Dynamics of Two Anadromous Species in a Dam Intersected River: Analysis of Two 100-Year Datasets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data
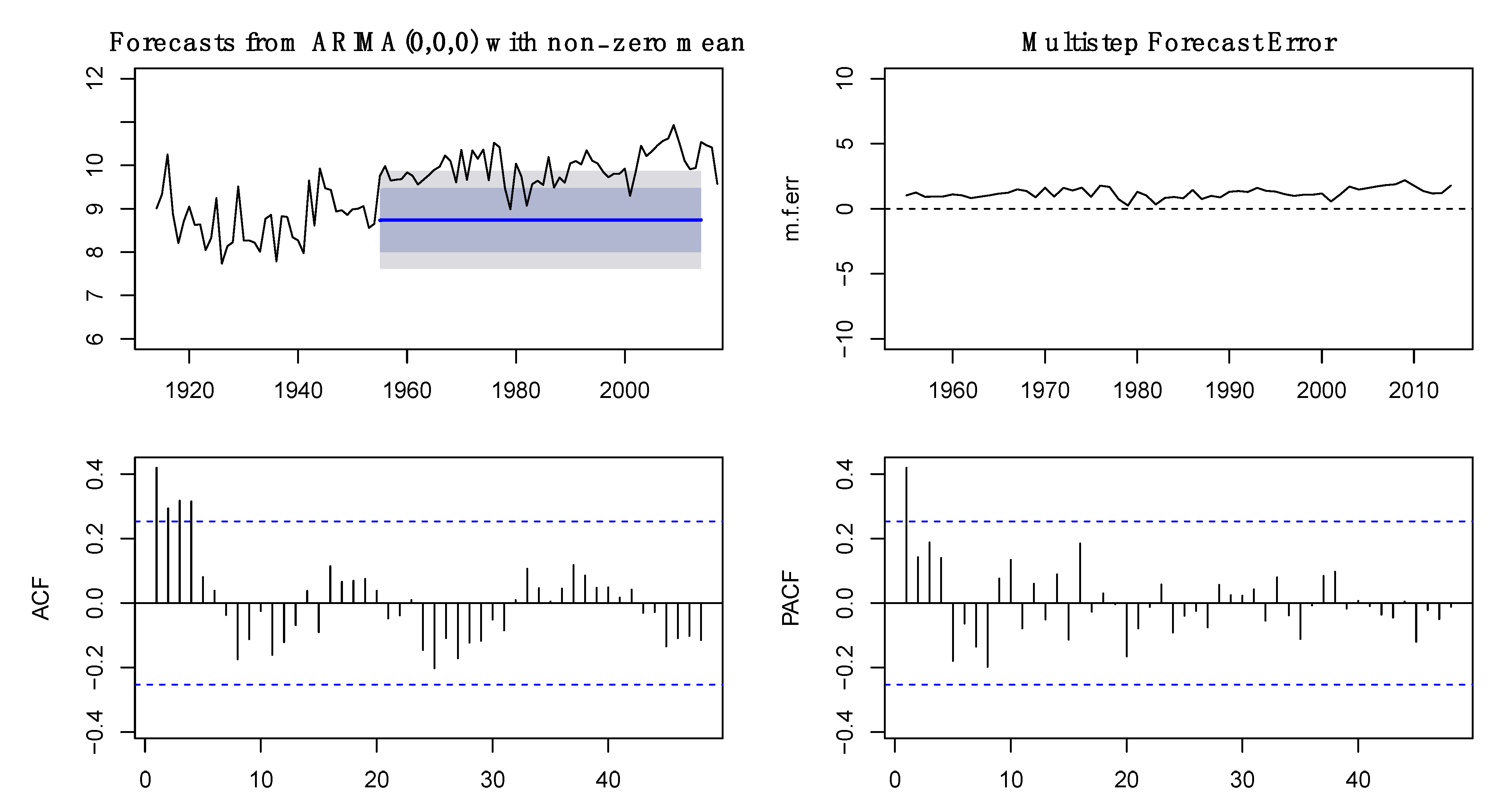
2.2. Analysis of Time Series
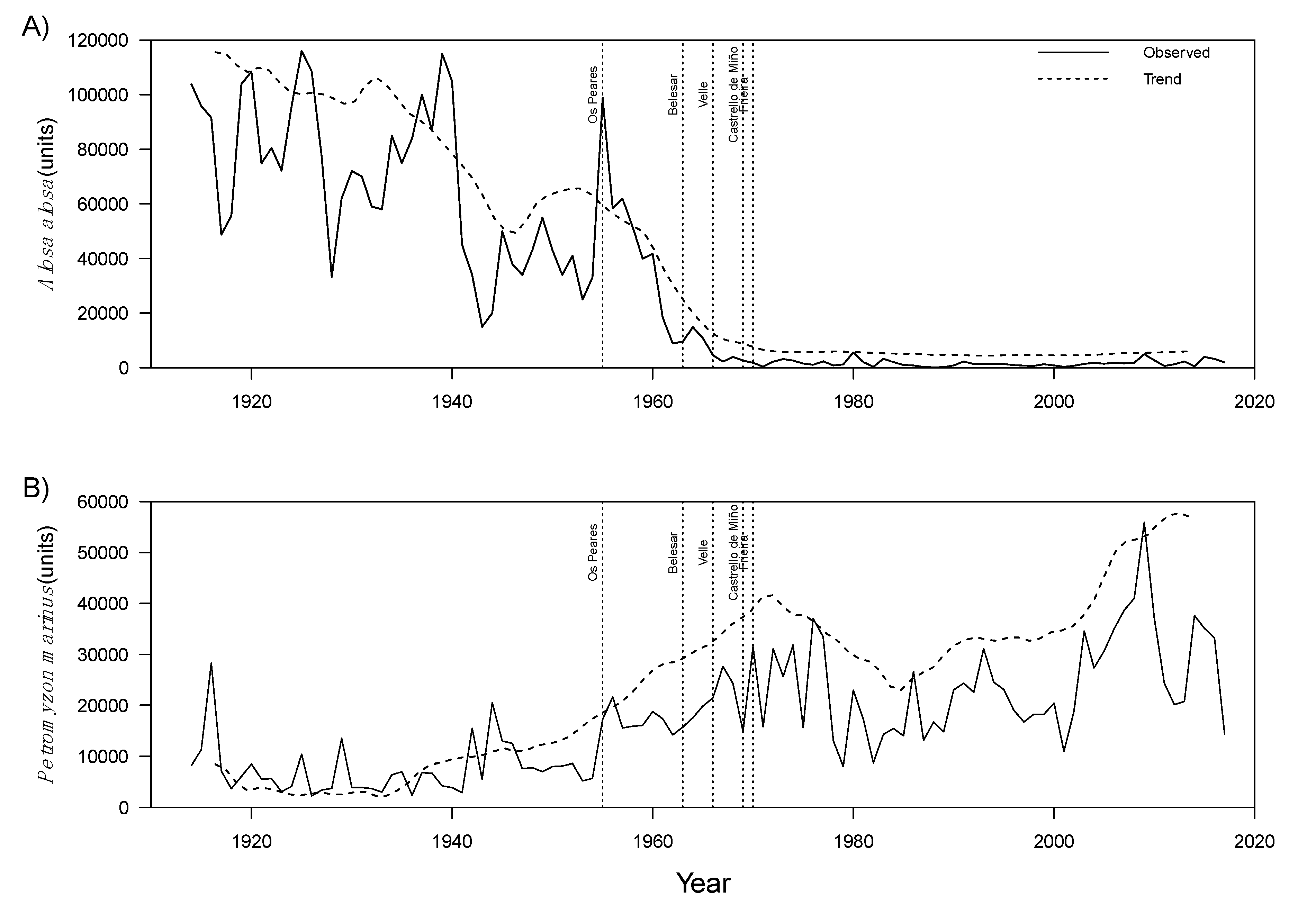
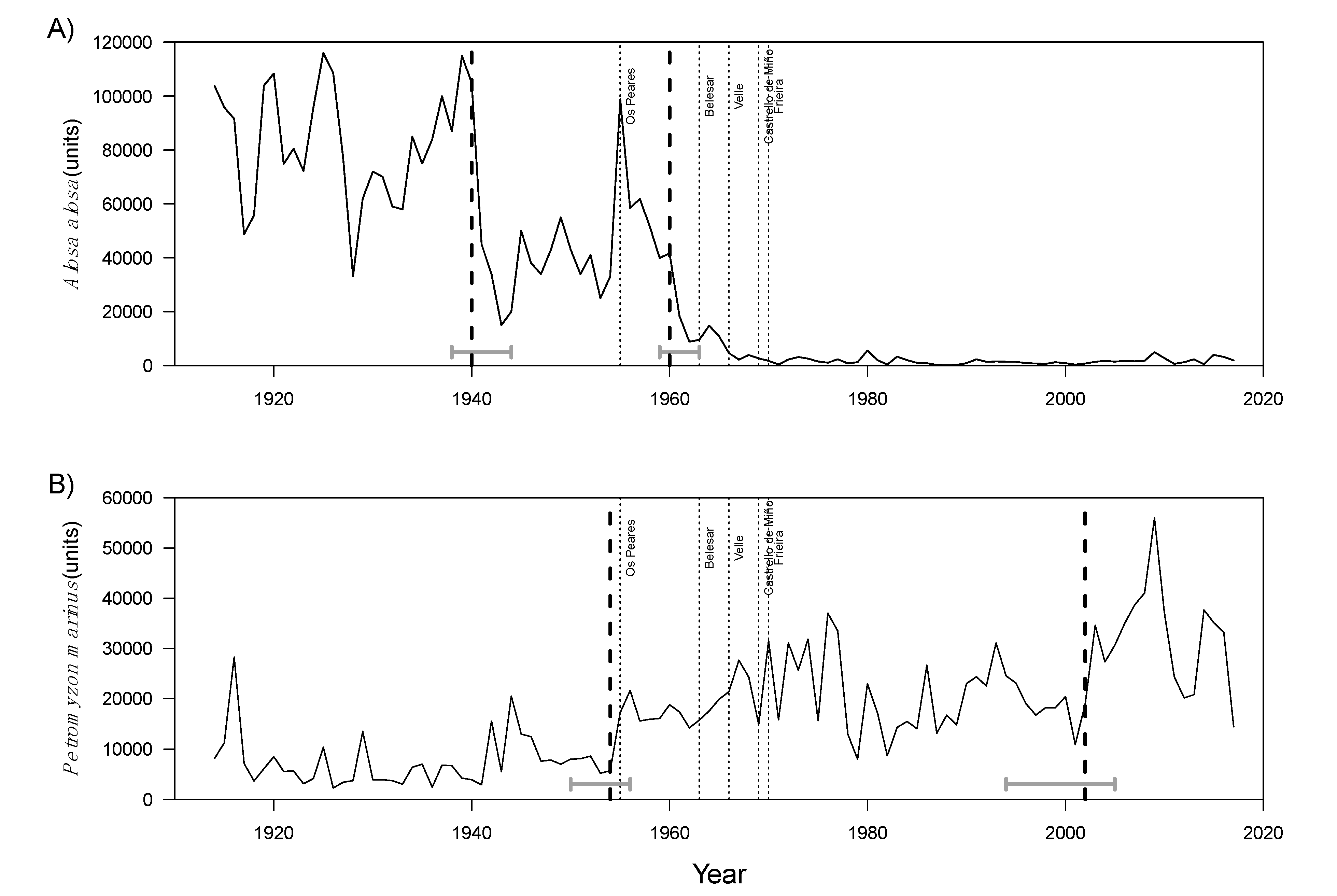
3. Results
3.1. Time Series Analysis of Fish Capture and Environmental Data
3.2. Intervention Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Limburg, K.E.; Waldman, J.R. Dramatic Declines in North Atlantic Diadromous Fishes. BioScience 2009, 59, 955–965. [Google Scholar] [CrossRef]
- Waldman, J.; Wilson, K.A.; Mather, M.; Snyder, N. A Resilience Approach Can Improve Anadromous Fish Restoration. Fisheries 2016, 41, 116–126. [Google Scholar] [CrossRef]
- Chaparro-Pedraza, P.C.; de Roos, A.M. Environmental change effects on life-history traits and population dynamics of anadromous fishes. J. Anim. Ecol. 2019, 88, 1178–1190. [Google Scholar] [CrossRef] [Green Version]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater Biodiversity: Importance, Threats, Status and Conservation Challenges. Biol. Rev. Camb. Philos. Soc. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global Threats to Human Water Security and River Biodiversity. Nature 2010, 468, 334. [Google Scholar] [CrossRef]
- Drouineau, H.; Bau, F.; Alric, A.; Deligne, N.; Gomes, P.; Sagnes, P. Silver eel downstream migration in fragmented rivers: Use of a Bayesian model to track movements triggering and duration. Aquat. Living Resour. 2017, 30, 5. [Google Scholar] [CrossRef] [Green Version]
- Schmutz, S.; Moog, O. Dams: Ecological Impacts and Management. In Riverine Ecosystem Management: Science for Governing Towards a Sustainable Future; Schmutz, S., Sendzimir, J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 111–127. [Google Scholar]
- Lassalle, G.; Rochard, E. Impact of twenty-first century climate change on diadromous fish spread over Europe, North Africa and the Middle East. Glob. Chang. Biol. 2009, 15, 1072–1089. [Google Scholar] [CrossRef]
- Beamish, R.J. Response of Anadromous Fish to Climate Change in the North Pacific; Taylor & Francis: Washington, DC, USA, 1995; pp. 123–136. [Google Scholar]
