Acceptance of a Protein Concentrate from Alfalfa (Medicago sativa) by Yellow Perch (Perca flavescens) Fed a Formulated Diet
Abstract
:1. Introduction
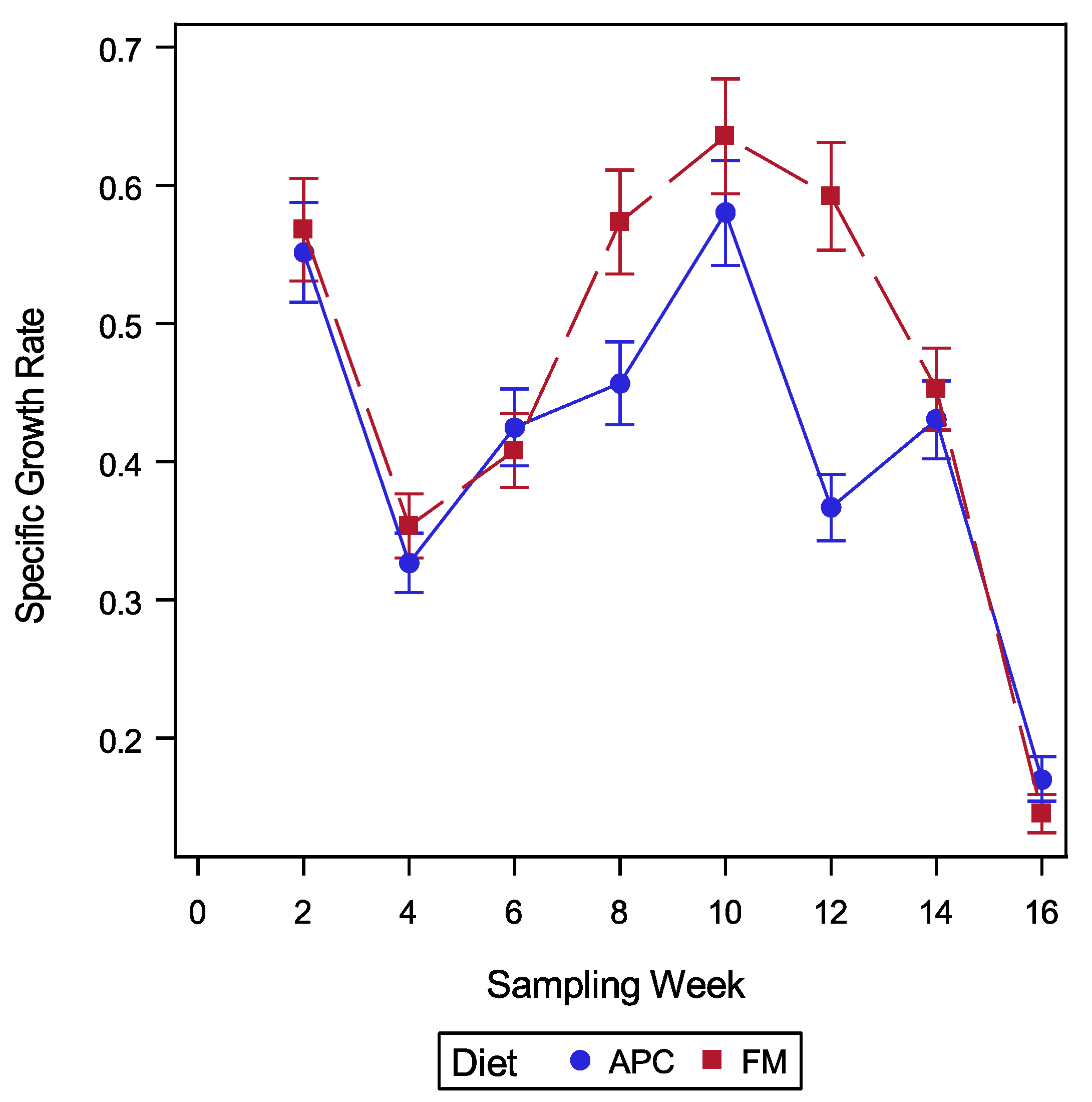
2. Results
3. Discussion
4. Materials and Methods
4.1. Experimental Diets
4.2. Feeding Trial
4.3. Analytical Procedures
4.4. Statistical Analysis and Calculations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interests
References
- Market Data Forecast. Aquafeed Market—Segmented by End Use (Fish-Carp, Salmon, Tilapia, Catfish, and Others; Mollusca-Oyster, Mussel, and Others; Crustaceans-Shrimp, Crab, and Others), by Additives (Vitamins, Minerals, Antioxidants, Amino Acids, Enzymes, Acidifiers, Binders) & by Region—Global Forecast—2025. Available online: https://www.marketdataforecast.com/market-reports/aquafeed-market (accessed on 24 March 2021).
