Cryobank of Mediterranean Brown Trout Semen: Evaluation of the Use of Frozen Semen up to Six Hours Post-Collection
Abstract
:1. Introduction
2. Results
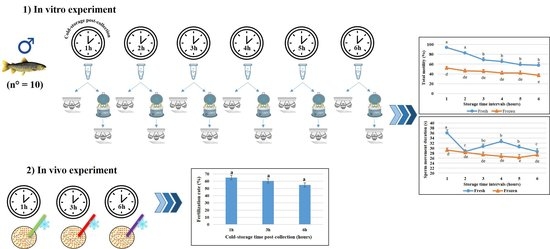
2.1. Effect of Storage Time Post-Semen Collection on Fresh Sperm Motility Parameters
2.2. Effect of Storage Time Post-Collection on Frozen Sperm Motility Parameters
2.3. Effect of Storage Time Post-Collection on Post-Thaw Fertilization Rate
3. Discussion
4. Materials and Methods
4.1. Animal Capture and Sperm and Eggs Collection
4.2. In Vitro Experimental Design and Cryopreservation Procedure
4.3. Sperm Analysis
4.4. Fertilizing Ability Trials of Cryopreserved Semen
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crivelli, A.J.; Poizat, G.; Berrebi, P.; Jesensek, D.; Rubin, J.F. Conservation biology applied to fish: The example of a project for rehabilitating the Marble trout (Salmo marmoratus) in Slovenia. Cybium 2000, 24, 211–230. [Google Scholar]
- Araguas, R.M.; Sanz, N.; Fernández, R.; Utter, F.M.; Pla, C.; García-Marín, J.-L. Genetic refuges for a self-sustained fishery: Experience in wild brown trout populations in the eastern Pyrenees. Ecol. Freshw. Fish 2008, 17, 610–616. [Google Scholar] [CrossRef]
- Araguas, R.M.; Sanz, N.; Fernández, R.; Utter, F.M.; Pla, C.; García-Marín, J.-L. Role of Genetic Refuges in the Restoration of Native Gene Pools of Brown Trout. Conserv. Biol. 2009, 23, 871–878. [Google Scholar] [CrossRef]
- Caudron, A.; Champigneulle, A.; Guyomard, R.; Largiadèr, C.R. Assessment of three strategies practiced by fishery managers for restoring native brown trout (Salmo trutta) populations in Northern French Alpine Streams. Ecol. Freshw. Fish 2011, 20, 478–491. [Google Scholar] [CrossRef]
- Caputo Barucchi, V.; Carosi, A.; Giovannotti, M.; La Porta, G.; Splendiani, S.; Lorenzoni, M. Life+ Trout Project (LIFE12 NAT/IT/0000940) for the Recovery and Conservation of Mediterranean Trout (Salmo Trutta Complex) in the Central Apennines (Italy). Front. Mar. Sci. Conference Abstract. In Proceedings of the XV European Congress of Ichthyology, Porto, Portugal, 7–11 September 2015. [Google Scholar]
- Bianco, P.G.; Caputo Barucchi, V.; Ferrito, V.; Lorenzoni, M.; Nonnis Marzano, F.; Stefani, F.; Sabatini, A.; Tancioni, L. Pesci d’acqua dolce. In Lista Rossa IUCN dei Vertebrati Italiani; Rondinini, C., Battistoni, A., Peronace, V., Teofili, C., Eds.; Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare: Roma, Italy, 2013; p. 54. [Google Scholar]
- Berrebi, P.; Povz, M.; Jesensek, D.; Cattaneo-Berrebi, G.; Crivelli, A.J. The genetic diversity of native, stocked and hybrid populations of marble trout in the Soča river, Slovenia. Heredity 2000, 85, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Berrebi, P.; Jesenšek, D.; Crivelli, A.J. Natural and domestic introgressions in the marble trout population of Soča River (Slovenia). Hydrobiologia 2017, 785, 277–291. [Google Scholar] [CrossRef]
- Marzano, F.N.; Corradi, N.; Papa, R.; Tagliavini, J.; Gandolfi, G. Molecular Evidence for Introgression and Loss of Genetic Variability in Salmo (trutta) macrostigma as a Result of Massive Restocking of Apennine Populations (Northern and Central Italy). Environ. Boil. Fishes 2003, 68, 349–356. [Google Scholar] [CrossRef]
- Caputo, V.; Giovannotti, M.; Cerioni, P.N.; Caniglia, M.L.; Splendiani, A. Genetic diversity of brown trout in central Italy. J. Fish Biol. 2004, 65, 403–418. [Google Scholar] [CrossRef]