- Allen, M.R.; Dube, O.P.; Solecki, W.; Aragón-Durand, F.; Cramer, W.; Humphreys, S.; Kainuma, M.; Kala, J.; Mahowald, N.; Mulugetta, Y.; et al. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Mattocks, S.; Hall, C.J.; Jordaan, A. Damming, Lost Connectivity, and the Historical Role of Anadromous Fish in Freshwater Ecosystem Dynamics. BioScience 2017, 67, 713–728. [Google Scholar] [CrossRef] [Green Version]
- Gordon, T.A.C.; Harding, H.R.; Clever, F.K.; Davidson, I.K.; Davison, W.; Montgomery, D.W.; Weatherhead, R.C.; Windsor, F.M.; Armstrong, J.D.; Bardonnet, A.; et al. Fishes in a changing world: Learning from the past to promote sustainability of fish populations. J. Fish Biol. 2018, 92, 804–827. [Google Scholar] [CrossRef] [Green Version]
- Soranno, P.A.; Schimel, D. Macrosystems ecology: Big data, big ecology. Front. Ecol. Environ. 2014, 12, 3. [Google Scholar] [CrossRef] [Green Version]
- Wilson, K.; Veneranta, L. Data-limited diadromous species—Review of European status. In ICES Cooperative Research Report No. 348; ICES Working Group: Copenhagen, Denmark, 2019; p. 273. [Google Scholar]
- Silva, S.; Servia, M.J.; Vieira-Lanero, R.; Nachón, D.J.; Cobo, F. Haematophagous feeding of newly metamorphosed European sea lampreys Petromyzon marinus on strictly freshwater species. J. Fish Biol. 2013, 82, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.; Araújo, M.J.; Antunes, C. Habitat modifications by sea lampreys (Petromyzon marinus) during the spawning season: Effects on sediments. J. Appl. Ichthyol. 2012, 28, 766–771. [Google Scholar] [CrossRef]
- Potter, I.C. Ecology of larval and metamorphosing lampreys. Can. J. Fish. Aquat. Sci. 1980, 37, 1641–1657. [Google Scholar] [CrossRef]
- Quintella, B.R.; Andrade, N.O.; Almeida, P.R. Distribution, larval stage duration and growth of the sea lamprey ammocoetes, Petromyzon marinus L., in a modified river basin. Ecol. Freshw. Fish 2003, 12, 286–293. [Google Scholar] [CrossRef]
- Mota, M.; Antunes, C. A preliminary characterisation of the habitat use and feeding of Allis shad (Alosa alosa) juveniles in the Minho River tidal freshwater wetlands. Limnetica 2012, 31, 165–172. [Google Scholar]
