Biosensor-Mediated In Situ Imaging Defines the Availability Period of Assimilatory Glutamine in Maize Seedling Leaves Following Nitrogen Fertilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Maize Growth Conditions
2.2. Two Hour Nitrogen Pulse and Leaf Tissue Sampling
2.3. Generating Leaf In Situ Images of Free Glutamine
3. Results and Discussion
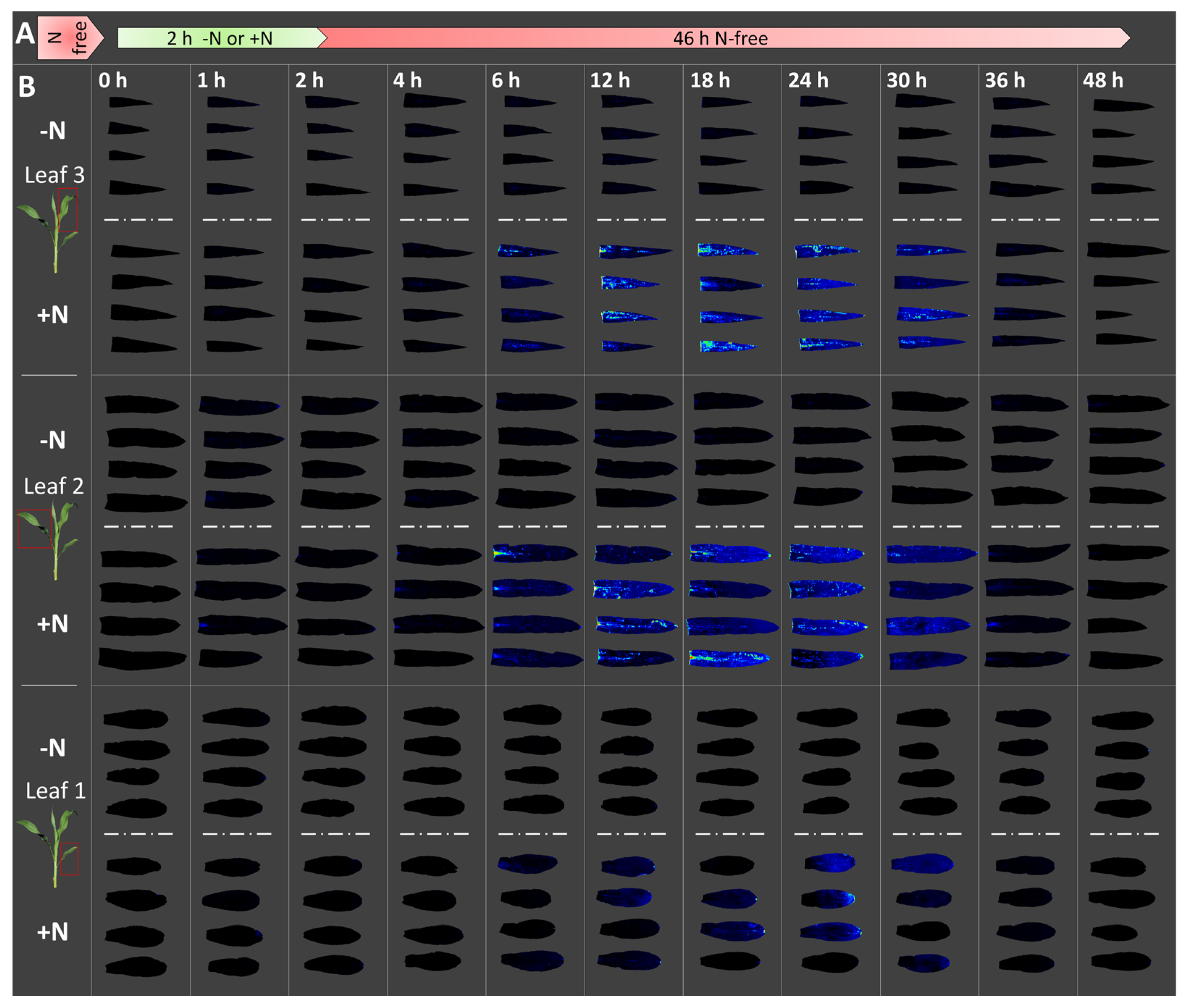
3.1. GlnLux Imaging Demonstrates the Availability Period of Assimilatory Gln
3.2. The Timing of Gln Accumulation and Depletion Occurs Symmetrically along the Leaf-Age Sink Gradient
3.3. The Timing of Gln Availability May Be Specific to the Conditions Used
3.4. Implications of a Limited Gln-Availability Period in Maize Leaf Tissue
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.M.; Habash, D. The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytol. 2009, 182, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Miflin, B.J.; Lea, P.J. The pathway of nitrogen assimilation in plants. Phytochemistry 1976, 15, 873–885. [Google Scholar] [CrossRef]
- Joy, K.W. Ammonia, glutamine, and asparagine: A carbon-nitrogen interface. Can. J. Bot. 1988, 66, 2103–2109. [Google Scholar] [CrossRef]
- Cren, M.; Hirel, B. Glutamine synthetase in higher plants: Regulation of gene and protein expression from the organ to the cell. Plant Cell Physiol. 1999, 40, 1187–1193. [Google Scholar] [CrossRef]
- Lam, H.M.; Coschigano, K.T.; Oliveira, I.C.; Melo-Oliveira, R.; Coruzzi, G.M. The molecular-genetics of nitrogen assimilation into amino acids in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 569–593. [Google Scholar] [CrossRef] [PubMed]
- Sugiharto, B.; Sugiyama, T. Effects of nitrate and ammonium on gene expression of phosphoenolpyruvate carboxylase and nitrogen metabolism in maize leaf tissue during recovery from nitrogen stress. Plant Physiol. 1992, 98, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Cañas, R.A.; Yesbergenova-Cuny, Z.; Simons, M.; Chardon, F.; Armengaud, P.; Quilleré, I.; Cukier, C.; Gibon, Y.; Limami, A.M.; Nicolas, S.; et al. Exploiting the genetic diversity of maize using a combined metabolomic, enzyme activity profiling, and metabolic modeling approach to link leaf physiology to kernel yield. Plant Cell 2017, 29, 919–943. [Google Scholar] [CrossRef] [PubMed]
- Tessaro, M.J.; Soliman, S.S.M.; Raizada, M.N. Bacterial whole-cell biosensor for glutamine with applications for quantifying and visualizing glutamine in plants. Appl. Environ. Microbiol. 2012, 78, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Goron, T.L.; Raizada, M.N. Biosensor-based spatial and developmental mapping of maize leaf glutamine at vein-level resolution in response to different nitrogen rates and uptake/assimilation durations. BMC Plant Biol. 2016, 16, 230. [Google Scholar] [CrossRef] [PubMed]
