas, N.J.; Lasiak, T.A.; Naylor, E. Salinity preference behavior in Carcinus. Mar. Behav. Physiol. 1981, 7, 277–282. [Google Scholar] [CrossRef]
- McGaw, I.J.; Kaiser, M.J.; Naylor, E.; Hughes, R.N. Intraspecific morphological variation related to the moult-cycle in colour forms of the shore crab Carcinus maenas. J. Zool. Lond. 1992, 228, 351–359. [Google Scholar] [CrossRef]
- Anger, K.; Spivak, E.; Luppi, T. Effects of reduced salinities on development and bioenergetics of early larval shore crab, Carcinus maenas. J. Exp. Mar. Biol. Ecol. 1998, 220, 287–304. [Google Scholar] [CrossRef] [Green Version]
- Lance, J. The salinity tolerance of some estuarine planktonic crustaceans. Biol. Bull. 1964, 127, 108–118. [Google Scholar] [CrossRef]
- Darbyson, E.A. Local Vectors of Spread of the Green Crab (Carcinus maenas) and the Clubbed Tunicate (Styela Clava) in the Southern Gulf of St. Lawrence, Canada. Master’s Thesis, University of Halifax, Halifax, NS, Canada, 2006. [Google Scholar]
- Bohn, G. Sur le renversement du courant respiratoire chez les Décapodes. CR. Acad. Sci. 1897, 125, 539–542. [Google Scholar]
- Reid, D.G.; Aldrich, J.C. Variation in responses to environmental hypoxia of different colour forms of the shore crab Carcinus maenas (L.). Comp. Biochem. Physiol. 1989, 92, 535–539. [Google Scholar] [CrossRef]
- Taylor, A.C.; Butler, P.J. The behavior and physiological responses of the shore crab Carcinus maenas during changes in environmental oxygen tension. Neth. J. Sea Res. 1973, 7, 496–505. [Google Scholar] [CrossRef]
- Aldrich, J.C. The influence of individual variations in metabolic rate and tidal conditions on the response to hypoxia in Carcinus maenas (L.). Comp. Biochem. Physiol. 1986, 83, 53–60. [Google Scholar] [CrossRef]
- Taylor, A.C. The respiratory responses of Carcinus maenas to declining oxygen tension. J. Exp. Biol. 1976, 65, 309–322. [Google Scholar]
- Jungblut, S.; Boos, K.; McCarthy, M.L.; Saborowski, R.; Hagen, W. Invasive versus native brachyuran crabs in a European rocky intertidal: Respiratory performance and energy expenditures. Mar. Biol. 2018, 165, 54. [Google Scholar] [CrossRef] [Green Version]
- McMahon, B.R. Physiological responses to oxygen depletion in intertidal animals. Am. Zool. 1988, 28, 39–53. [Google Scholar] [CrossRef]
- Souza, A.T.; Ilarri, M.I.; Campos, J.; Marques, J.C.; Martins, I. Differences in the neighborhood: Structural variations in the carapace of shore crabs Carcinus maenas (Decapoda: Portunidae). Estuar. Coast. Shelf Sci. 2011, 95, 424–430. [Google Scholar] [CrossRef]
- Wolf, F. Red and green colour forms in the common shore crab Carcinus maenas (L.) (Crustacea: Brachyura: Portunidae): Theoretical predictions and empirical data. J. Nat. Hist. 1998, 32, 1807–1812. [Google Scholar] [CrossRef]
- Styrishave, B.; Rewitz, K.; Andersen, O. Frequency of moulting by shore crabs Carcinus maenas (L.) changes their colour and their success in mating and physiological performance. J. Exp. Mar. Biol. Ecol. 2004, 313, 317–336. [Google Scholar] [CrossRef]
- Styrishave, B.; Aagaard, A.; Andersen, O. In situ studies on physiology and behavior in two colour forms of the shore crab Carcinus maenas in relation to season. Mar. Ecol. Prog. Ser. 1999, 189, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Rewitz, K.; Styrishave, B.; Depledge, M.H.; Andersen, O. Spatial and temporal distribution of shore crabs Carcinus maenas in a small tidal estuary (Looe Estuary), Cornwall, England. J. Crustac. Biol. 2004, 24, 178–187. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, M.J.; Hughes, R.N.; Reid, D.G. Chelal morphometry, prey-size selection and aggressive competition in green and red forms of Carcinus maenas (L.). J. Exp. Mar. Biol. Ecol. 1990, 140, 121–134. [Google Scholar] [CrossRef]
- Lee, K.T.; Vespoli, J.L. Tracking color change in individual green crabs, Carcinus maenas (L.). Northeast. Nat. 2015, 22, 413–423. [Google Scholar] [CrossRef]
- Queiroga, H. An analysis of the size structure of Carcinus maenas (L.)(Decapoda, Brachyura) from Canal de Mira, Ria de Aveiro, Portugal. Biosis 1993, 1, 89–106. [Google Scholar]
- Poirier, L.A.; Mohan, J.; Speare, R.; Davidson, J.; Quijón, P.A.; St-Hilaire, S. Moulting synchrony in green crabs (Carcinus maenas) from Prince Edward Island, Canada. Mar. Biol. Res. 2016, 12, 969–977. [Google Scholar] [CrossRef]
- Fulton, B.A.; Fairchild, E.A.; Warner, R. Carcinus maenas in two New Hampshire estuaries. Part 2: Assessment of average intermolt period. J. Crustac. Biol. 2013, 33, 339–347. [Google Scholar] [CrossRef] [Green Version]
- McVean, A.; Findlay, I. The incidence of autotomy in an estuarine population of the crab Carcinus maenas. J. Mar. Biol. Assoc. UK 1979, 59, 341–354. [Google Scholar] [CrossRef]
- Bliss, D.E. Autotomy and regeneration. In Physiology of Crustacea, Vol. 1; Waterman, T.H., Ed.; Academic Press: New York, NY, USA, 1961; pp. 561–589. [Google Scholar]
- Sekkelsten, G.I. Effect of handicap on mating success in male shore crabs Carcinus maenas. Oikos 1988, 51, 131–134. [Google Scholar] [CrossRef]
- Brian, J.V. Inter-Population Variability in the Shore Crab (Carcinus maenas L.) and Its Potential Use as a Biomarker of Anthropogenic Effects. Ph.D. Thesis, Napier University, Edinburgh, UK, 2002. [Google Scholar]
- van der Meeren, G.I. Sex- and size-dependent mating tactics in a natural population of shore crabs Carcinus maenas. J. Anim. Ecol. 1994, 63, 307–314. [Google Scholar] [CrossRef]
- d’Acoz, C.D.U. Activités reproductrices saisonnieres des differentes classe de tailes d’une population de crabs verts Carcinus maenas (Linnaeus, 1758) dans le sud de la mer du Nord. Cah. Biol. Mar. 1993, 35, 1–13. [Google Scholar]
- van der Meeren, G.I.; Van der Meeren, C.I. Location of spawning shore crabs, Carcinus maenas (L., 1758) (Decapoda, Brachyura). Crustaceana 1992, 63, 92–94. [Google Scholar] [CrossRef]
- Williamson, H.C. On the larval and early young stages, and rate of growth, of the shore crab (Carcinus maenas Leach). Rep. Fish. Board. Scotl. 1903, 21, 136–139. [Google Scholar]
- Rasmussen, E. Behaviour of sacculinized shore crabs (Carcinus maenas Pennant). Nature 1959, 183, 479–480. [Google Scholar] [CrossRef]
- Wheatly, M.G. The provision of oxygen to developing eggs by female shore crabs (Carcinus maenas). J. Mar. Biol. Assoc. UK 1981, 61, 117–128. [Google Scholar] [CrossRef]
- Almaça, C. Note sur la biologie des populations de Carcinus maenas (L.) de la zone intertidale du Portugal occidental. Quad. Lab. Tecnol. Pesca 1982, 3, 179–185. [Google Scholar]
- Sprung, M. Larval abundance and recruitment of Carcinus maenas L. close to its southern geographic limit: A case of match and mismatch. In Advances in Decapod Crustacean Research; Springer: Berlin/Heidelberg, Germany, 2001; pp. 153–158. [Google Scholar]
- Queiroga, H.; Costlow, J.D.; Moreira, M.H. Larval abundance patterns of Carcinus maenas (Decapoda, Brachyura) in Canal de Mira (Ria de Aveiro, Portugal). Mar. Ecol. Prog. Ser. 1994, 111, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Best, K.; McKenzie, C.H.; Couturier, C. Reproductive biology of an invasive population of European green crab, Carcinus maenas, in Placentia Bay, Newfoundland. Manag. Biol. Invasions 2017, 8, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Sharp, G.; Semple, R.; Connolly, K.; Blok, R.; Audet, D.; Cairns, D.; Courtenay, S. Ecological assessment of the Basin Head Lagoon: A proposed Marine Protected Area. Can. Manuscr. Rep. Fish. Aquat. Sci. 2003, 2641, 76. [Google Scholar]
- Gillespie, G.E.; Phillips, A.C.; Paltzat, D.L.; Therriault, T.W. Status of the European green crab, Carcinus maenas, in British Columbia—2006. Can. Tech. Rep. Fish. Aquat. Sci. 2007, 2700, 39. [Google Scholar]
- Mabin, C.; Wilson, J.R.U.; Le Roux, J.J.; Robinson, T.B. Reassessing the invasion of South African waters by the European shore-crab Carcinus maenas. Afr. J. Mar. Sci. 2017, 39, 259–267. [Google Scholar] [CrossRef]
- Lyons, J.L.; O’Riordan, M.R.; Cross, F.T.; Culloty, C.S. Reproductive biology of the shore crab Carcinus maenas (Decapoda, Portunidae): A macroscopic and histological view. Invertebr. Reprod. Dev. 2012, 56, 144–156. [Google Scholar] [CrossRef]