- Baglinière, J.L.; Sabatié, R.; Rochard, E.; Alexandrino, P.; Aprahamian, M.W. Theballis shad Alosa alosa: Biology, ecology, range, and status of populations. Am. Fish. Soc. 2003, 35, 85–102. [Google Scholar]
- Mota, M.; Antunes, C. First report on the status of Allis shad (Alosa alosa) in the Minho River (Northwestern Iberian Peninsula). J. Appl. Ichthyol. 2011, 27, 56–59. [Google Scholar] [CrossRef]
- Baglinière, J.L.; Elie, P. Les aloses (Alosa alosa et Alosa fallax spp.): Écobiologie et Variabilité des Populations; INRA-Cemagref: Paris, France, 2000; p. 275. [Google Scholar]
- Aprahamian, M.; Alexandrino, P.; Antunes, C.; Cobo, F.; King, J.; Lambert, P.; Martin, J. Shads state of the art. In Report of the Workshop on Lampreys and Shads (WKLS), 27–29 November 2014, Lisbon, Portugal; ICES Document CM 2014/SSGEF: 13; International Council for the Exploration of the Sea: Copenhagen, Denmark, 2015; pp. 45–103. 206p. [Google Scholar]
- Mateus, C.S.; Rodríguez-Muñoz, R.; Quintella, B.R.; Alves, M.J.; Almeida, P.R. Lampreys of the Iberian Peninsula: Distribution, Population Status and Conservation. Endanger. Species Res. 2012, 16, 183–198. [Google Scholar] [CrossRef]
- Mota, M.; Rochard, E.; Antunes, C. Status of the Diadromous Fish of the Iberian Peninsula: Past, Present and Trends. Limnetica 2016, 29, 1–18. [Google Scholar]
- Braga, H.O.; Pereira, M.J.; Morgado, F.; Soares, A.M.V.M.; Azeiteiro, U.M. Ethnozoological Knowledge of Traditional Fishing Villages about the Anadromous Sea Lamprey (Petromyzon marinus) in the Minho River, Portugal. J. Ethnobiol. Ethnomedicine 2019, 15, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braga, H.O.; Pereira, M.J.; Musiello-Fernandes, J.; Morgado, F.; Soares, A.M.V.M.; Azeiteiro, U.M. The Role of Local Ecological Knowledge for the Conservation and Sustainable Fisheries of the Sea Lamprey (Petromyzon marinus Linnaeus, 1758) in the Iberian Peninsula. Ocean Coast. 2020, 198, 105345. [Google Scholar] [CrossRef]
- Stratoudakis, Y.; Mateus, C.S.; Quintella, B.R.; Antunes, C.; de Almeida, P.R. Exploited anadromous fish in Portugal: Suggested direction for conservation and management. Mar. Policy 2016, 73, 92–99. [Google Scholar] [CrossRef]
