Oxidative Stress Produced by Paraquat Reduces Nitrogen Fixation in Soybean-Bradyrhizobium diazoefficiens Symbiosis by Decreasing Nodule Functionality
Abstract
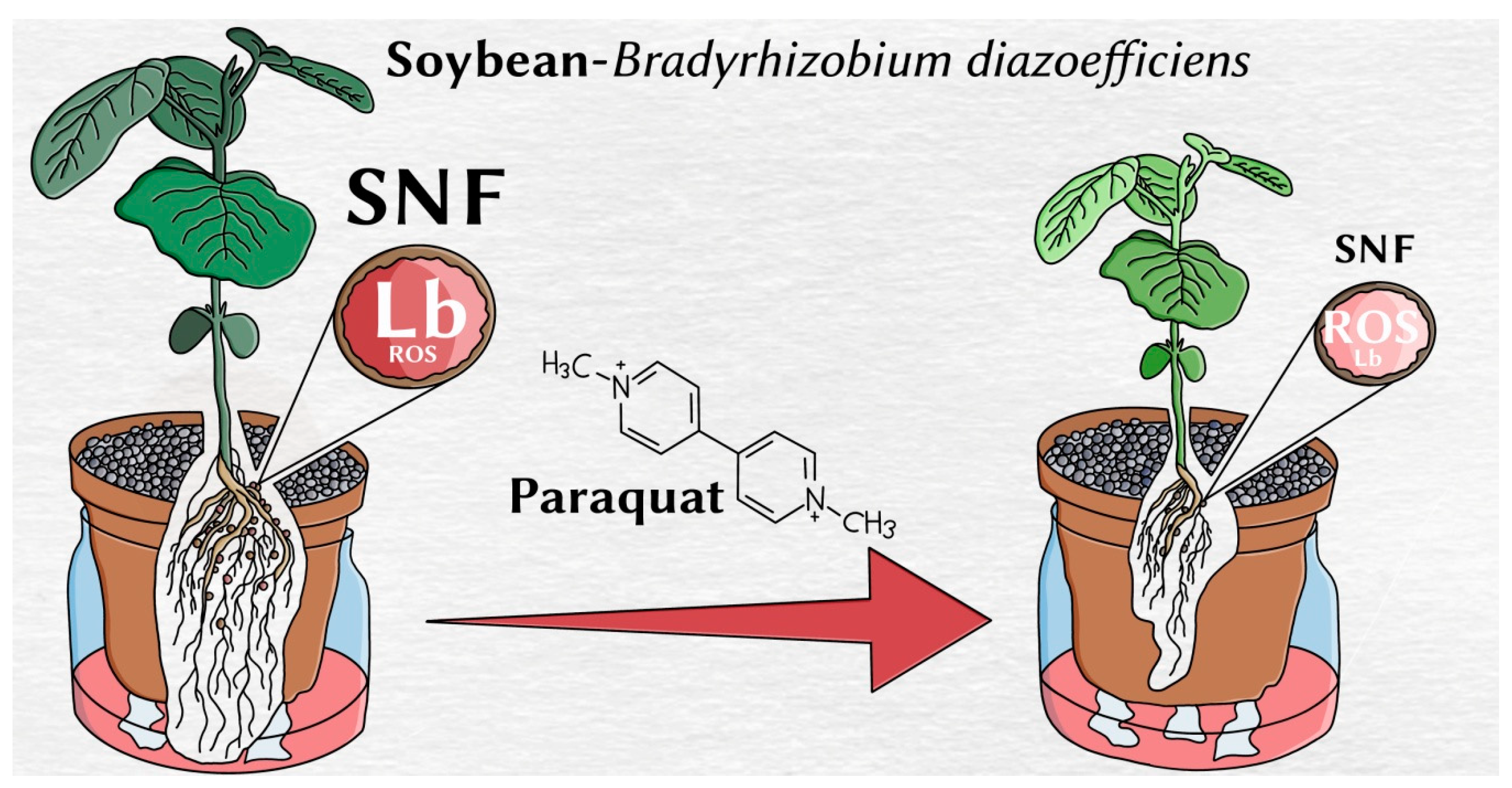
:1. Introduction
2. Materials and Methods
2.1. Bacterial Culture
2.2. Plant Inoculation and Growth
2.3. Analyses
2.4. Experimental Design and Statistical Analysis
3. Results
3.1. Bacterial Growth
3.2. Plant Physiology
3.3. N Content and Symbiotic Nitrogen Fixation (SNF)
3.4. Lb Content and Lipid Peroxidation in Nodules
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Graham, P.H.; Vance, C.P. Legumes: Importance and constraints to greater use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voisin, A.; Guéguen, J.; Huyghe, C.; Jeuffroy, M.H.; Magrini, M.B.; Meynard, J.M.; Mougel, C.; Pellerin, S.; Pelzer, E. Legumes for feed, food, biomaterials and bioenergy in Europe: A review. Agron. Sustain. 2014, 34, 361–380. [Google Scholar] [CrossRef]
- Foyer, C.; Lam, H.M.; Nguyen, H.T.; Siddique, K.H.M.; Varshney, R.K.; Colmer, T.D.; Cowling, W.; Bramley, H.; Mori, T.A.; Hodgson, J.M.; et al. Neglecting legumes has compromised human health and sustainable food production. Nat. Plants 2016, 2, 16112. [Google Scholar] [CrossRef] [PubMed]
- Siddique, K.H.M.; Johansen, C.; Turner, N.C.; Jeuffroy, M.H.; Hashem, A.; Sakar, D.; Gan, Y.; Alghamdi, S.S. Innovations in agronomy for food legumes. A review. Agron. Sustain. Dev. 2012, 32, 45–64. [Google Scholar] [CrossRef] [Green Version]
- Cernay, C.; Pelzer, E.; Makowski, D. A global experimental dataset for assessing grain legume production. Sci. Data 2016, 27, 160084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD. Crop Production (Indicator). Available online: https://data.oecd.org/agroutput/crop-production.htm (accessed on 27 September 2020). [CrossRef]
- SoyStats. Available online: http://soystats.com/international-world-soybean-production/ (accessed on 27 September 2020).
- Mylona, P.; Pawlowski, K.; Bisseling, T. Symbiotic nitrogen fixation. Plant Cell 1995, 7, 869–885. [Google Scholar] [CrossRef]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Peix, A.; Ramírez-Bahena, M.H.; Velázquez, E.; Bedmar, E.J. Bacterial Associations with Legumes. Crit. Rev. Plant Sci. 2015, 34, 17–42. [Google Scholar] [CrossRef]