- Hines, A.H.; Ruiz, G.M.; Hitchcock, N.G.; deRivera, C. Projecting Range Expansion of Invasive European Green Crabs (Carcinus maenas) to Alaska: Temperature and Salinity Tolerance of Larvae; Prince William Sound Regional Citizens’ Advisory Council: Anchorage, AK, USA, 2004; p. 22. [Google Scholar]
- Flores, A.A.V.; Gomes, C.C.; Villano, W.F. Source populations in coastal crabs: Parameters affecting egg production. Aquat. Biol. 2009, 7, 31–43. [Google Scholar] [CrossRef]
- Bate, C.S. On the development of Decapoda Crustacea. Philos. Trans. R. Soc. Lond. 1858, 148, 2–589. [Google Scholar]
- Griffen, B.D. Linking individual diet variation and fecundity in an omnivorous marine consumer. Oecologia 2014, 174, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Klein Breteler, W. Laboratory experiments on the influence of environmental factors on the frequency of moulting and the increase in size at moulting of juvenile shore crabs, Carcinus maenas. Neth. J. Sea Res. 1975, 9, 100–120. [Google Scholar] [CrossRef]
- Starr, M.; Himmelman, J.H.; Therriault, J.-C. Direct coupling of marine invertebrate spawning with phytoplankton blooms. Science 1990, 247, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Mohamedeen, H.; Hartnoll, R.G. Larval and postlarval growth of individually reared specimens of the common shore crab Carcinus maenas (L.). J. Exp. Mar. Biol. Ecol. 1989, 134, 1–24. [Google Scholar] [CrossRef]
- Epifanio, C.E.; Cohen, J.H. Behavioral adaptations in larvae of brachyuran crabs; a review. J. Exp. Mar. Biol. Ecol. 2016, 482, 85–105. [Google Scholar] [CrossRef]
- Rasmussen, E. Systematics and ecology of the Isefjord marine fauna (Denmark) with a survey of the eelgrass (Zostera) vegetation and its communities. Ophelia 1973, 11, 1–507. [Google Scholar] [CrossRef]
- Zeng, C.; Naylor, E. Occurrence in coastal water and endogenous tidal swimming rhythms of late megalopae of the shore crab Carcinus maenas: Implications for onshore recruitment. Mar. Ecol. Prog. Ser. 1966, 136, 69–79. [Google Scholar] [CrossRef]
- Queiroga, H. Processos de dispersão e recrutamento das larvas do caranguejo Carcinus maenas (L.) na Ria de Aveiro. Ph.D. Thesis, Universidade de Aveiro, Aveiro, Portugal, 1995. [Google Scholar]
- Queiroga, H. Distribution and drift of the crab Carcinus maenas (L.) (Decapoda, Portunidae) larvae over the continental shelf off northern Portugal in April 1991. J. Plank. Res. 1996, 18, 1981–2000. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.; Naylor, E. Endogenous tidal rhythms of vertical migration in field collected zoea-1 larvae of the shore crab Carcinus maenas: Implications for ebb tide offshore dispersal. Mar. Ecol. Prog. Ser. 1966, 132, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Crowe, T.P.; Underwood, A.J. Testing behavioural “preferences” for suitable microhabitat. J. Exp. Mar. Biol. Ecol. 1998, 225, 1–11. [Google Scholar] [CrossRef]
- Therriault, T.W.; Herborg, L.M.; Locke, A.; McKindsey, C.W. Risk Assessment for European Green Crab (Carcinus maenas) in Canadian Waters; Fisheries and Oceans Canada, Science Branch: Nanaimo, BC, Canada, 2008. [Google Scholar]
- Orton, J.H. Experiments in the sea on the rate of growth of some Crustacea Decapoda. J. Mar. Biol. Assoc. UK 1936, 20, 673–689. [Google Scholar] [CrossRef] [Green Version]
- Lützen, J. Growth, reproduction, and life span in Sacculina carcini Thompson (Cirripedia: Rhizocephala) in the Isefjord, Denmark. Sarsia 1984, 69, 91–105. [Google Scholar] [CrossRef]
- Behrens Yamada, S.; Dumbauld, B.R.; Kalin, A.; Hunt, C.E.; Figlar-Barnes, R.; Randall, A. Growth and persistence of a recent invader Carcinus maenas in estuaries of the northeastern Pacific. Biol. Invasions 2005, 7, 309–321. [Google Scholar] [CrossRef]
- Werner, M. Prevalence of the parasite Sacculina carcini Thompson 1836 (Crustacea, Rhizocephala) on its host crab Carcinus maenas (L.) on the west coast of Sweden. Ophelia 2001, 55, 101–110. [Google Scholar] [CrossRef]
- Munch-Petersen, S.; Sparre, P.; Hoffman, E. Abundance of the shore crab, Carcinus maenas (L) estimated from mark-recapture experiments. Dana 1982, 2, 97–121. [Google Scholar]
- Heath, J.R.; Barnes, H. Some changes in biochemical composition with season and during the moulting cycle of the common shore crab, Carcinus maenas (L.). J. Exp. Mar. Biol. Ecol. 1970, 5, 199–233. [Google Scholar] [CrossRef]
- Garm, A. Mechanosensory properyies of the mouthpart setae of the European shore crab Carcinus maenas. Mar. Biol. 2005, 147, 1179–1190. [Google Scholar] [CrossRef]
- Pedersen, K.L.; Pedersen, S.N.; Højrup, P.; Andersen, J.S.; Roepstorff, P.; Knudsen, J.; Depledge, M.H. Purification and characterization of a cadmium-induced metallothionein from the shore crab Carcinus maenas (L.). Biochem. J. 1994, 297, 609–614. [Google Scholar] [CrossRef] [Green Version]
- Sneddon, L.U.; Huntingford, F.A.; Taylor, A.C. Weapon size versus body size as a predictor of winning in fights between shore crabs, Carcinus maenas (L.). Behav. Ecol. Sociobiol. 1997, 41, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Abelló, P.; Aagaard, A.; Warman, C.G.; Depledge, M.H. Spatial variability in the population structure of the shore crab Carcinus maenas (Crustacea: Brachyura) in a shallow-water, weakly tidal fjord. Mar. Ecol. Prog. Ser. 1997, 147, 97–103. [Google Scholar] [CrossRef]
- McVean, A. The incidence of autotomy in Carcinus maenas (L.). J. Exp. Mar. Biol. Ecol. 1976, 24, 177–187. [Google Scholar] [CrossRef]
- Fletcher, N.; Hardege, J.D. The cost of conflict: Agonistic encounters influence responses to chemical signals in the European shore crab. Anim. Behav. 2009, 77, 357–361. [Google Scholar] [CrossRef]
- Walne, P.R.; Dean, G.J. Experiments on predation by the shore crab Carcinus maenas on Mytilus and Mercenaria. ICES J. Mar. Sci. 1972, 34, 190–199. [Google Scholar] [CrossRef]
- Elner, R.W. The mechanics of predation by the shore crab, Carcinus maenas (L.), on the edible mussel, Mytilus edulis L. Oecologia 1978, 36, 333–344. [Google Scholar] [CrossRef]
- Elner, R.W.; Hughes, R.N. Energy maximization in the diet of the shore crab, Carcinus maenas. J. Anim. Ecol. 1978, 47, 103–116. [Google Scholar] [CrossRef]
- Huxley, J.S.; Richards, O.W. Relative growth of the abdomen and the carapace of the shore-crab Carcinus maenas. J. Mar. Biol. Assoc. UK 1931, 17, 1001–1015. [Google Scholar] [CrossRef] [Green Version]
- Kamermans, P.; Blankendaal, M.; Perdon, J. Predation of shore crabs (Carcinus maenas (L.)) and starfish (Asterias rubens L.) on blue mussel (Mytilus edulis L.) seed from wild sources and spat collectors. Aquaculture 2009, 290, 256–262. [Google Scholar] [CrossRef]
- Stevens, M.; Lown, A.E.; Wood, L.E. Camouflage and individual variation in shore crabs (Carcinus maenas) from different habitats. PLoS ONE 2014, 9, e115586. [Google Scholar] [CrossRef]
- Spooner, E.H.; Coleman, R.A.; Attrill, M.J. Sex differences in body morphology and multitrophic interactions involving the foraging behavior of the crab Carcinus maenas. Mar. Ecol. 2007, 28, 394–403. [Google Scholar] [CrossRef]
- Tremblay, M.J.; Thompson, A.; Paul, K. Recent Trends in the Abundance of the Invasive Green Crab (Carcinus maenas) in Bras d’Or Lakes and Eastern Nova Scotia Based on Trap Surveys; Fisheries and Ocean Canada, Bedford Institute of Oceanography: Dartmouth, NS, Canada, 2006. [Google Scholar]
- Tummon Flynn, P.; Lynn, K.D.; Cairns, D.K.; Quijon, P.A. The role of the non-indigenous green crab (Carcinus maenas) in the decline of a unique strain of Irish moss (Chondrus crispus): Direct and indirect effects. ICES J. Mar. Sci. 2019. [Google Scholar] [CrossRef]
- Rayner, G. The Behavioural Interactions between the American Lobster (Homarus Americanus) and the Invasive Green Crab (Carcinus maenas). Ph.D. Thesis, Memorial University of Newfoundland, St. John’s, NL, Canada, 2018. [Google Scholar]
- Rayner, G.; McGaw, I.J. Effects of the invasive green crab (Carcinus maenas) on American lobster (Homarus americanus): Food acquisition and trapping behavior. J. Sea Res. 2019, 144, 95–104. [Google Scholar] [CrossRef]