- Mota, M.; Sousa, R.; Bio, A.; Araujo, J.; Braga, C.; Antunes, C. Seasonal changes in fish assemblages in the River Minho tidal freshwater wetlands, NW of the Iberian Peninsula. Ann. De Limnol. Int. J. Limnol. 2014, 50, 185–198. [Google Scholar] [CrossRef]
- Mota, M.; Bio, A.; Bao, M.; Pascual, S.; Rochard, E.; Antunes, C. New insights into biology and ecology of the Minho River Allis shad (Alosa alosa L.): Contribution to the conservation of one of the last European shad populations. Rev. Fish Biol. Fish. 2015, 25, 395–412. [Google Scholar] [CrossRef]
- Sousa, R.; Dias, S.; Guilhermino, L.; Antunes, C. Minho River tidal freshwater wetlands: Threats to faunal biodiversity. Aquat. Biol. 2008, 3, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Mil-Homens, M.; Costa, A.M.; Fonseca, S.; Trancoso, M.A.; Lopes, C.; Serrano, R.; Sousa, R. Characterization of heavy-metal contamination in surface sediments of the Minho River Estuary by way of factor analysis. Arch. Environ. Contam. Toxicol. 2013, 64, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.H.; Sousa, M.C.; Morgado, F.; Dias, J.M. Modeling the Impact of Extreme River Discharge on the Nutrient Dynamics and Dissolved Oxygen in Two Adjacent Estuaries (Portugal). J. Mar. Sci. Eng. 2019, 7, 412. [Google Scholar] [CrossRef] [Green Version]
- Van Puijenbroek, P.J.T.M.; Buijse, A.D.; Kraak, M.H.S.; Verdonschot, P.F.M. Species and river specific effects of river fragmentation on European anadromous fish species. River Res. Appl. 2019, 35, 68–77. [Google Scholar] [CrossRef]
- Almeida, P.; Quintella, B.; Dias, N. Movement of radio-tagged anadromous sea lamprey during the spawning migration in the River Mondego (Portugal). Hydrobiologia 2002, 483, 1–8. [Google Scholar] [CrossRef]
- Paumier, A.; Drouineau, H.; Boutry, S.; Sillero, N.; Lambert, P. Assessing the relative importance of temperature, discharge, and day length on the reproduction of an anadromous fish (Alosa alosa). Freshw. Biol. 2020, 65, 253–263. [Google Scholar] [CrossRef]
- Huang, Z. Drifting with Flow versus Self-Migrating—How Do Young Anadromous Fish Move to the Sea? iScience 2019, 19, 772–785. [Google Scholar] [CrossRef] [Green Version]