- Stacey, G. The rhizobium-legume nitrogen-fixing symbiosis. In Biology of the Nitrogen Cycle; Bothe, H., Ferguson, S., Newton, W.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 147–163. ISBN 9780444528575. [Google Scholar] [CrossRef]
- Olivares, J.; Bedmar, E.J.; Sanjuán, J. Biological nitrogen fixation in the context of global change. Mol. Plant Microbe Interact. 2013, 26, 486–494. [Google Scholar] [CrossRef] [Green Version]
- Rutten, P.J.; Poole, P.S. Oxygen regulatory mechanisms of nitrogen fixation in rhizobia. Adv. Microb. Physiol. 2019, 75, 325–389. [Google Scholar] [CrossRef]
- Poole, P.; Ramachandran, V.; Terpolilli, J. Rhizobia: From saprophytes to endosymbionts. Nat. Rev. Microbiol. 2018, 16, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Udvardi, M.; Poole, P.S. Transport and metabolism in legume-rhizobia symbioses. Annu. Rev. Plant Biol. 2013, 64, 781–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crews, T.E.; Peoples, M.B. Legume versus fertilizer sources of nitrogen: Ecological tradeoffs and human needs. Agri. Ecosyst. Environ. 2004, 102, 279–297. [Google Scholar] [CrossRef]
- Hungria, M.; Vargas, M.A.T. Environmental factors affecting N2 fixation in grain legumes in the tropics, with an emphasis on Brazil. Field Crop. Res. 2000, 65, 151–164. [Google Scholar] [CrossRef]
- Kucey, R.M.N.; Chaiwanakupt, P.; Arayangkool, T.; Snitwongse, P.; Siripaibool, C.; Wadisirisuk, P.; Bookerd, N. Nitrogen fixation (15N dilution) with soybeans under Thai field conditions. II. Effect of herbicides and water application schedule. Plant Soil 1988, 108, 87–92. [Google Scholar] [CrossRef]
- Kucey, R.M.N.; Chaiwanakupt, P.; Snitwongse, P.; Toomsan, B.; Boonkerd, N.; Siripaibool, C.; Rennie, R.J.; Rungrattanakasin, W.; Wadisirisak, P. Nitrogen fixation (15N dilution) with soybeans under Thai field conditions. III. Effect of Bradyrhizobium japonicum strains and herbicides in northeast Thailand. J. Gen. Appl. Microbiol. 1988, 34, 243–253. [Google Scholar] [CrossRef]
- Dalton, D.A. Effects of Paraquat on the Oxygen Free Radical Biology of Soybean Root Nodules. Bull. Environ. Contain. Toxicol. 1992, 48, 721–726. [Google Scholar] [CrossRef]
- Donati, A.J.; Jeon, J.M.; Sangurdekar, D.; So, J.S.; Chang, W.S. Genome-wide transcriptional and physiological responses of Bradyrhizobium japonicum to paraquat-mediated oxidative stress. Appl. Environ. Microbiol. 2011, 77, 3633–3643. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Hatzios, K.K. Differential response of two soybean cultivars to paraquat. Z. Nat. C 1993, 48, 379–384. [Google Scholar] [CrossRef]