- Khanal, R.; Earl, H.; Lee, E.A.; Lukens, L. The genetic architecture of flowering time and related traits in two early flowering maize lines. Crop Sci. 2011, 51, 146–156. [Google Scholar] [CrossRef]
- Goron, T.L.; Bhosekar, V.K.; Shearer, C.R.; Watts, S.; Raizada, M.N. Whole plant acclimation responses by finger millet to low nitrogen stress. Front. Plant Sci. 2015, 6, 652. [Google Scholar] [CrossRef] [PubMed]
- Monselise, E.B.I.; Kost, D. 15N NMR spectroscopic study of ammonium ion assimilation by Spirodela oligorrhiza—Lemnaceae—As affected by light and carbon supply in green and extoliated plants. Israel J. Plant Sci. 1998, 46, 255–264. [Google Scholar] [CrossRef]
- Oaks, A.; Sivasankar, S.; Goodfellow, V.J. The specificity of methionine sulfoximine and azaserine inhibition in plant tissues. Phytochemistry 1998, 49, 355–357. [Google Scholar] [CrossRef]
- Meiri, A.; Silk, W.K.; Lauchli, A. Growth and deposition of inorganic nutrient elements in developing leaves of Zea mays L. Plant Physiol. 1992, 99, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Poethig, R.S. Phase change and the regulation of developmental timing in plants. Science 2003, 301, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Neves-Piestun, B.G.; Bernstein, N. Salinity-induced changes in the nutritional status of expanding cells may impact leaf growth inhibition in maize. Funct. Plant Biol. 2005, 32, 141–152. [Google Scholar] [CrossRef]
- Hu, Y.; Burucs, Z.; von Tucher, S.; Schmidhalter, U. Short-term effects of drought and salinity on mineral nutrient distribution along growing leaves of maize seedlings. Environ. Exp. Bot. 2007, 60, 268–275. [Google Scholar] [CrossRef]
- Andrews, M. The partitioning of nitrate assimilation between root and shoot of higher plants. Plant Cell Environ. 1986, 9, 511–519. [Google Scholar]
- Sakakibara, H.; Kawabata, S.; Takahashi, H.; Hase, T.; Sugiyama, T. Molecular cloning of the family of glutamine synthetase genes from maize: Expression of genes for glutamine synthetase and ferredoxin-dependent glutamate synthase in photosynthetic and non-photosynthetic tissues. Plant Cell Physiol. 1992, 33, 49–58. [Google Scholar]
- Redinbaugh, M.G.; Campbell, W.H. Glutamine synthetase and ferredoxin-dependant glutamate synthase expression in the maize (Zea mays) root primary response to nitrate. Plant Physiol. 1993, 101, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Sukanya, R.; Li, M.; Snustad, D.P. Root- and shoot-specific responses of individual glutamine synthetase genes of maize to nitrate and ammonium. Plant Mol. Biol. 1994, 26, 1935–1946. [Google Scholar] [CrossRef] [PubMed]