- Aman, J.; Grimes, K.W. Measuring Impacts of Invasive European Green Crabs on Maine Salt Marshes: A Novel Approach. In Report to the Maine Outdoor Heritage Fund; Wells National Estuarine Research Reserve: Wells, ME, USA, 2016; p. 19. [Google Scholar]
- Jamieson, G.S.; Foreman, M.G.G.; Cherniawsky, J.Y.; Levings, C.D. European green crab (Carcinus maenas) dispersal: The Pacific experience. In Crabs in Cold Water Regions: Biology, Management and Economics; Paul, A.J., Dawe, E.G., Elner, R., Jamieson, G.S., Kruse, G.H., Otto, R.S., Sainte-Marie, B., Shirley, T.C., Woodby, D., Eds.; College Program AK-SG-02-01; University of Alaska Sea Grant: College, AK, USA, 2002; pp. 561–576. [Google Scholar]
- Kelley, A.L.; deRivera, C.E.; Grosholz, E.D.; Ruiz, G.M.; Behrens Yamada, S.; Gillespie, G. Thermogeographic variation in body size of Carcinus maenas, the European green crab. Mar. Biol. 2015, 162, 1625–1635. [Google Scholar] [CrossRef]
- DiBacco, C.; Therriault, T.W. Reproductive periodicity and larval vertical migration behavior of European green crab Carcinus maenas in a non-native habitat. Mar. Ecol. Prog. Ser. 2015, 536, 123–134. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Baron, P.J.; Orensanz, J.M. A prediction come true: The green crab invades the Patagonian coast. Biol. Invasions 2005, 7, 547–552. [Google Scholar] [CrossRef]
- Campbell, R.T.; Baring, R.J.; Dittmann, S. Cracking the cuisine: Invasive European shore crabs (Carcinus maenas) select a menu of soft-shelled mussels over cockles. J. Exp. Mar. Biol. Ecol. 2019, 517, 25–33. [Google Scholar] [CrossRef]
- Jensen, G.C.; McDonald, P.S.; Armstrong, D.A. Biotic resistance to green crab, Carcinus maenas, in California bays. Mar. Biol. 2007, 151, 2231–2243. [Google Scholar] [CrossRef]
- Behrens Yamada, S.; Gillespie, G.E. Will the European green crab (Carcinus maenas) persist in the Pacific Northwest? ICES J. Mar. Sci. 2008, 65, 725–729. [Google Scholar] [CrossRef] [Green Version]
- Grosholz, E.D.; Ruiz, G.M. Biological invasions drive size increases in marine and estuarine invertebrates. Ecol. Lett. 2003, 6, 700–705. [Google Scholar] [CrossRef]
- Bloch, C.P.; Curry, K.D.; Jahoda, J.C. Long-term effects of an invasive shore crab on Cape Cod, Massachusetts. Northeast. Nat. 2015, 22, 178–191. [Google Scholar] [CrossRef] [Green Version]
- Scherer, B.; Reise, K. Significant predation on micro- and macrobenthos by the crab Carcinus maenas L. in the Wadden Sea. Kiel Meeresforsch Sonderh 1981, 5, 490–500. [Google Scholar]
- Jackson, D.; Mason, C.F.; Long, S.P. Macro-invertebrate populations and production on a salt-marsh in east England dominated by Spartina anglica. Oecologia 1985, 65, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J. Effectiveness of crab and lobster traps. Can. J. Fish. Aquat. Sci. 1990, 47, 1228–1251. [Google Scholar] [CrossRef]
- Grosholz, E.D.; Ruiz, G.M.; Dean, C.A.; Shirley, K.A.; Maron, J.L.; Connors, P.G. The impacts of a nonindigenous marine predator in a California bay. Ecology 2000, 81, 1206–1224. [Google Scholar] [CrossRef]
- Miller, R.J. Saturation of crab traps: Reduced entry and escapement. ICES J. Mar. Sci. 1979, 38, 338–345. [Google Scholar] [CrossRef]
- Bergshoeff, J.A.; McKenzie, C.H.; Best, K.; Zargarpour, N.; Favaro, B. Using underwater video to evaluate the performance of the Fukui trap as a mitigation tool for the invasive European green crab (Carcinus maenas) in Newfoundland, Canada. PeerJ 2018, 6, e4223. [Google Scholar] [CrossRef] [Green Version]
- Bergshoeff, J.A.; McKenzie, C.H.; Favaro, B. Improving the efficiency of the Fukui trap as a capture tool for the invasive European green crab (Carcinus maenas) in Newfoundland, Canada. PeerJ 2019, 7, e6308. [Google Scholar] [CrossRef] [Green Version]
- Waser, A.M.; Dekker, R.; Witte, J.I.; McSweeney, N.; Ens, B.J.; van der Meer, J. Quantifying tidal movements of the shore crab Carcinus maenas on to complex epibenthic bivalve habitats. Estuaries Coasts 2018, 41, 507–520. [Google Scholar] [CrossRef] [Green Version]
- Edwards, E. Observations on growth of the edible crab Cancer pagurus. Rapports et Procés-Verbaux des Réunions 1965, 156, 62–70. [Google Scholar]
- Therriault, T. European green crab in British Columbia—Population and impact. In Proceedings of the Regional Advisory Process on Green Crab, Carcinus maenas, Populations and Mitigations in the Newfoundland and Labrador Region, Canada, 17 March 2010; Can. Sci. Advis. Sec. Proceed. Ser. 2011/020; Clovelly Golf Club: St. John’s, NL, Canada, 2011; pp. 5–7. [Google Scholar]
- Mathieson, S.; Berry, A.J. Spatial, temporal and tidal variation in crab populations in the Forth Estuary, Scotland. J. Mar. Biol. Assoc. UK 1997, 77, 167–183. [Google Scholar] [CrossRef]
- Grundstrom, J.; McAneney, B.; Fregeau, M.; Weston, S.; Buttner, J.; Walton, W.C.; Murphy, D.C. Characterization of Green Crab (Carcinus maenas) Populations in Coastal Waters of Massachusetts; 2004; p. 16, Unpublished report. [Google Scholar]
- Aagaard, A. In situ variation in heart rate of the shore crab Carcinus maenas in relation to environmental factors and physiological condition. Mar. Biol. 1996, 125, 765–772. [Google Scholar] [CrossRef]
- Baeta, A.; Cabral, H.N.; Marques, J.C.; Pardal, M.A. Feeding ecology of the green crab, Carcinus maenas (L., 1758) in a temperate estuary, Portugal. Crustaceana 2006, 79, 1181–1193. [Google Scholar]
- McGaw, I.J.; (Memorial University, St. John’s, NL, Canada). Personal communication, 2018.
- McKenzie, C. Ecological assessment of the invasive European green crab, Carcinus maenas, in Newfoundland: 2007–2009 population dynamics ecological impact. In Proceedings of the Regional Advisory Process on Green Crab, Carcinus maenas, Populations and Mitigations in the Newfoundland and Labrador Region, Canada, 17 March 2010; Can. Sci. Advis. Sec. Proceed. Ser. 2011/020; Clovelly Golf Club: St. John’s, NL, Canada, 2011; pp. 1–3. [Google Scholar]
- Goldstein, J.S.; Morrissey, E.M.; Moretti, E.D.; Watson, W.H. A comparison of the distribution and abundance of European green crabs and American lobsters in the Great Bay Estuary, New Hampshire, USA. Fish. Res. 2017, 189, 10–17. [Google Scholar] [CrossRef]
- Young, T.; Komarow, S.; Deegan, L.; Garritt, R. Population size and summer home range of the green crab, Carcinus maenas, in salt marsh tidal creeks. Biol. Bull. 1999, 197, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Gee, J.M.; Warwick, R.M.; Davey, J.T.; George, C.L. Field experiments on the role of epibenthic predation in determining prey densities in an estuarine mudflat. Estuar. Coast. Shelf Sci. 1985, 21, 439–448. [Google Scholar] [CrossRef]
- Raffaelli, D.; Conacher, A.; McLaclan, H.; Emes, C. The role of epibenthic crustacean predators in an estuarine food web. Estuar. Coast. Shelf Sci. 1989, 28, 149–160. [Google Scholar] [CrossRef]
- Zwarts, L.; Wanink, J.H. How the food supply harvestable by waders in the Wadden Sea depends on the variation in energy density, body weight, biomass, burying depth and behavior of tidal-flat invertebrates. Neth. J. Sea Res. 1993, 31, 441–476. [Google Scholar] [CrossRef]
- Ameyaw-Akumfi, C.; Hughes, R.N. Behaviour of Carcinus maenas feeding on large Mytilus edulis. How do they assess the optimum diet? Mar. Ecol. Prog. Ser. 1987, 38, 213–216. [Google Scholar] [CrossRef]
- Smith, O.R.; Chin, E. The effects of predation on soft clams, Mya arenaria. Natl Shellfish. Assoc. Conv. Address 1951, 42, 37–44. [Google Scholar]
- MacPhail, J.S.; Lord, E.I.; Dickie, L.M. The green crab—A new clam enemy. Fish. Res. Bull. Can. Atl. Prog. Rep. 1955, 63, 3–12. [Google Scholar]
- Jensen, K.T.; Jensen, J.N. The importance of some epibenthic predators on the density of juvenile benthic macrofauna in the Danish Wadden Sea. J. Exp. Mar. Biol. Ecol. 1985, 89, 157–174. [Google Scholar] [CrossRef]
- Beal, B.F. Evaluating factors contributing to mortalities of juveniles of the soft-shell clam (Mya arenaria L.) in Hampton/Seabrook Harbor, New Hampshire. Final Rep. New Hamps. Estuaries Proj. 2002, 111p. Available online: http://scholars.unh.edu/cgi/viewcontent.cgi?article=1315context=prep (accessed on 27 December 2017).