- Querci, G.; Pecchioli, E.; Leonzio, C.; Frati, F.; Nardi, F. Molecular characterization and hybridization in Salmo (trutta) macrostigma morphotypes from Central Italy. Hydrobiologia 2012, 702, 191–200. [Google Scholar] [CrossRef]
- Gratton, P.; Allegrucci, G.; Sbordoni, V.; Gandolfi, A. The evolutionary jigsaw puzzle of the surviving trout (Salmo trutta L. complex) diversity in the Italian region. A multilocus Bayesian approach. Mol. Phylogenet. Evol. 2014, 79, 292–304. [Google Scholar] [CrossRef]
- Splendiani, A.; Ruggeri, P.; Giovannotti, M.; Pesaresi, S.; Occhipinti, G.; Fioravanti, T.; Lorenzoni, M.; Cerioni, P.N.; Barucchi, V.C. Alien brown trout invasion of the Italian peninsula: The role of geological, climate and anthropogenic factors. Biol. Invasions 2016, 18, 2029–2044. [Google Scholar] [CrossRef]
- Iaffaldano, N.; Di Iorio, M.; Manchisi, A.; Esposito, S.; Gibertoni, P.P. Effective freezing rate for semen cryopreservation in endangered Mediterranean brown trout (Salmo trutta macrostigma) inhabiting the Biferno river (South Italy). Zygote 2015, 24, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, M.; Esposito, S.; Rusco, G.; Roncarati, A.; Miranda, M.; Gibertoni, P.P.; Cerolini, S.; Iaffaldano, N. Semen cryopreservation for the Mediterranean brown trout of the Biferno River (Molise-Italy): Comparative study on the effects of basic extenders and cryoprotectants. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusco, G.; Di Iorio, M.; Gibertoni, P.P.; Esposito, S.; Penserini, M.; Roncarati, A.; Cerolini, S.; Iaffaldano, N. Optimization of Sperm Cryopreservation Protocol for Mediterranean Brown Trout: A Comparative Study of Non-Permeating Cryoprotectants and Thawing Rates In Vitro and In Vivo. Animals 2019, 9, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusco, G.; Di Iorio, M.; Iampietro, R.; Esposito, S.; Gibertoni, P.P.; Penserini, M.; Roncarati, A.; Iaffaldano, N. A Simple and Efficient Semen Cryopreservation Method to Increase the Genetic Variability of Endangered Mediterranean Brown Trout Inhabiting Molise Rivers. Animals 2020, 10, 403. [Google Scholar]
- Jawahar, K.T.P.; Betsy, J. Cryopreservation of Fish Gametes: An Overview. In Cryopreservation of Fish Gametes; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2020; pp. 151–175. [Google Scholar]
- Lahnsteiner, F.; Weismann, T.; Patzner, R.A. Aging Processes of Rainbow Trout Semen during Storage. Progress. Fish Cult. 1997, 59, 272–279. [Google Scholar] [CrossRef]
- Cremades, T.; Vazquez, J.M.; Martínez, E.A.; Roca, J.; Rodriguez-Martinez, H.; Abáigar, T. Kinematic Changes during the Cryopreservation of Boar Spermatozoa. J. Androl. 2005, 26, 610–618. [Google Scholar] [CrossRef] [Green Version]
- Katz, D.F.; Yanagimachi, R. Movement Characteristics of Hamster and Guinea Pig Spermatozoa upon Attachment to the Zona Pellucida. Biol. Reprod. 1981, 25, 785–791. [Google Scholar] [CrossRef] [Green Version]
- Suarez, S.S.; Katz, D.F.; Overstreet, J.W. Movement Characteristics and Acrosomal Status of Rabbit Spermatozoa Recovered at the Site and Time of Fertilization. Biol. Reprod. 1983, 29, 1277–1287. [Google Scholar] [CrossRef] [Green Version]
- Suarez, S.S. Hamster sperm motility transformation during development of hyperactivation in vitro and epididymal maturation. Gamete Res. 1988, 19, 51–65. [Google Scholar] [CrossRef]