- Prinsi, B.; Espen, L. Mineral nitrogen sources differently affect root glutamine synthetase isoforms and amino acid balance among organs in maize. BMC Plant Biol. 2015, 15, 96. [Google Scholar] [CrossRef] [PubMed]
- Lillo, C. Signalling cascades integrating light-enhanced nitrate metabolism. Biochem. J. 2008, 415, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Okumoto, S.; Zhang, X.; Ervin, E. Circadian patterns of the major nitrogen metabolism-related enzymes and metabolites in creeping bentgrass and the influence of cytokinin and nitrate. Crop Sci. 2011, 51, 2145–2154. [Google Scholar] [CrossRef]
- Magalhães, J.R.; Ju, G.C.; Rich, P.J.; Rhodes, D. Kinetics of 15NH4+ Assimilation in Zea mays. Plant Physiol. 1990, 94, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Vidmar, J.J.; Zhuo, D.; Siddiqi, M.Y.; Schjoerring, J.K.; Touraine, B.; Glass, A.D.M. Regulation of high-affinity nitrate transporter genes and high-affinity nitrate influx by nitrogen poolis in roots of barley. Plant Physiol. 2000, 123, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Ivanko, S.; Ingversen, J. Investigation on the assimilation of nitrogen by maize roots and the transport of some major nitrogen compounds by xylem sap. III. Transport of nitrogen compounds by xylem sap. Physiol. Plant 1971, 24, 355–363. [Google Scholar] [CrossRef]
- Millard, P. The accumulation and storage of nitrogen by herbaceous plants. Plant Cell Environ. 1988, 11, 1–8. [Google Scholar] [CrossRef]
- Miller, A.J.; Fan, X.; Orsel, M.; Smith, S.J.; Wells, D.M. Nitrate transport and signalling. J. Exp. Bot. 2007, 58, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Brien, A.O.; Vega, A.; Bouguyon, E.; Krouk, G.; Gojon, A.; Coruzzi, G.; Gutie, R.A. Nitrate transport, sensing, and responses in plants. Mol. Plant 2016, 9, 837–856. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, Z.; Ding, G.; Shi, L.; Xu, F.; Cai, H. A natural light/dark cycle regulation of carbon-nitrogen metabolism and gene expression in rice shoots. Front. Plant Sci. 2016, 7, 1318. [Google Scholar] [CrossRef] [PubMed]
- Coruzzi, G.M.; Zhou, L. Carbon and nitrogen sensing and signaling in plants: Emerging “matrix effects”. Curr. Opin. Plant Biol. 2001, 4, 247–253. [Google Scholar] [CrossRef]
- Touraine, B.; Daniel-Vedele, F.; Forde, B.G. Nitrate uptake and its regulation. In Plant Nitrogen; Lea, P.J., Morot-Gaudry, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1–36. [Google Scholar]