- Rust, M.B.; Barrows, F.T.; Hardy, R.W.; Lazur, A.; Naughten, K.; Silverstein, J. The Future of Aquafeeds. NOAA Technical Memorandum 2011 NMFS F/SPO-124. Available online: https://spo.nmfs.noaa.gov/sites/default/files/tm124.pdf (accessed on 24 March 2021).
- Chatzifotis, S.; Esteban, A.G.; Divanach, P. Fishmeal replacement by alfalfa protein concentrate in sharp snout sea bream Diplodus puntazzo. Fish. Sci. 2006, 72, 1313–1315. [Google Scholar] [CrossRef]
- Olvera-Novoa, M.; Campos, S.G.; Sabido, M.G.; Martínez Palacios, C.A. The use of alfalfa leaf protein concentrates as a protein source in diets for tilapia (Oreochromis mossambicus). Aquaculture 1990, 90, 291–302. [Google Scholar] [CrossRef]
- Rechulicz, J.; Ognik, K.; Grela, E.R. The effect of adding protein-xanthophylls concentrate (PX) from lucerne (Medicago sativa) on growth parameters and redox profile in muscles of carp, Cyprinus carpio (L.). Turk. J. Fish. Aquat. Sci. 2014, 14, 697–703. [Google Scholar] [CrossRef]
- Robert, N.; Coulmier, D.; Divanach, P.; Cuzon, G. PX aqua, an alfalfa protein and pigment concentrate for fish and shrimp feeds. In Proceedings of the 11th International Symposium on Nutrition and Feeding in Fish (Vol. NL203), Phuket, Thailand, 2–7 May 2004. [Google Scholar]
- Koegel, R.G.; Straub, R.J. Fractionation of alfalfa for food, feed, biomass, and enzymes. Trans. ASAE 1996, 39, 769–774. [Google Scholar] [CrossRef]
- Ingredients by Nature. Vitalfa, an Alfalfa Nutrient Concentrate, Composition Sheet. Montclair, CA: Ingredients by Nature. Available online: http://ingredientsbynature.com/storage/app/media/pdfs/IBN-BrewsterBrand-Vitalfa-TechSheet-042018d.pdf (accessed on 24 March 2021).
- Francis, G.; Makkar, H.P.S.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 199, 197–227. [Google Scholar] [CrossRef]
- Gaweł, E.; Grzelak, M. The effect of a protein-xanthophyll concentrate from alfalfa (phytobiotic) on animal production—A current review. Ann. Anim. Sci. 2012, 12, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Barrows, F.T.; Gaylord, T.G.; Sealey, W.M.; Rawles, S.D. Database of Nutrient Digestibility of Traditional and Novel Feed Ingredients for Trout and Hybrid Striped Bass. USDA-ARS, Trout-Grains Project; 2015. Available online: https://www.ars.usda.gov/pacific-west-area/aberdeen-id/small-grains-and-potato-germplasm-research/docs/fish-ingredient-database/ (accessed on 24 March 2021).
- Yost, M.A.; Russelle, M.P.; Coulter, J.A. Field-specific fertilizer nitrogen requirements for first-year corn following alfalfa. Agron. J. 2014, 106, 645–658. [Google Scholar] [CrossRef]
- Fernandez, A.L.; Sheaffer, C.C.; Tautges, N.E.; Putnam, D.H.; Hunter, M.C. Alfalfa, Wildlife, and the Environment, 2nd ed.; National Alfalfa and Forage Alliance: St. Paul, MN, USA, 2019. [Google Scholar]
- Hart, S.D.; Garling, D.L.; Malison, J.A. (Eds.) Yellow Perch (Perca flavescens) Culture Guide. NCRAC Culture Series 103. North Central Regional Aquaculture Center, Ames, IA. 2006. Available online: https://www.ncrac.org/files/biblio/YellowPerchPub.pdf (accessed on 24 March 2021).