- Marques, S.C.; Pardal, M.Â.; Primo, A.L.; Martinho, F.; Falcão, J.; Azeiteiro, U.; Molinero, J.C. Evidence for changes in estuarine zooplankton fostered by increased climate variance. Ecosystems 2018, 21, 56–67. [Google Scholar] [CrossRef] [Green Version]
- Vicente-Serrano, S.; Trigo, R. Hydrological, Socioeconomic and Ecological Impacts of the North Atlantic Oscillation in the Mediterranean Region; Springer Science & Business Media: Berlin, Germany, 2011. [Google Scholar]
- Segurado, P.; Branco, P.; Avelar, A.P.; Ferreira, M.T. Historical changes in the functional connectivity of rivers based on spatial network analysis and the past occurrences of diadromous species in Portugal. Aquat. Sci. 2014, 77, 427–440. [Google Scholar] [CrossRef]
- Trigo, R.M.; Pozo-Vázquez, D.; Osborn, T.J.; Castro-Díez, Y.; Gámiz-Fortis, S.; Esteban-Parra, M.J. North Atlantic Oscillation influence on precipitation, river flow and water resources in the Iberian Peninsula. Int. J. Climatol. J. R. Meteorol. Soc. 2004, 24, 925–944. [Google Scholar] [CrossRef]
- DeCastro, M.; Lorenzo, N.; Taboada, J.; Sarmiento, M.; Alvarez, I.; Gómez-Gesteira, M. Influence of teleconnection patterns on precipitation variability and on river flow regimes in the Miño River basin (NW Iberian Peninsula). Clim. Res. 2006, 32, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Fatela, F.; Moreno, J.; Leorri, E.; Corbett, R. High marsh foraminiferal assemblages’ response to intra-decadal and multi-decadal precipitation variability, between 1934 and 2010 (Minho, NW Portugal). J. Sea Res. 2014, 93, 118–132. [Google Scholar] [CrossRef]
- Mendes, R.; Saldías, G.S.; Decastro, M.; Gómez-Gesteira, M.; Vaz, N.; Dias, J.M. Seasonal and interannual variability of the Douro turbid river plume, northwestern Iberian Peninsula. Remote Sens. Environ. 2017, 194, 401–411. [Google Scholar] [CrossRef]
- Costa, M.; Almeida, P.R.; Domingos, I.; Costa, J.; Correia, M.; Chaves, M.; Teixeira, C. Present status of the main shad’s populations in Portugal. BFPP Bull. Fr. Peche Prot. Milieux Aquat. Bull. Fr. Peche Piscic. 2001, 362/363, 1109–1116. [Google Scholar] [CrossRef]
- Donoso, M.C.; Vargas, N. Water Interactions with Energy, Environment, Food, and Agriculture; EOLSS Publishers Co. Ltd: Paris, France, 2009. [Google Scholar]