- Tyrell, M.; Harris, L.G. Potential impact of the introduced Asian shore crab, Hemigrapsus sanguineus, in northern New England: Diet, feeding preferences, and overlap with the green crab, Carcinus maenas. In Marine Bioinvasions, Proceedings of the First National Conference, Cambridge, MA, USA, 24–27 January 1999; Pederson, Ed.; MIT SeaGrant College Program: Cambridge, MA, USA, 1999; pp. 208–220. [Google Scholar]
- DeGraaf, J.D.; Tyrrell, M.C. Comparison of the feeding rates of two introduced crab species, Carcinus maenas and Hemigrapsus sanguineus, on the blue mussel, Mytilus edulis. Northeast. Nat. 2004, 11, 163–166. [Google Scholar] [CrossRef]
- Whitlow, W.L. Changes in survivorship, behavior, and morphology in native soft-shell clams induced by invasive green crab predators. Mar. Ecol. 2010, 31, 418–430. [Google Scholar] [CrossRef]
- Department of Fisheries and Oceans (DFO). Ecological Assessment of the Invasive European Green Crab (Carcinus maenas) in Newfoundland 2007–2009. In DFO Canadian Science Advisory Secretariat Science Advisory Report 2010/033; DFO: St. John’s, NL, Canada, 2011; p. 10. [Google Scholar]
- Trussell, G.C.; Ewanchuk, P.J.; Bertness, M.D.; Silliman, B.R. Trophic cascades in rocky shore tide pools: distinguishing lethal and nonlethal effects. Oecologia 2004, 139, 427–432. [Google Scholar] [CrossRef]
- Sigurdsson, G.M.; Rochette, R. Predation by green crab and sand shrimp on settling and recently settled American lobster postlarvae. J. Crustac. Biol. 2013, 33, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Gregory, G.J.; Quijon, P.A. The impact of a coastal invasive predator on infaunal communities: Assessing the roles of density and a native counterpart. J. Sea Res. 2011, 66, 181–186. [Google Scholar] [CrossRef]
- Le Calvez, J.C. Location of the shore crab Carcinus maenas L., in the food web of a managed estuary ecosystem: The Rance Basin (Brittany, France). Investigación Pesquera 1987, 51 (Suppl. 1), 431–442. [Google Scholar]
- Ruiz, G.M.; Rodriguez, L. Preliminary evaluation and predictions of impacts of Carcinus maenas on native crabs in Tasmania. In Proceedings of the First International Workshop on the Demography, Impacts and Management of Introduced Populations of the European Crab, Carcinus maenas, Hobart, Australia, 20–21 March 1997; Thresher, R.E., Ed.; Technical Report 11. Centre for Research on Introduced Marine Pests: Hobart, TAS, Australia, 1997; pp. 51–55. [Google Scholar]
- Griffen, B.D.; Guy, T.; Buck, J.C. Inhibition between invasives: A newly introduced predator moderates the impacts of a previously established invasive predator. J. Anim. Ecol. 2008, 77, 32–40. [Google Scholar] [CrossRef]
- Wallace, J.C. Feeding, starvation and metabolic rate in the shore crab Carcinus maenas. Mar. Biol. 1973, 20, 277–281. [Google Scholar] [CrossRef]
- Crothers, J.H. Dale Fort Marine Fauna. Field Stud. 1966, 2, 169. [Google Scholar]
- Crothers, J.H. The distribution of crabs on rocky shores around the Dale Peninsula. Field. Stud. 1970, 23, 263–274. [Google Scholar]
- Mascaró, M.; Seed, R. Foraging behavior of juvenile Carcinus maenas (L.) and Cancer pagurus L. Mar. Biol. 2001, 139, 1135–1145. [Google Scholar]
- van den Brink, A.; Hutting, S. Clash of the crabs: Interspecific, inter-cohort competition between the native European green crab, Carcinus maenas and the exotic brush clawed crab Hemigrapsus takanoi on artificial oyster reefs. J. Sea Res. 2017, 128, 41–51. [Google Scholar] [CrossRef]
- Schubart, C.D. The East Asian shore crab Hemigrapsus sanguineus (Brachyura: Varunidae) in the Mediterranean Sea: An independent human-mediated introduction. Sci. Mar. 2003, 67, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Dauvin, J.C.; Rius, A.T.; Ruellet, T. Recent expansion of two invasive crab species Hemigrapsus sanguineus (de Haan, 1835) and H. takanoi Asakura and Wataabe 2005 along the Opal Coast, France. Aquat. Invasions 2009, 4, 451–465. [Google Scholar] [CrossRef] [Green Version]
- Miron, G.; Audet, D.; Landry, T.; Moriyasu, M. Predation potential of the invasive green crab (Carcinus maenas) and other common predators on commercial bivalve species found on Prince Edward Island. J. Shellfish Res. 2005, 24, 579–586. [Google Scholar]
- Sungail, J.; Brown, A.C.; Alpert, K.; Maurukas, J. Prey selection by Gulf of Maine green crabs (Carcinus maenas), rock crabs (Cancer irroratus) and American lobsters (Homarus americanus): A laboratory study. J. Exp. Mar. Biol. Ecol. 2013, 449, 294–303. [Google Scholar] [CrossRef]
- Matheson, K.; Gagnon, P. Effects of temperature, body size, and chela loss on competition for a limited food resource between indigenous rock crab (Cancer irroratus Say) and recently introduced green crab (Carcinus maenas L.). J. Exp. Mar. Biol. Ecol. 2012, 428, 49–56. [Google Scholar] [CrossRef]
- Donahue, M.J.; Nichols, A.; Santamaria, C.A.; League-Pike, P.E.; Krediet, C.J.; Perez, K.O.; Shulman, M.J. Predation risk, prey abundance, and the vertical distribution of three brachyuran crabs on Gulf of Maine shores. J. Crustac. Biol. 2009, 29, 523–531. [Google Scholar] [CrossRef] [Green Version]
- Belair, M.-C.; Miron, G. Predation behavior of Cancer irroratus and Carcinus maenas during conspecific and heterospecific challenges. Aquat. Biol. 2009, 6, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Breen, E.; Metaxas, A. Effects of juvenile non-indigenous Carcinus maenas on the growth and condition of juvenile Cancer irroratus. J. Exp. Mar. Biol. Ecol. 2009, 377, 12–19. [Google Scholar] [CrossRef]
- Williams, P.J.; Floyd, T.A.; Rossong, M.A. Agonistic interactions between invasive green crabs, Carcinus maenas (Linnaus), and sub-adult American lobsters, Homarus americanus (Milne Edwards). J. Exp. Mar. Biol. Ecol. 2006, 329, 66–74. [Google Scholar] [CrossRef]
- Rossong, M.A.; Williams, P.J.; Comeau, M.; Mitchell, S.C.; Apaloo, J. Agonistic interactions between the invasive green crab, Carcinus maenas (Linnaeus) and juvenile American lobster, Homarus americanus (Milne Edwards). J. Exp. Mar. Biol. Ecol. 2006, 329, 281–288. [Google Scholar] [CrossRef]
- Rossong, M.A.; Quijon, P.A.; Williams, P.J.; Snelgrove, P.V.R. Foraging and shelter behavior of juvenile American lobster (Homarus americanus): The influence of a non-indigenous crab. J. Exp. Mar. Biol. Ecol. 2011, 403, 75–80. [Google Scholar] [CrossRef]
- Haarr, M.L.; Rochette, R. The effect of geographic origin on interactions between adult invasive green crabs carcinus maenas and juvenile American lobsters Homarus americanus in Atlantic Canada. J. Exp. Mar. Biol. Ecol. 2012, 422, 88–100. [Google Scholar] [CrossRef]
- McDonald, P.S.; Jensen, G.C.; Armstrong, D.A. The competitive and predatory impacts of the nonindigenous crab Carcinus maenas (L.) on early benthic phase Dungeness crab Cancer magister Dana. J. Exp. Mar. Biol. Ecol. 2001, 258, 39–54. [Google Scholar] [CrossRef]
- McDonald, P.S.; Jensen, G.C.; Armstrong, D.A. Green crabs and native predators; possible limitations on the west coast invasion. (Abstract). J. Shellfish Res. 1998, 17, 1283. [Google Scholar]
- MacDonald, J.A.; Roudez, R.; Glover, T.; Weis, J.S. The invasive green crab and Japanese shore crab: Behavioral interactions with a native crab species, the blue crab. Biol. Invasions 2007, 9, 837–848. [Google Scholar] [CrossRef]
- Ahi, R.S.; Moss, S.P. Status of the nonindigenous crab, Hemigrapsus sanguineus, at Greenwich Point, Connecticut. Northeast. Nat. 1999, 6, 221–224. [Google Scholar]
- Lohrer, A.M.; Whitlatch, R.B. Interactions among aliens: Apparent replacement of one exotic species by another. Ecology 2002, 83, 719–732. [Google Scholar] [CrossRef]
- Lohrer, A.M.; Whitlatch, R.B. Ecological studies on the recently introduced Japanese shore crab (Hemigrapsus sanguineus), in eastern Long Island Sound. In Proceedings of the Second Northeast Conference on Nonindigenous Aquatic Nuisance Species, Burlington, VT, USA, 18–19 April 1997; Balcoln, N., Ed.; Connecticut Sea Grant College Program CTSG-97-02. University of Connecticut, Avery Point: Groton, CT, USA, 1997; pp. 49–60. [Google Scholar]
- McDermott, J.J. The western Pacific brachyuran (Hemigrapsus sanguineus: Grapsidae), in its new habitat along the Atlantic coast of the United States: Geographic distribution and ecology. ICES J. Mar. Sci. 1998, 55, 289–298. [Google Scholar] [CrossRef]
- Gerard, V.A.; Cerrato, R.M.; Larson, A.A. Potential impacts of a grapsid crab on intertidal communities of the northwestern Atlantic Ocean. Biol. Invasions 1999, 1, 353–361. [Google Scholar] [CrossRef]
- Kraemer, G.P.; Sellberg, M.; Gordon, A.; Main, J. Eight-year record of Hemigrapsus sanguineus (Asian shore crab) invasion in western Long Island Sound estuary. Northeast. Nat. 2007, 14, 207–224. [Google Scholar] [CrossRef]
- Griffen, B.D. Ecological impacts of replacing one invasive species with another in rocky intertidal areas. In In the Wrong Place—Alien Marine Crustaceans: Distribution, Biology and Impacts. Invading Nature—Springer Series in Invasion Ecology, Vol. 6; Galil, B.S., Clark, P.F., Carlton, J.T., Eds.; Springer: New York, NY, USA, 2011; pp. 687–701. [Google Scholar]
- Young, A.M.; (Salem State University, Salem, MA, USA). Personal observation, 2014.