- Schaeffer, T.W.; Brown, M.L.; Rosentrater, K.A. Effects of dietary distillers dried grains with solubles and soybean meal on extruded pellet characteristics and growth responses of juvenile yellow perch. N. Am. J. Aquacult. 2011, 73, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Brown, P.B.; Dabrowski, K.; Garling, D.L. Nutrition and feeding of yellow perch (Perca flavescens). J. Appl. Ichthyol. 1996, 12, 171–174. [Google Scholar] [CrossRef]
- Hart, S.D.; Brown, B.J.; Gould, N.L.; Robar, M.L.; Witt, E.M.; Brown, P.B. Predicting the optimal dietary essential amino acid profile for growth of juvenile yellow perch with whole body amino acid concentrations. Aquac. Nutr. 2010, 16, 248–253. [Google Scholar] [CrossRef]
- Kasper, C.S.; Watkins, B.A.; Brown, P.B. Evaluation of two soybean meals fed to yellow perch (Perca flavescens). Aquac. Nutr. 2007, 13, 431–438. [Google Scholar] [CrossRef]
- Schaeffer, T.W.; Hennen, M.J.; Brown, M.L.; Rosentrater, K.A. Nutritional composition and use of common carp muscle in yellow perch diets. N. Am. J. Aquacult. 2012, 74, 297–305. [Google Scholar] [CrossRef]
- Malison, J.A.; Held, J.A. Effects of fish size at harvest, initial stocking density and tank lighting conditions on the habituation of pond-reared yellow perch (Perca flavescens) to intensive culture conditions. Aquaculture 1992, 104, 67–78. [Google Scholar] [CrossRef]
- Andriani, Y.; Dhahiyat, Y.; Zahidah, Z.; Subhan, U.; Iskandar, I.; Zidni, I.; Mawardiani, T. Effect of water irrigation volume on Capsicum frutescens growth and plankton abundance in aquaponics system. IOP Conf. Ser. Earth Environ. Sci. 2018, 139, 012001. [Google Scholar] [CrossRef]
- Pinho, S.M.; Molinari, D.; de Mello, G.L.; Fitzsimmons, K.M.; Coelho Emerenciano, M.G. Effluent from a biofloc technology (BFT) tilapia culture on the aquaponics production of different lettuce varieties. Ecol. Eng. 2017, 103, 146–153. [Google Scholar] [CrossRef]
- Tidwell, J.H.; Coyle, S.D.; Evans, J.; Weibel, C.; McKinney, J.; Dodson, K.; Jones, H. Effect of culture temperature on growth, survival, and biochemical composition of yellow perch Perca flavescens. J. World Aquacult. Soc. 1999, 30, 324–330. [Google Scholar] [CrossRef]
- Sen, S.; Makkar, H.P.S.; Becker, K. Alfalfa saponins and their implication in animal nutrition. J. Agric. Food Chem. 1998, 46, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Tava, A.; Odoardi, M.; Oleszek, W. Seasonal changes of saponin content in five alfalfa (Medicago sativa) cultivars. Agricoltura Mediterranea 1999, 129, 111–116. [Google Scholar]
- Coburn, J.E.M. Manufacture and Use of Alfalfa (Medicago sativa L.) Leaf Protein Concentrate as a Protein Supplement in Fish Culture Diets. Master’s Thesis, University of Minnesota, St. Paul, MN, USA, 2019. [Google Scholar]
- Gaylord, T.G.; Sealey, W.M.; Barrows, F.T.; Myrick, C.A.; Fornshell, G. Evaluation of ingredient combinations from differing origins (fishmeal, terrestrial animal and plants) and two different formulated nutrient targets on rainbow trout growth and production efficiency. Aquac. Nutr. 2017, 23, 1319–1328. [Google Scholar] [CrossRef]
- Latimer, G.W.J. (Ed.) Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Rockville, MD, USA, 2012. [Google Scholar]

| Component | APC | SBM | MFM |
|---|---|---|---|
| Crude protein | 520 | 519 | 705 |
| Crude lipid | 104 | 17 | 104 |
| Ash | 140 | ND § | ND |
| Carbohydrate | 172 | ND | ND |
| Crude fiber | 27 | ND | ND |
| Amino Acids | |||