- De la Rosa, J.; Araújo, M.; González-Pérez, J.; González-Vila, F.; Soares, A.; Martins, J.; Leorri, E.; Corbett, R.; Fatela, F. Organic matter sources for tidal marsh sediment over the past two millennia in the Minho River estuary (NW Iberian Peninsula). Org. Geochem. 2012, 53, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Larinier, M.; Travade, F. The design of fishways for shad. Bull. Fr. De La Peche Et De La Piscic. 2012, 364, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Neiva, A. Portuguese granites associated with Sn-W and Au mineralizations. Bull. Geol. Soc. Finl. 2002, 74, 79–101. [Google Scholar] [CrossRef]
- Andrade, N.; Quintella, B.; Ferreira, J.; Pinela, S.; Povoa, I.; Pedro, S.; Almeida, P. Sea lamprey (Petromyzon marinus L.) spawning migration in the Vouga river basin (Portugal): Poaching impact, preferential resting sites and spawning grounds. Hydrobiologia 2007, 582, 121–132. [Google Scholar] [CrossRef]
- Hansen, M.J.; Madenjian, C.P.; Slade, J.W.; Steeves, T.B.; Almeida, P.R.; Quintella, B.R. Population ecology of the sea lamprey (Petromyzon marinus) as an invasive species in the Laurentian Great Lakes and an imperiled species in Europe. Rev. Fish Biol. Fish. 2016, 26, 509–535. [Google Scholar] [CrossRef] [Green Version]
- Beaulaton, L.; Taverny, C.; Castelnaud, G. Fishing, abundance and life history traits of the anadromous sea lamprey (Petromyzon marinus) in Europe. Fish. Res. 2008, 92, 90–101. [Google Scholar] [CrossRef]
- Piet, G.J.; Quirijns, F.J.; Robinson, L.; Greenstreet, S.P.R. Potential pressure indicators for fishing, and their data requirements. ICES J. Mar. Sci. 2006, 64, 110–121. [Google Scholar] [CrossRef] [Green Version]
- Stewart, K.R.; Lewison, R.L.; Dunn, D.C.; Bjorkland, R.H.; Kelez, S.; Halpin, P.N.; Crowder, L.B. Characterizing fishing effort and spatial extent of coastal fisheries. PLoS ONE 2010, 5, e14451. [Google Scholar] [CrossRef] [Green Version]
- FAO. Report of the Expert Workshop to Estimate the Magnitude of Illegal, Unreported and Unregulated Fishing Globally; Food and Agricultureorganization of the United Nations: Rome, Italy, 2015. [Google Scholar]


| Variable | Time Coverage | Source |
|---|---|---|
| Alosa alosa Annual Landings (number of fish) | 1914–2017 | Port Authority of Caminha |