- Richards, B.; Chief Scientist, Weedoo Greenboat Inc., West Palm Beach, FL, USA. Personal communication, 2018.
- Menge, B.A. Components of predation intensity in the low zone of the New England rocky intertidal region. Oecologia 1983, 58, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Epifanio, C.E. Invasion biology of the Asian shore crab Hemigrapsus sanguineus: A review. J. Exp. Mar. Biol. Ecol. 2013, 441, 33–49. [Google Scholar] [CrossRef]
- Baillie, C.; Grabowski, J.H. Invasion dynamics: Interactions between the European green crab Carcinus maenas and the Asian shore crab Hemigrapsus sanguineus. Biol. Invasions 2019, 21, 787–802. [Google Scholar] [CrossRef]
- Wong, M.C.; Dowd, M. Role of invasive green crabs in the food web of an intertidal sand flat determined from field observations and a dynamic simulation model. Estuaries Coasts 2014, 37, 1004–1016. [Google Scholar] [CrossRef]
- Moksnes, P.-O. Self-regulating mechanisms in cannibalistic populations of juvenile shore crabs Carcinus maenas. Ecology 2004, 85, 1343–1354. [Google Scholar] [CrossRef] [Green Version]
- Dunstone, N.; Birks, J.D.S. The feeding ecology of mink (Mustela vison) in coastal habitat. J. Zool. 1987, 212, 69–83. [Google Scholar] [CrossRef]
- Mason, C.F.; MacDonald, S.M. The winter diet of otters (Lutra zutra) on a Scottish sea loch. J. Zool. 1980, 192, 558–561. [Google Scholar] [CrossRef]
- Sergeant, D.E. The status of the common seal (Phoca vitulina L.) on the East Anglian coast. J. Mar. Biol. Assoc. UK 1951, 29, 707–717. [Google Scholar] [CrossRef] [Green Version]
- Lynch, B.R.; Rochette, R. Spatial overlap and interaction between sub-adult American lobsters, Homarus americanus, and the invasive European green crab Carcinus maenas. J. Exp. Mar. Biol. Ecol. 2009, 369, 127–135. [Google Scholar] [CrossRef]
- Skonberg, D.I.; Perkins, D.L. Nutrient composition of green crab (Carcinus maenas) leg meat and claw meat. Food Chem. 2002, 77, 401–404. [Google Scholar] [CrossRef]
- McNiven, M.A.; Quijon, P.A.; Mitchell, A.W.; Ramsey, A.; St-Hilaire, S. Composition and distribution of the European green crab in Prince Edward Island, Canada. Open J. Anim. Sci. 2013, 3, 295–298. [Google Scholar] [CrossRef] [Green Version]
- St-Hilaire, S. Assessing the Potential for a Soft-Shell Green Crab Industry in PEI. Ph.D. Thesis, University of Prince Edward Island, Charlottetown, PE, Canada, 2016. [Google Scholar]
- Warner, R. Little green invaders. We have met the enemy and his name is Carcinus maenas. Now shall we eat him? The Boston Globe 2015, 287, 16–23. Available online: https://www.bostonglobe.com/magazine/2015/02/12/the-green-crab-problem-shall-eat-enemy/Ahtg6L87Gpxs0RMKntYAoN/story.html (accessed on 22 July 2017).
- Galetti, J.A.; Calder, B.L.; Skonberg, D.I. Mechanical separation of green crab (Carcinus maenas) meat and consumer acceptability of a value-added food product. J. Aquat. Food Prod. Tech. 2017, 26, 172–180. [Google Scholar] [CrossRef]
- McMahon, M. Investigating the viability of a soft-shell green crab industry in New England. In Proceedings of the Green Crab Working Summit Presentation, Portland, ME, USA, 6 June 2018. [Google Scholar]
- Parks, M. Bait to delicacy: Navigating and redefining the green crab market. In Proceedings of the Green Crab Working Summit Presentation, Portland, ME, USA, 6 June 2018. [Google Scholar]
- Skonberg, D. Toward full utilization of the green crab Biomass: Food and feed ingredients. In Proceedings of the Green Crab Working Summit Presentation, Portland, ME, USA, 6 June 2018. [Google Scholar]
- Kang, B.; Myracle, A.D.; Skonberg, D.I. Potential of recovered proteins from invasive green crabs (Carcinus maenas) as a functional food ingredient. J. Sci. Food Agric. 2019, 99, 1748–1754. [Google Scholar] [CrossRef]
- Parks, M.; Thái, T. The Green Crab Cookbook; Ingram Spark Publishing: La Vergne, TN, USA, 2018. [Google Scholar]
- Lee, K.T.; Jivoff, P.; Bishop, R.E. A low cost, reliable method for quantifying coloration in Carcinus maenas (Linnaeus, 1758) (Decapoda, Brachyura). Crustaceana 2005, 78, 579–590. [Google Scholar]
- Nagaraju, G.D.; Borst, D. Methyl farnesoate couples environmental changes to testicular development in the crab Carcinus maenas. J. Exp. Biol. 2008, 211, 2773–2778. [Google Scholar] [CrossRef] [Green Version]
- Abuhagr, A.M.; Blindert, J.L.; Nimitkul, S.; Zander, I.A.; LaBere, S.M.; Chang, S.A.; MacLea, K.S.; Chang, E.S.; Mykles, D.L. Molt regulation in green and red color morphs of the crab Carcinus maenas: Gene expression of molt-inhibiting hormone signaling components. J. Exp. Biol. 2014, 217, 796–808. [Google Scholar] [CrossRef] [Green Version]
- Deese, H.; Arnold, S. Green crabs working as lobster bait in Nova Scotia. Waterfr. Arch. 2014. Available online: http://www.workingwaterfrontarchives.org/2014/03/18/green-crabs-as-lobster-bait-working-in-nova-scotia/ (accessed on 18 March 2014).
- Ghosh, B.; Urban, M.W. Self-repairing oxetane-substituted chitosan polyurethane networks. Science 2009, 323, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Li, Y.H. Innovation in cosmetic and medical science. The role of chitin nanofibrils composites. J. Appl. Cosmetol. 2015, 33, 9–24. [Google Scholar]
- Gao, Y.; Cranston, R. Recent advances in antimicrobial treatments of textiles. Text. Res. J. 2008, 78, 60–72. [Google Scholar]
- Varshosaz, J.; Jaffari, F.; Karimzadeh, S. Development of bioadhesive chitosan gels for topical delivery of lidocaine. Sci. Pharm. 2006, 74, 209–223. [Google Scholar] [CrossRef] [Green Version]
- Steiger, M.G.; Mach-Aigner, A.R.; Gorsche, R.; Rosenberg, E.E.; Mihovilovic, M.D.; Mach, R.L. Synthesis of an antiviral drug precursor from chitin using a saprophyte as a whole-cell catalyst. Microb. Cell Fact. 2011, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Teotia, S.; Lata, R.; Gupta, M. Chitosan as a macroaffinity ligand: Purification of chitinases by affinity precipitation and aqueous two-phase extractions. J. Chromatogr. A 2004, 1052, 85–91. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C. Estuary Therapy: Advances in Coastal Restoration at Kejimkujik National Park Seaside. In Green Crab Summit Presentation; University of Maine: Orono, ME, USA, 2013; Available online: http://www.seagrant.umaine.edu/green-crab-summit (accessed on 16 December 2013).