| Methionine | 11.4 | 6.6 | 20.1 |
| Lysine | 33.8 | 27.2 | 52.8 |
| Threonine | 25.5 | 23.4 | 29.8 |
| Histidine | 12.5 | 13.1 | 18.7 |
| Leucine | 45.8 | 45.3 | 51.5 |
| Phenylalanine | 29.1 | 29.2 | 28.2 |
| Valine | 29.6 | 28.0 | 35.4 |
| Arginine | 31.2 | 44.3 | 51.3 |
| Isoleucine | 25.0 | 23.4 | 30.7 |
| Tryptophan | 10.4 | ND | ND |
| Cysteine | 4.7 | ND | ND |
| Glutamic Acid | 54.6 | 94.0 | 86.8 |
| Proline | 23.4 | 28.3 | 31.2 |
| Tyrosine | 23.4 | 21.9 | 23.3 |
| Alanine | 30.2 | 23.9 | 43.0 |
| Aspartic Acid | 50.4 | 61.0 | 61.1 |
| Glycine | 26.5 | 22.2 | 43.6 |
| Serine | 23.4 | 30.3 | 26.7 |
| Ingredient | Fishmeal Control | APC |
|---|---|---|
| Menhaden Fishmeal, Omega Protein, Special Select | 150.0 | 0.0 |
| Alfalfa Nutrient Concentrate | 0.0 | 184.0 |
| Soybean Meal 48% crude protein | 150.0 | 150.0 |
| Poultry Meal 1 | 150.0 | 150.0 |
| Corn Protein Concentrate, Empyreal 75 2 | 50.0 | 50.0 |
| Wheat Gluten Meal | 3.2 | 5.6 |
| Pastry Flour | 342.6 | 300.6 |
| Lecithin | 10.0 | 10.0 |
| Stay-C | 1.5 | 1.5 |
| Vitamin premix ARS 702 3 | 10.0 | 10.0 |
| Trace Mineral Mix ARS 640 4 | 1.0 | 1.0 |
| Sodium Chloride | 2.8 | 2.8 |
| Magnesium Oxide | 0.6 | 0.6 |
| Potassium Chloride | 5.6 | 5.6 |
| Monocalcium Phosphate | 18.0 | 32.0 |
| Choline Chloride 50% | 10.0 | 10.0 |
| DL-Methionine | 4.2 | 5.0 |
| Lysine Hydrochloride | 18.1 | 18.8 |
| Threonine | 3.2 | 3.2 |
| Taurine | 5.0 | 5.0 |
| Yttrium Oxide | 1.0 | 1.0 |
| Menhaden Fish Oil | 63.2 | 53.3 |
| Calculated Composition | ||
| Crude Protein | 404 | 401 |
| Crude Lipid | 120 | 120 |
| Digestible Energy (Kcal/g) | 4.08 | 4.02 |
| Proximate Chemical Composition | ||
| Crude Protein | 411 | 399 |
| Crude Lipid | 97.0 | 93.7 |
| Ash | 72.9 | 70.6 |
| Parameter | Diets | SE | p-Value | |
|---|---|---|---|---|
| FM | APC | |||
| Growth Performance Indexes | ||||
| IBW (g) | 23.4 | 21.7 | -- | -- |
| FBW (g) | 40.4 a | 33.3 b | 3.13 | <0.01 |
| WG (%) | 67.14 a | 54.67 b | 3.80 | 0.04 |
| SGR (%/day) | 0.46 a | 0.39 b | 0.02 | 0.04 |
| K | 1.10 | 1.08 | 0.02 | 0.35 |
| Survival (%) | 87 | 83 | 4 | 0.26 |
| FI (% BW/day) | 0.59 b | 0.65 a | 0.02 | 0.05 † |
| FCR | 1.67 b | 1.99 a | 0.23 | 0.03 |
| FY (%) | 39.82 | 39.81 | 0.77 | 0.97 |
| VSI | 7.58 b | 8.13 a | 0.78 | 0.01 |
| HSI | 2.30 | 2.40 | 0.10 | 0.24 |
| Fillet Composition | ||||
| Moisture (g/kg) | 788.6 b | 792.6 a | 4.2 | 0.05 |
| Crude Protein (g/kg) | 196.3 | 193.8 | 5.3 | 0.20 |
| Crude Fat (g/kg) | 1.6 | 1.4 | 0.2 | 0.18 |
| Ash (g/kg) | 12.8 | 12.7 | 0.5 | 0.72 |
| Gross Energy (kcal/g) | 1.04 | 1.03 | 0.01 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coburn, J.; Wells, M.S.; Phelps, N.B.D.; Gaylord, T.G.; Samac, D.A. Acceptance of a Protein Concentrate from Alfalfa (Medicago sativa) by Yellow Perch (Perca flavescens) Fed a Formulated Diet. Fishes 2021, 6, 9. https://0-doi-org.brum.beds.ac.uk/10.3390/fishes6020009
Coburn J, Wells MS, Phelps NBD, Gaylord TG, Samac DA. Acceptance of a Protein Concentrate from Alfalfa (Medicago sativa) by Yellow Perch (Perca flavescens) Fed a Formulated Diet. Fishes. 2021; 6(2):9. https://0-doi-org.brum.beds.ac.uk/10.3390/fishes6020009
Chicago/Turabian StyleCoburn, Jessica, M. Scott Wells, Nicholas B. D. Phelps, T. Gibson Gaylord, and Deborah A. Samac. 2021. "Acceptance of a Protein Concentrate from Alfalfa (Medicago sativa) by Yellow Perch (Perca flavescens) Fed a Formulated Diet" Fishes 6, no. 2: 9. https://0-doi-org.brum.beds.ac.uk/10.3390/fishes6020009