- Shivaji, S.; Peedicayil, J.; Devi, L.G. Analysis of the motility parameters of in vitro hyperactivated hamster spermatozoa. Mol. Reprod. Dev. 1995, 42, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Casas, I.; Sancho, S.; Briz, M.D.; Pinart, E.; Bussalleu, E.; Yeste, M.; Bonet, S. Freezability prediction of boar ejaculates assessed by functional sperm parameters and sperm proteins. Theriogenology 2009, 72, 930–948. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.; Kirkman-Brown, J.C.; Connolly, T.; Gaffney, E. Modelling a tethered mammalian sperm cell undergoing hyperactivation. J. Theor. Biol. 2012, 309, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S.; Ho, H.C. Hyperactivation of mammalian sperm. Cell. Mol. Boil. 2003, 49, 351–356. [Google Scholar]
- Yanagimachi, R. In Vitro Capacitation of Hamster Spermatozoa by Follicular Fluid. Reproduction 1969, 18, 275–286. [Google Scholar] [CrossRef]
- Ho, H.-C.; Suarez, S.S. An Inositol 1,4,5-Trisphosphate Receptor-Gated Intracellular Ca2+ Store Is Involved in Regulating Sperm Hyperactivated Motility. Biol. Reprod. 2001, 65, 1606–1615. [Google Scholar] [CrossRef] [Green Version]
- Watson, P. The causes of reduced fertility with cryopreserved semen. Anim. Reprod. Sci. 2000, 60–61, 481–492. [Google Scholar] [CrossRef]
- Andrabi, S.M.H. Fundamental principles of cryopreservation of Bos taurus and Bos indicus bull spermatozoa (Minireview). Int. J. Agric. Biol. 2007, 9, 367–369. [Google Scholar]
- Green, C.; Watson, P. Comparison of the capacitation-like state of cooled boar spermatozoa with true capacitation. Reprodoction 2001, 122, 889–898. [Google Scholar] [CrossRef]
- Harrison, R.A.; Gadella, B.M. Bicarbonate-induced membrane processing in sperm capacitation. Theriogenology 2005, 63, 342–351. [Google Scholar] [CrossRef]
- Saravia, F.; Hernández, M.; Wallgren, M.; Johannisson, A.; Rodriguez-Martinez, H. Controlled cooling during semen cryopreservation does not induce capacitation of spermatozoa from two portions of the boar ejaculate. Int. J. Androl. 2007, 30, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, S.; Morisawa, M. Roles for potassium and calcium channels in the initiation of sperm motility in rainbow trout (K+/Ca2+/channel/sperm motility/rainbow trout). Develop. Growth Differ. 1988, 30, 117–124. [Google Scholar] [CrossRef]
- Tanimoto, S.; Nakazawa, T.; Kudo, Y.; Morisawa, M. Implication that potassium flux and increase in intracellular calcium are necessary for the initiation of sperm motility in salmonid fishes. Mol. Reprod. Dev. 1994, 39, 409–414. [Google Scholar] [CrossRef]
- Kho, K.H.; Tanimoto, S.; Inaba, K.; Oka, Y.; Morisawa, M. Transmembrane Cell Signaling for the Initiation of Trout Sperm Motility: Roles of Ion Channels and Membrane Hyperpolarization for Cyclic AMP Synthesis. Zool. Sci. 2001, 18, 919–928. [Google Scholar] [CrossRef]
- Krasznai, Z.; Morisawa, M.; Morisawa, S.; Krasznai, Z.T.; Trón, L.; Gáspár, R.; Márián, T. Role of ion channels and membrane potential in the initiation of carp sperm motility. Aquat. Living Resour. 2003, 16, 445–449. [Google Scholar] [CrossRef]
- Detweiler, C.; Thomas, P. Role of ions and ion channels in the regulation of Atlantic croaker sperm motility. J. Exp. Zool. 1998, 281, 139–148. [Google Scholar] [CrossRef]
- Bondarenko, O.; Dzyuba, B.; Rodina, M.; Cosson, J. Role of Ca2+ in the IVM of spermatozoa from the sterlet Acipenser ruthenus. Reprod. Fertil. Dev. 2017, 29, 1319–1328. [Google Scholar] [CrossRef]
- Yanagimachi, R.; Harumi, T.; Matsubara, H.; Yan, W.; Yuan, S.; Hirohashi, N.; Iida, T.; Yamaha, E.; Arai, K.; Matsubara, T.; et al. Chemical and physical guidance of fish spermatozoa into the egg through the micropyle†,‡. Biol. Reprod. 2017, 96, 780–799. [Google Scholar] [CrossRef] [Green Version]