- Lützen, J.; Jensen, K.H.; Glenner, H. Life history of Sacculina carcini Thompson, 1836 (Cirripedia: Rhizocephala: Sacculinidae) and the intermoult cycle of its host, the shore crab Carcinus maenas (Linnaeus, 1758) (Decapoda: Brachyura: Carcinidae). J. Crustac. Biol. 2018, 38, 413–419. [Google Scholar] [CrossRef]
- Mouritsen, K.N.; Jensen, T. The effect of Sacculina carcini infections on the fouling, burying, behavior and condition of the shore crab, Carcinus maenas. Mar. Biol. Res. 2006, 2, 270–275. [Google Scholar] [CrossRef]
- Bojko, J.; Subramaniam, K.; Waltzek, T.B.; Stentiford, G.D.; Behringer, D.C. Genomic and developmental characterisation of a novel bunyavirus infecting the crustacean Carcinus maenas. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Bojko, J.; Clark, F.; Bass, D.; Dunn, A.M.; Stewart-Clark, S.; Stebbing, P.D.; Stentiford, G.D. Parahepatospora carcini n. gen., n. sp., a parasite of invasive Carcinus maenas with intermediate features of sporogony between the Enterocytozoon clade and other Microsporidia. J. Invertebr. Pathol. 2017, 143, 124–134. [Google Scholar] [CrossRef]
- Torchin, M.E.; Lafferty, K.D.; Kuris, A.M. Release from parasites as natural enemies: Increased performance of a globally introduced marine crab. Biol. Invasions 2001, 3, 333–345. [Google Scholar] [CrossRef]
- Costa, S.; Bessa, F.; Pardal, M.A. The parasite Sacculina carcini Thompson, 1936 (Cirrepedia, Rhizocephala) in the crab Carcinus maenas (Linnaeus, 1758) (Decapoda, Portunidae): Influence of environmental conditions, colour morphotype and sex. Crustaceana 2013, 86, 34–47. [Google Scholar]
- Waser, A.M.; Goedknegt, M.A.; Dekker, R.; McSweeney, N.; Witte, J.I.J.; van der Meer, J.; Thieltges, D.W. Tidal elevation and parasitism: Patterns of infection by the rhizocephalan parasite Sacculina carcini in shore crabs Carcinus maenas. Mar. Ecol. Prog. Ser. 2016, 545, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Rytter Jensen, A.; Schneider, M.R.; Høeg, J.T.; Glenner, H.; Lützen, J. Variation in juvenile stages and success of male acquisition in Danish and French populations of the parasitic barnacle Sacculina carcini (Cirripedia: Rhizocephala) parasitizing the shore crab Carcinus maenas. Mar. Biol. Res. 2019, 15, 191–203. [Google Scholar] [CrossRef]
- Høeg, J.T.; Lützen, J. Life cycle and reproduction in the Cirripedia Rhizocephala. Oceanog. Mar. Biol. Ann. Rev. 1995, 33, 427–485. [Google Scholar]
- Torchin, M.E.; Mitchell, C.E. Parasites, pathogens, and invasions by plants and animals. Front. Ecol. Environ. 2004, 2, 183–190. [Google Scholar] [CrossRef]
- Blakeslee, A.M.H.; Keogh, C.L.; Fowler, A.E.; Griffen, B.D. Assessing the effects of trematode infection on invasive green crabs in eastern North America. PLoS ONE 2015, 10, e0128674. [Google Scholar] [CrossRef] [Green Version]
- Kuris, A. Nemertean egg predators as potential biocontrol agents for Carcinus maenas. In Proceedings of the First International Workshop on the Demography, Impacts and Management of Introduced Populations of the European crab, Carcinus maenas, Hobart, Australia, 20–21 March 1997; Thresher, R.E., Ed.; Technical Report 11. Centre for Research on Introduced Marine Pests: Hobart, TAS, Australia, 1997; pp. 88–90. [Google Scholar]
- Minchin, D. The influence of the parasitic cirripede Sacculina carcini on its brachyuran host Carcinus maenas within its home range. In Proceedings of the First International Workshop on the Demography, Impacts and Management of Introduced Populations of the European crab, Carcinus maenas, Hobart, Australia, 20–21 March 1997; Thresher, R.E., Ed.; Technical Report 11. Centre for Research on Introduced Marine Pests: Hobart, TAS, Australia, 1997; pp. 81–86. [Google Scholar]
- Goddard, J.H.R.; Torchin, M.E.; Kuris, A.M.; Lafferty, K.D. Host specificity of Sacculina carcini, a potential biological control agent of the introduced European green crab Carcinus maenas in California. Biol. Invasions 2005, 7, 895–912. [Google Scholar] [CrossRef]
- Crooks, J.A. Characterizing ecosystem-level consequences of biological invasions: The role of ecosystem engineers. Oikos 2002, 97, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Garbary, D.J.; Miller, A.G.; Williams, J.; Seymour, N.R. Drastic decline of an extensive eelgrass bed in Nova Scotia due to the activity of the invasive green crab (Carcinus maenas). Mar. Biol. 2014, 161, 3–15. [Google Scholar] [CrossRef]
- McDonald, P.S.; Holsman, K.K.; Beauchamp, D.A.; Dumbauld, B.R.; Armstrong, D.A. Bioenergetics modeling to investigate habitat use by the nonindigenous crab, Carcinus maenas, in Willipa Bay, Washington. Estuar. Coast. 2006, 29, 1132–1149. [Google Scholar] [CrossRef]
- Davis, R.C.; Short, F.T.; Burdick, D.M. Quantifying the effects of green crab damage to eelgrass transplants. Rest. Ecol. 1998, 6, 297–302. [Google Scholar] [CrossRef]
- Garbary, D.J.; Miller, A.G. Green crabs (Carcinus maenas) as the grim reaper: Destruction of eelgrass beds in Nova Scotia. J. Shellfish Res. 2006, 25, 728. [Google Scholar]
- Matheson, K.; McKenzie, C.H.; Gregory, R.S.; Robichaud, D.A.; Bradbury, I.A.; Snelgrove, P.V.; Rose, G.A. Linking eelgrass decline and impacts on associated fish communities to European green crab Carcinus maenas invasion. Mar. Ecol. Prog. Ser. 2016, 548, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Trussell, G.C.; Ewanchuk, P.J.; Bertness, M.D. Trait-mediated effects in rocky intertidal food chains: Predator risk cues alter prey feeding rates. Ecology 2003, 84, 629–640. [Google Scholar] [CrossRef]
- Dare, P.J.; Edwards, D.B. Experiments on the survival, growth and yield of relaid seed mussels (Mytilus edulis L.) in the Menai Straits, North Wales. ICES J. Mar. Sci. 1976, 37, 16–28. [Google Scholar] [CrossRef]
- Richards, M.G.; Huxman, M.; Bryant, A. Predation: A causal mechanism for variability in intertidal bivalve populations. J. Exp. Mar. Biol. Ecol. 1999, 241, 159–177. [Google Scholar] [CrossRef]
- Camphuysen, C.J.; Berrevoets, C.M.; Cremers, H.J.W.M.; Dekinga, A.; Dekker, R.; Ens, B.J.; van der Have, T.M.; Kats, R.K.H.; Kuiken, T.; Leopold, M.F.; et al. Mass mortality of common eiders (Somateria mollissima) in the Dutch Wadden Sea, winter 1999/2000: Starvation in a commercially exploited wetland of international imporE.B.ce. Biol. Conserv. 2002, 106, 303–317. [Google Scholar] [CrossRef]
- Welch, W.R. Changes in abundance of the green crab, Carcinus maenas (L.), in relation to recent temperature changes. Fish. Bull. 1968, 67, 337–345. [Google Scholar]
- Tan, E.B.P.; Beal, B.F. Interactions between the invasive European green crab, Carcinus maenas (L.), and juveniles of the soft-shell clam, Mya arenaria L., in eastern Maine, USA. J. Exp. Mar. Biol. Ecol. 2015, 462, 62–73. [Google Scholar] [CrossRef]
- McClenachan, L.; O’Connor, G.; Reynolds, T. Adaptive capacity of co-management systems in the face of environmental change: The soft-shell clam fisher and invasive green crab in Maine. Mar. Policy 2015, 52, 26–32. [Google Scholar] [CrossRef]
- Atlantic Coastal Cooperative Statistics Program. Available online: http://www.accsp.org (accessed on 7 July 2018).