- Regensburger, B.; Hennecke, H. RNA polymerase from Rhizobium japonicum. Arch. Microbiol. 1983, 135, 103–109. [Google Scholar] [CrossRef]
- Fernández, N.; Cabrera, J.J.; Varadarajan, A.R.; Lutz, S.; Ledermann, R.; Roschitzki, B.; Eberl, L.; Bedmar, E.J.; Fischer, H.M.; Pessi, G.; et al. An integrated systems approach unveils new aspects of microoxia-mediated regulation in Bradyrhizobium diazoefficiens. Front. Microbiol. 2019, 10, 924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesa, S.; Hauser, F.; Friberg, M.; Malaguti, E.; Fischer, H.; Hennecke, H. Comprehensive assessment of the regulons controlled by the FixLJ-FixK2-FixK1 cascade in Bradyrhizobium japonicum. J. Bacteriol. 2008, 190, 6568–6579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tortosa, G.; Pacheco, P.J.; Hidalgo-García, A.; Granados, A.; Delgado, A.; Mesa, S.; Bedmar, E.J.; Delgado, M.J. Copper modulates nitrous oxide emissions from soybean root nodules. Environ. Exp. Bot. 2020, 180, 104262. [Google Scholar] [CrossRef]
- Trung, B.C.; Yoshida, S. Improvement of Leonard jar assembly for screening of effective rhizobium. Soil Sci. Plant Nutr. 1983, 29, 97–100. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.M. A Manual for the Practical Study of the Root-Nodule Bacteria; Blackwell Scientific Publications: Oxford, UK, 1970. [Google Scholar]
- Fehr, W.R.; Aviness, C.E.; Burmood, D.T.; Pennington, J.S. Stage of development descriptions for soybeans, Glycine max L. Merril. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Hardarson, G.; Danso, S.K.A. Methods for measuring biological nitrogen fixation in grain legumes. Plant Soil 1993, 152, 19–23. [Google Scholar] [CrossRef]
- LaRue, T.A.; Child, J.J. Sensitive fluorometric assay for leghemoglobin. Anal. Biochem. 1979, 92, 11–15. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1984. [Google Scholar]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Del Río, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef] [Green Version]