| Petromyzon marinus Annual Captures (number of fish) | 1914–2017 | Port Authority of Caminha |
| Median sea surface temperature | 1914–2017 | COBE SST (https://www.esrl.noaa.gov/psd/data/gridded/data.cobe.html); (accessed date 1 October 2019) Minho River mouth |
| Median air temperature | 1914–2017 | Global Historical Climatology Network (https://www.esrl.noaa.gov/psd/data/gridded/data.UDel_AirT_Precip.html#detail) for the Minho River mouth (accessed date 1 October 2019) |
| Median Upwelling index | 1967–2017 | 1967 to 2017: Instituto Español de Oceanografia (http://www.indicedeafloramiento.ieo.es/index_UI_en.html) (accessed date 1 October 2019); for the Rias Baixas region (Spain) |
| Total yearly precipitation | 1914–2017 | 1914 to 2013: Global Precipitation Climatology Centre (https://www.esrl.noaa.gov/psd/data/) (accessed date 1 July 2019); Minho River mouth.2013 to 2017: Era-Interim (https://www.ecmwf.int/en/forecasts/datasets/archive-datasets/reanalysis-datasets/era-interim) (accessed date 1 July 2019); Minho River mouth |
| North Atlantic Oscillation index | 1914–2017 | NOAA’s National Centers for Environmental Information (https://www.esrl.noaa.gov/psd/gcos_wgsp/Timeseries/NAO) (accessed date 1 October 2019) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azeiteiro, U.M.; Pereira, M.J.; Soares, A.M.V.M.; Braga, H.O.; Morgado, F.; Sousa, M.C.; Dias, J.M.; Antunes, C. Dynamics of Two Anadromous Species in a Dam Intersected River: Analysis of Two 100-Year Datasets. Fishes 2021, 6, 21. https://0-doi-org.brum.beds.ac.uk/10.3390/fishes6020021
Azeiteiro UM, Pereira MJ, Soares AMVM, Braga HO, Morgado F, Sousa MC, Dias JM, Antunes C. Dynamics of Two Anadromous Species in a Dam Intersected River: Analysis of Two 100-Year Datasets. Fishes. 2021; 6(2):21. https://0-doi-org.brum.beds.ac.uk/10.3390/fishes6020021
Chicago/Turabian StyleAzeiteiro, Ulisses M., Mário J. Pereira, Amadeu M. V. M. Soares, Heitor O. Braga, Fernando Morgado, Magda C. Sousa, João M. Dias, and Carlos Antunes. 2021. "Dynamics of Two Anadromous Species in a Dam Intersected River: Analysis of Two 100-Year Datasets" Fishes 6, no. 2: 21. https://0-doi-org.brum.beds.ac.uk/10.3390/fishes6020021