- Beal, B.F.; Kraus, M.G. Interactive effects of initial size, stocking density, and type of predator deterrent netting on survival and growth of cultured juveniles of the soft-shell clam, Mya arenaria L, in eastern Maine. Aquaculture 2002, 208, 81–111. [Google Scholar] [CrossRef]
- Beal, B.F. Biotic and abiotic factors influencing growth and survival of wild and cultured individuals of the softshell clam (Mya arenaria L.) in eastern Maine. J. Shellfish Res. 2006, 25, 461–474. [Google Scholar] [CrossRef]
- Beal, B.F. Relative importance of predation and intraspecific competition in regulating growth and survival of juveniles of the softshell clam, Mya arenaria L., at several spatial scales. J. Exp. Mar. Biol. Ecol. 2006, 336, 1–17. [Google Scholar] [CrossRef]
- Beal, B.F.; Coffin, C.R.; Randall, S.F.; Goodenow, C.A.; Pepperman, K.E.; Ellis, B.W.; Jourdet, C.B.; Protopopescu, G.C. Spatial variability in recruitment of an infaunal bivalve: Experimental effects of predator exclusion on the softshell clam (Mya arenaria L.) along three tidal estuaries in southern Maine, USA. J. Shellfish Res. 2018, 37, 1–27. [Google Scholar] [CrossRef]
- Williams, P.J.; Macsween, C.; Rossong, M.A. Competition between invasive green crab (Carcinus maenas) and American lobster (Homarus americanus). N. Z. J. Mar. Freshw. Res. 2009, 44, 37–41. [Google Scholar] [CrossRef]
- Malyshev, A.; Quijón, P.A. Disruption of essential habitat by a coastal invader: New evidence of the effects of green crabs on eelgrass beds. ICES J. Mar. Sci. 2011, 68, 1852–1856. [Google Scholar] [CrossRef] [Green Version]
- Neckles, H.A. Loss of eelgrass in Casco Bay, linked to green crab disturbance. Northeast. Nat. 2015, 22, 478–500. [Google Scholar] [CrossRef]
- Howard, B.R.; Francis, F.T.; Côté, I.M.; Therriault, T.W. Habitat alteration by invasive European green crab (Carcinus maenas) causes eelgrass loss in British Columbia, Canada. Biol. Invasions 2019, 21, 3607–3618. [Google Scholar] [CrossRef]
- Finger, J. Concerns for the molluscan shellfish industry. In Proceedings of the Exotic Green Crab Presentation Abstract, Vancouver, WA, USA, 9–10 February 1998. [Google Scholar]
- Grosholz, E.D.; Olin, P.G.; Williams, B.; Tinsman, R. Reducing predation on Manila clams by nonindigenous European green crabs. J. Shellfish Res. 2001, 20, 913–919. [Google Scholar]
- Bojko, J.; Stebbing, P.D.; Dunn, A.M.; Bateman, K.S.; Clark, F.; Kerr, R.C.; Stewart-Clark, S.; Johannesen, Á.; Stentiford, G.D. Green crab Carcinus maenas symbiont profiles along a North Atlantic invasion route. Dis. Aquat. Org. 2018, 128, 147–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonard, G.H.; Bertness, M.D.; Yund, P.O. Crab predation, waterborne cues, and inducible defenses in the blue mussel, Mytilus edulis. Ecology 1999, 80, 1–14. [Google Scholar] [CrossRef]
- Trussell, G.C. Phemotypic plasticity in an intertidal snail – the role of a common crab predator. Evolution 1996, 50, 448–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeley, R. Intense natural selection caused a rapid morphological transition in a living marine snail. Proc. Natl. Acad. Sci. USA 1986, 83, 6897–6901. [Google Scholar] [CrossRef] [Green Version]
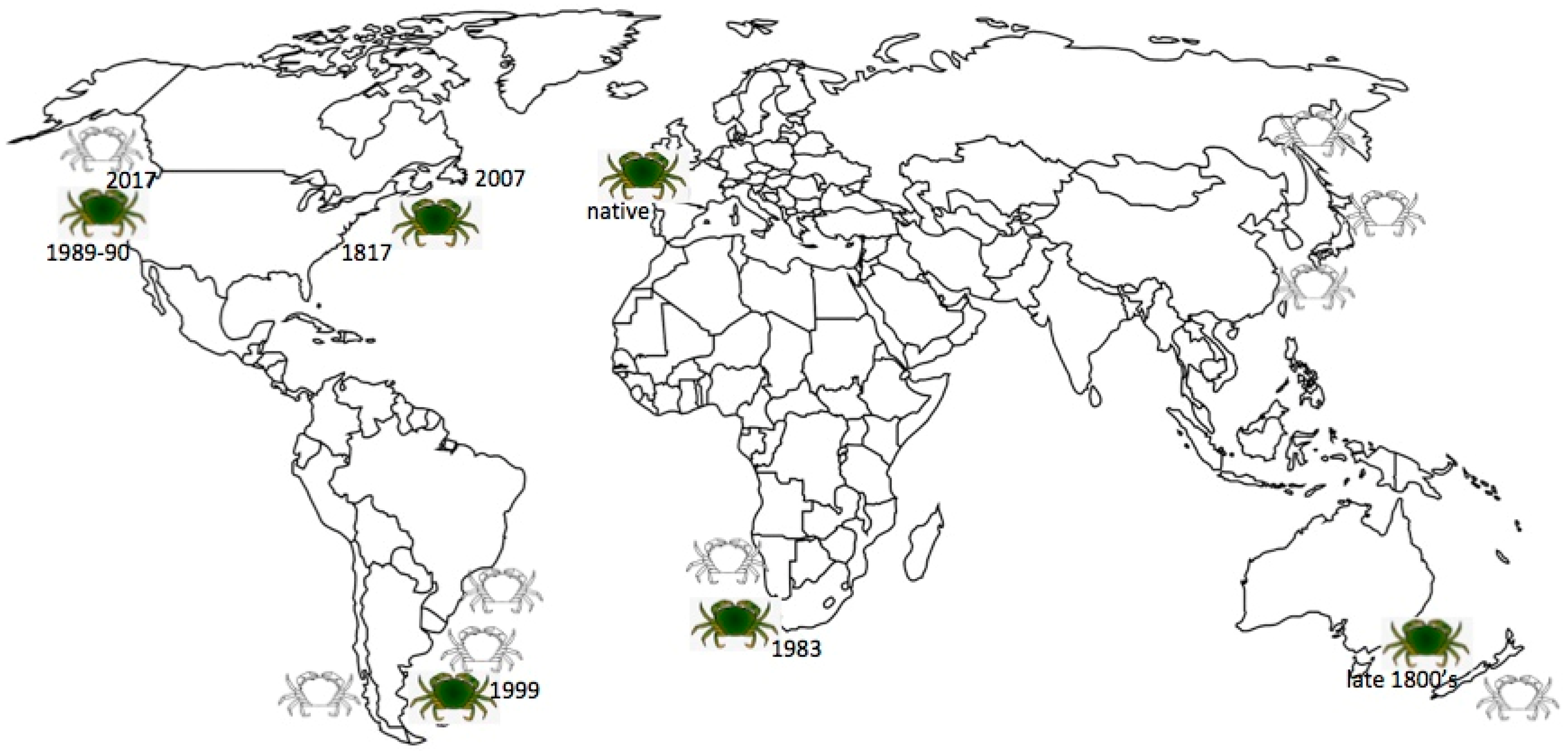
| Location | Copulation | Females Ovigerous | Larvae Released |
|---|---|---|---|
| Native European Population | |||
| Norway | January [189] | ||
| Scotland | All year (Peaks October to Spring Months) [190] | April to end of July [190] | |
| Denmark | May and June [191] | ||
| Baltic Germany | August [142] | ||
| United Kingdom | July-September [192] | November to December [192] | May-June [192] |
| Wales | August to September [5] | February to June (Peak in March and April) [5] | February to July [5] |
| The Netherlands | July to September (Peaks in August) [3] | November and December, and during the spring and early summer [3] | After winter season and in June [3] |
| Belgium | June to October [188] | December to August [188] | March to mid-October [188] |
| France | May to November (Peak in August and September) [7] | November to July (Peak in April) [7] | |
| Portugal | April-May and September-December [193] | All year [150]; October to June (Peaks in January-February) [193] | All year [150]; October through May [194]; February to June [195] |
| Northwestern Atlantic Nonindigenous Population | |||
| Newfoundland, Canada | Spring and Late Summer [196] | July and August [196] | Late May or Early June to August (Peak in June and July) [196] |
| Prince Edward Island, Canada | Peak from mid-August to September [147] | July to mid-September; (Peak in early July) [147,197] | August to December [147,197] |
| Maine, US | July to October (Peaks in August) [139] | April to August (Peaks from May to June) [139]; December to August, peak in summer months [125] | Peaks in September [139] |
| Massachusetts, US | November to January, and May to August [113] | ||
| Northeastern Pacific Nonindigenous Population | |||
| British Columbia, Canada | July [9,198] | April and May [9,198] | May [9,198] |
| South Africa Nonindigenous Population | |||
| Bloubergstrand and Sea Point | July to December [65] | ||
| Table Bay | June to January, peak in October [199] | ||
| Argentina Nonindigenous Population | |||
| San Jorge Gulf, Patagonia, Argentina | May to September [82] | ||
| Location | Latitude | Maximum Size (mm) | Sexual Maturity (mm) | Reference | ||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||
| Native European Population | ||||||
| Herdla, NO | 61° N | 80 | 44 | 28–30 | [189] | |
| Herdla, NO | 61° N | 80 | >20 | [185] | ||
| west coast of Sweden | 58° N | 72 | [219] | |||
| Western Sweden | 58–59° N | 100 | Moksnes, unpublished data, cited in Behrens Yamada [218] | |||
| Kattegat, DK | 57° N | 71 | 62 | [220] | ||
| Isefjord, DK | 55–56° N | 92 | [209] | |||
| Millport, SL | 56° N | 80 | [221] | |||
| Orensund, DK | 56° N | 80 (C) | [222] | |||
| Odense Fjord, DK | 56° N | 80 | [223] | |||
| Clyde Sea Area, SL | 56° N | 80 | [224] | |||
| Kerteminde Fjord, DK | 55° N | 81 | [225] | |||
| Schlei Fjord, Baltic Sea, DE | 54° N | 75 | 60 | [142] | ||
| Wadden Sea, DE | 54° N | 71 | [174] | |||
| Whitby Harbor and Robin Hood’s Bay, ENG | 53° N | 82.5 | 75 | 16–31 | [226] | |
| Boggle Hole, ENG | 54° N | 75 | [227] | |||
| Isle of Man, UK | 54° N | 27 | [207] | |||
| Llandudno and Conway, North WAL | 53° N | 75 | [228] | |||
| Menai Straits, North WAL | 53° N | 83.1 | 74.1 | [52] | ||
| Menai Straits, North WAL | 53° N | 75 | [229] | |||
| Menai Straits, North WAL | 53° N | 74 | [230] | |||
| Den Helder, NL | 53° N | 86 | 70 | 36–42 | [3] | |
| Essex, ENG | 52° N | 62 | [231] | |||
| Dale Peninsula, SW WAL | 51° N | 86 | 70 | 25 | 15 | [1] |
| Oosterschelde, NL | 52° N | 63 | [232] | |||
| Bullens Bay, IE | 52° N | 86.5 | 38.6 | [200] | ||
| Swansea, South WAL | 52° N | 86 | 70 | 44 | [5] | |
| Ostend, BE | 51° N | 44 | 23–45 | [188] | ||
| Cornwall, ENG | 50–51° N | 21 | 28 | [233] | ||
| Plymouth, ENG | 50° N | 57 | 65 | [231] | ||
| Mountbatten, Plymouth, ENG | 50° N | 65 (C) | [234] | |||
| Baltic Germany | 50° N | 75 | 60 | [8] | ||
| Luc-sur-Mer, FR | 49° N | 12 | [7] | |||