- Zhuo, D.; Okamoto, M.; Vidmar, J.J.; Glass, A.D.M. Regulation of a putative high-affinity nitrate transporter (Nrt2;1At) in roots of Arabidopsis thaliana. Plant J. 1999, 17, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Pal’ove-Balang, P.; Mistrik, I. Control of nitrate uptake by phloem-translocated glutamine in Zea mays L. seedlings. Plant Biol. 2002, 4, 440–445. [Google Scholar] [CrossRef]
- Nazoa, P.; Vidmar, J.J.; Tranbarger, T.J.; Mouline, K.; Damiani, I.; Tillard, P.; Zhuo, D.; Glass, A.D.M.; Touraine, B. Regulation of the nitrate transporter gene AtNRT2.1 in Arabidopsis thaliana: Responses to nitrate, amino acids and developmental stage. Plant Mol. Biol. 2003, 52, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.; Chung, T.; Juo, Y.; Hsieh, M. Glutamine rapidly induces the expression of key transcription factor genes involved in nitrogen and stress responses in rice roots. BMC Genom. 2015, 16, 731. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.; Chung, T.; Hsieh, M. Gene expression profiling of rice seedlings in response to glutamine treatment. Genom. Data 2015, 6, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Karmoker, J.L.; Clarkson, D.T.; Saker, L.R.; Rooney, J.M.; Purves, J.V. Sulphate deprivation depresses the transport of nitrogen to the xylem and the hydraulic conductivity of barley (Hordeum vulgare L.) roots. Planta 1991, 185, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Sugiharto, B.; Suzuki, I.; Burnell, J.N.; Sugiyama, T. Glutamine induces the N-dependent accumulation of mRNAs encoding phosphoenolpyruvate carboxylase and carbonic anhydrase in detached maize leaf tissue. Plant Physiol. 1992, 100, 2066–2070. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goron, T.L.; Raizada, M.N. Biosensor-Mediated In Situ Imaging Defines the Availability Period of Assimilatory Glutamine in Maize Seedling Leaves Following Nitrogen Fertilization. Nitrogen 2020, 1, 3-11. https://0-doi-org.brum.beds.ac.uk/10.3390/nitrogen1010002
Goron TL, Raizada MN. Biosensor-Mediated In Situ Imaging Defines the Availability Period of Assimilatory Glutamine in Maize Seedling Leaves Following Nitrogen Fertilization. Nitrogen. 2020; 1(1):3-11. https://0-doi-org.brum.beds.ac.uk/10.3390/nitrogen1010002
Chicago/Turabian StyleGoron, Travis L., and Manish N. Raizada. 2020. "Biosensor-Mediated In Situ Imaging Defines the Availability Period of Assimilatory Glutamine in Maize Seedling Leaves Following Nitrogen Fertilization" Nitrogen 1, no. 1: 3-11. https://0-doi-org.brum.beds.ac.uk/10.3390/nitrogen1010002