- Lissabet, J.F.B.; Belén, L.H.; Lee-Estevez, M.; Risopatrón, J.; Valdebenito, I.; Figueroa, E.; Farias, J. The CatSper channel is present and plays a key role in sperm motility of the Atlantic salmon (Salmo salar). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 241, 110634. [Google Scholar] [CrossRef]
- Kirichok, Y.; Navarro, B.; Clapham, D. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nat. Cell Biol. 2006, 439, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Darszon, A.; Guerrero, A.; Galindo, B.E.; Nishigaki, T.; Wood, C.D. Sperm-activating peptides in the regulation of ion fluxes, signal transduction and motility. Int. J. Dev. Biol. 2008, 52, 595–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labbé, C.; Maisse, G. Influence of rainbow trout thermal acclimation on sperm cryopreservation: Relation to change in the lipid composition of the plasma membrane. Aquaculture 1996, 145, 281–294. [Google Scholar] [CrossRef]
- Martínez-Pastor, F.; Fernández-Santos, M.R.; Del Olmo, E.; Domínguez-Rebolledo, A.E.; Esteso, M.C.; Montoro, V.; Garde, J.J. Mitochondrial activity and forward scatter vary in necrotic, apoptotic and membrane-intact spermatozoan subpopulations. Reprod. Fertil. Dev. 2008, 20, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Gibertoni, P.P.; Jelli, F.; Bracchi, P. Allevamento, riproduzione e reintroduzione in ambiente naturale di trote fario di “ceppo mediterraneo”, Salmo (trutta) trutta, L. Ann. Della Fac. Med. Vet. 1998, 18, 1–20. [Google Scholar]
- Jelli, F.; Gibertoni, P.P. Recupero e Reintroduzione di Ceppi Autoctoni di Trota Fario, Salmo (trutta) trutta L., nel Bacino del Fiu-me Secchia. Atti del Convegno: La Trota Fario, Salmo (trutta) trutta L. di Ceppo Mediterraneo; Esperienze Gestionali a Confronto: Reggio Emilia, Italy, 1999; pp. 21–28.
- Penserini, M.; Nonnis Marzano, F.; Gandolfi, G.; Maldini, M. Fenotipi della trota mediterranea: Metodologia di indagine molecolare combinata e selezione morfologica per l’identificazione degli esemplari autoctoni. J. Freshwat. Biol. 2006, 34, 69–75. [Google Scholar]
- Billard, R. Reproduction in rainbow trout: Sex differentiation, dynamics of gametogenesis, biology and preservation of gametes. Aquaculture 1992, 100, 263–298. [Google Scholar] [CrossRef]



| Storage Time | Fertilization Rate (%) | |
|---|---|---|
| Means ± SE | Min—Max | |
| 1 h | 65.2 ± 6.3 a | 43.0—87.5 |
| 3 h | 60.4 ± 6.9 a | 40.8—79.3 |
| 6 h | 54.8 ± 3.5 a | 41.3—64.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusco, G.; Di Iorio, M.; Iampietro, R.; Roncarati, A.; Esposito, S.; Iaffaldano, N. Cryobank of Mediterranean Brown Trout Semen: Evaluation of the Use of Frozen Semen up to Six Hours Post-Collection. Fishes 2021, 6, 26. https://0-doi-org.brum.beds.ac.uk/10.3390/fishes6030026
Rusco G, Di Iorio M, Iampietro R, Roncarati A, Esposito S, Iaffaldano N. Cryobank of Mediterranean Brown Trout Semen: Evaluation of the Use of Frozen Semen up to Six Hours Post-Collection. Fishes. 2021; 6(3):26. https://0-doi-org.brum.beds.ac.uk/10.3390/fishes6030026
Chicago/Turabian StyleRusco, Giusy, Michele Di Iorio, Roberta Iampietro, Alessandra Roncarati, Stefano Esposito, and Nicolaia Iaffaldano. 2021. "Cryobank of Mediterranean Brown Trout Semen: Evaluation of the Use of Frozen Semen up to Six Hours Post-Collection" Fishes 6, no. 3: 26. https://0-doi-org.brum.beds.ac.uk/10.3390/fishes6030026