| Minho Estuary, PT | 42° N | 65 | 56 | 30 | [173] | |
| Canal de Mira, PT | 41° N | 27 | [195] | |||
| Mondego estuary, PT | 41° N | 71 | 65 | 29 | [150] | |
| Ericeira and Parede, PT | 39° N | 27 | 21.5 | [193] | ||
| Northwestern Atlantic Nonindigenous Population | ||||||
| Bras d’Or Lakes, Nova Scotia, CA | 46° N | 91 | 69 | 40 | [235] | |
| Placentia Bay, Newfoundland, CA | 48° N | 79 | 72 | 32 | 37 | [196] |
| Newfoundland west coast, CA | 48° N | 30 | McKenzie unpublished data, cited in Best et al. [196] | |||
| Basin Head, Prince Edward Island, CA | 46° N | 89.76 | 76.44 | 21.3 | 28.66 | [147] |
| Basin Head, Prince Edward Island, CA | 46° N | 80 | [236] | |||
| Basin Head Lagoon, Prince Edward Island, CA | 46° N | 42.7 | [197] | |||
| Long Harbor, Placentia Bay, Newfoundland, CA | 45° N | 78 | [237,238] | |||
| Boothbay Harbor, Maine, US | 44° N | 82 | 70 | 34 | 34 | [139] |
| Days Cove, Damariscotta, Maine, US | 44° N | 82 (C) | [239] | |||
| Broad Cove, Yarmouth, Maine, US | 44° N | 85 (C) | [239] | |||
| Webhannet River, Wells, Maine, US | 43° N | 78 (C) | [239] | |||
| Great Bay Estuary, New Hampshire, US | 43° N | 91 | 88 | [182] | ||
| Hampton-Seabrook Estuary, New Hampshire, US | 43° N | 36 | [182] | |||
| Pavilion Beach, Ipswich, Massachusetts, US | 42° N | 22 | A.M. Young, personal observation | |||
| Salem Sound, Massachusetts, US | 42° N | 80.9 | 85.9 | [113] | ||
| Northeastern Pacific Nonindigenous Population | ||||||
| Little Espinoza Inlet, British Columbia, CA | 50° N | 80.3 | 58.4 | [240] | ||
| Pipestem Inlet, Barkley Sound, British Columbia, CA | 49° N | 113 | 87 | [241] | ||
| Barkley Sound, British Columbia, CA | 49° N | 101.1 | 85.4 | [52] | ||
| Barkley Sound, British Columbia, CA | 49° N | 114 | 93 | 41 | [59] | |
| British Columbia, CA | 49 ° N | 106 | Therriault & DiBacco personal communication to Iain McGaw | |||
| Pipestem Inlet, Barkley Sound, British Columbia, CA | 49° N | 95 (C) | [242] | |||
| Vancouver Island, British Columbia, CA | 49° N | 98 | 76 | [198] | ||
| Oregon/Washington, US | 46–48° N | 99.6 | 79 | [218] | ||
| Tillamock Bay, Oregon, US | 46° N | 92 | 85 | [241] | ||
| Netarts Bay, Oregon, US | 45° N | 98 | 89 | [241] | ||
| Yaquina Bay, Oregon, US | 45° N | 96 | 83 | [241] | ||
| Yaquina Bay, Oregon, US | 45° N | 96 | 79 | [8] | ||
| Oregon, US | 45° N | 99 (C) | Behrens Yamada personal communication to Jamieson [240] | |||
| Coos Bay, Oregon, US | 43° N | 98 | 88 | [241] | ||
| Bodega Harbor, California, US | 38° N | 40 | [30] | |||
| Bodega Harbor, California, US | 38° N | 34 | [8] | |||
| Bodega Bay, California, US | 38° N | 102 | 88 | [241] | ||
| Tomales Bay, California, US | 38° N | 86 | 73 | [241] | ||
| Sea Drift Lagoon, California, US | 38° N | 88 | 88 | [241] | ||
| San Francisco Bay | 38° N | 83 | 80 | [241] | ||
| Elkhorn Slough, California, US | 37° N | 89 | 89 | [241] | ||
| Argentina Nonindigenous Population | ||||||
| Camarones Bay, AR | 45° S | 81.4 | 71.4 | 39.9 | [243] | |
| San Jorge Gulf, Patagonia, AR | 45° S | 73.4 | 64.3 | 45.8 | [82] | |
| South Africa Nonindigenous Population | ||||||
| Table Bay Docks, Cape Town, ZA | 34° S | 84 | 56 | [65] | ||
| Australia Nonindigenous Population | ||||||
| Gulf St. Vincent, South Australia, AU | 35° S | 80 | 67 | [244] | ||
| Location | Latitude (to Nearest Degree) | Sex Ratio (M:F) | Reference |
|---|---|---|---|
| Native European Population | |||
| Herdla, Norway | 61° N | 1.55; 1.48 | [187] |
| Herdia, Norway | 61° N | 1.49 | [185] |
| Kattegat, Denmark | 57° N | 51.63 * (shallow) 21.99 * (deeper) | [220] |
| Forth Estuary, Scotland | 56° N | 0.68 | [259] |
| Firth of Forth, Scotland1 | 56° N | 0.85 | [107] |
| Kerteminde Fjord, Denmark | 55° N | 2.99 | [145] |
| Kerteminde Fjord, Denmark | 55° N | 1.01 | [225] |
| Menai Strait, Anglesey, UK | 53° N | 1.86 | [263] |
| Whitby Harbor & Robin Hood’s Bay, England | 53° N | 0.45 | [226] |
| Ynys Faelog, Menai Strait, North Wales | 53° N | 2.33 | [143] |
| Den Helder, Netherlands | 53° N | 0.62 | [3] |
| Bullens Bay, Ireland | 51° N | 1.89 | [200] |
| Looe Estuary, Cornwall, England | 50° N | 0.64 | [233] |
| Mondego estuary, Portugal | 41° N | 0.89 | [150] |
| northwestern Atlantic nonindigenous population | |||
| Placentia Bay, Newfoundland, Canada | 48° N | 1.00 (September) > 1.00 (June) | [264] |
| Placentia Bay, Newfoundland, Canada | 48° N | 1.76 (fall) 1.33 (spring) 1.29 (summer) | [196] |
| Basin Head, PEI, Canada | 46° N | 1.63 | [147] |
| Bras d’Or Lakes, Nova Scotia, Canada | 46° N | 2.70 | [235] |
| Port L’Hebert, Nova Scotia, Canada | 44° N | 1.18 (May) 0.79 (August) | [122] |
| Boothbay Harbor, Maine | 44° N | 1.29 | [139] |
| Days Cove, Damariscotta, Maine | 44° N | 1.57 | [239] |
| Broad Cove, Yarmouth, Maine | 44° N | 0.60 | [239] |
| Biddeford Pool, Maine | 43° N | < 1.00 in 74.4% of samples | [125] |
| Webhannet River, Wells, Maine | 43° N | 0.94 | [239] |
| Great Bay Estuary, New Hampshire, US | 43° N | 1.00 | [265] |
| Great Bay Estuary, New Hampshire, US | 43° N | 0.37 | [182] |
| Houlton-Seabrook Estuary, New Hampshire, US | 43° N | 0.98 | [182] |
| Plum Island Sound, Massachusetts, US | 43° N | 0.34 (upstream) 0.90 (downstream) | [112] |
| Great Marsh, Rowley, Massachusetts, US | 43° N | 0.73 | [260] |
| Great Marsh, Rowley, Massachusetts, US | 43° N | 1.11 | [266] |
| Salem Sound, Massachusetts, US | 42° N | 0.37 | [113] |
| Nauset Marsh, Eastham, Massachusetts, US | 42° N | 0.26 | [260] |
| northeastern Pacific nonindigenous population | |||
| Nootka Sound, British Columbia, Canada | 49° N | 4.18 | [198] |
| Clayoquot Sound, British Columbia, Canada | 49 ° N | 3.88 | [198] |
| Barkley Sound, British Columbia, Canada | 49° N | 1.58 | [198] |
| Barkley Sound, British Columbia, Canada | 49° N | 2.45 | [263] |
| Barkley Sound, British Columbia, Canada | 49° N | 1.61 | [59] |
| Pipestem Inlet, British Columbia, Canada | 49° N | 1.71 | [241] |
| Tillamook Bay, Oregon, US | 45° N | 8.25 | [241] |
| Netarts Bay, Oregon, US | 45° N | 4.04 | [241] |
| Yaquina Bay, Oregon, US | 44° N | 6.00 | [241] |
| Coos Bay, Oregon, US | 43° N | 3.45 | [241] |
| Bodega Harbor, California, US | 38° N | 3.07 | [241] |
| Tomales Bay, California, US | 38° N | 2.36 | [241] |
| Seadrift lagoon, California, US | 38° N | 0.73 | [241] |
| San Francisco Bay, California, US | 37° N | 0.63 | [241] |
| Elkhorn Slough, California, US | 36° N | 0.72 | [241] |
| Argentina nonindigenous population | |||
| San Jorge Gulf, Patagonia, Argentina | 45° S | 2.36 | [82] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, A.M.; Elliott, J.A. Life History and Population Dynamics of Green Crabs (Carcinus maenas). Fishes 2020, 5, 4. https://0-doi-org.brum.beds.ac.uk/10.3390/fishes5010004
Young AM, Elliott JA. Life History and Population Dynamics of Green Crabs (Carcinus maenas). Fishes. 2020; 5(1):4. https://0-doi-org.brum.beds.ac.uk/10.3390/fishes5010004
Chicago/Turabian StyleYoung, Alan M., and James A. Elliott. 2020. "Life History and Population Dynamics of Green Crabs (Carcinus maenas)" Fishes 5, no. 1: 4. https://0-doi-org.brum.beds.ac.uk/10.3390/fishes5010004