- Salunkhe, P.; Topfer, T.; Buer, J.; Tummler, B. Genome-wide transcriptional profiling of the steady-state response of Pseudomonas aeruginosa to hydrogen peroxide. J. Bacteriol. 2005, 187, 2565–2572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesa, S.; Reutimann, L.; Fischer, H.M.; Hennecke, H. Posttranslational control of transcription factor FixK2, a key regulator for the Bradyrhizobium japonicum-soybean symbiosis. Proc. Natl. Acad. Sci. USA 2009, 106, 21860–21865. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Deng, K.; Zaremba, S.; Deng, X.; Lin, C.; Wang, Q.; Tortorello, M.L.; Zhang, W. Transcriptomic response of Escherichia coli O157:H7 to oxidative stress. Appl. Environ. Microbiol. 2009, 75, 6110–6123. [Google Scholar] [CrossRef] [Green Version]
- Masloboeva, N.; Reutimann, L.; Stiefel, P.; Follador, R.; Leimer, N.; Hennecke, H.; Mesa, S.; Fischer, H.M. Reactive oxygen species-inducible ECF sigma factors of Bradyrhizobium japonicum. PLoS ONE 2012, 7, e43421. [Google Scholar] [CrossRef]
- Hamim, H.; Violita, V.; Triadiati, T.; Miftahudin, M. Oxidative stress and photosynthesis reduction of cultivated (Glycine max L.) and wild soybean (G. tomentella L.) exposed to drought and paraquat. Asian J. Plant Sci. 2017, 16, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Marino, D.; González, E.M.; Arrese-Igor, C. Drought effects on carbon and nitrogen metabolism of pea nodules can be mimicked by paraquat: Evidence for the occurrence of two regulation pathways under oxidative stresses. J. Exp. Bot. 2006, 57, 665–673. [Google Scholar] [CrossRef]
- Kuzma, M.M.; Hunt, S.; Layzell, D.B. Role of oxygen in the limitation and inhibition of nitrogenase activity and respiration rate in individual soybean nodules. Plant Physiol. 1993, 101, 161–169. [Google Scholar] [CrossRef] [Green Version]



| Composition 1 | Concentration |
|---|---|
| g L−1 | |
| KH2PO4 | 0.3 |
| K2HPO4 | 0.3 |
| MgSO4 × 7H2O | 0.1 |
| Peptone | 3 |
| Yeast extract | 1 |
| mg L−1 | |
| CaCl2 × H2O | 5 |
| Na2MoO4 × 2H2O | 0.1 |
| H3BO3 | 10 |
| ZnSO4 × 7H2O | 1 |
| CuSO4 × 5H2O | 0.5 |
| FeCl3 | 1 |
| MnCl2 × 6H2O | 0.5 |
| Spectinomycin 2 | 100 µg mL−1 |
| Arabinose 2 | 0.1% (w/v) |
| Agar 3 | 15 g L−1 |
| Composition 1 | Concentration |
|---|---|
| g L−1 | |
| CaHPO4 | 0.42 |
| CaSO4 × 2H2O | 0.54 |
| K2HPO4 | 0.08 |
| MgSO4 × 7H2O | 0.08 |
| NaCl | 0.08 |
| FeCl3 × 6H2O | 0.07 |
| mg L−1 | |
| MnCl2 × 6H2O | 0.21 |
| Na2MoO4 × 2H2O | 0.04 |
| H3BO3 | 4.23 |
| ZnSO4 × 7H2O | 0.42 |
| CuSO4 × 5H2O | 0.21 |
| Paraquat Added to Mineral Solution (µM) | SDW (mg plant−1) | RDW (mg plant−1) | SDW/RDW (plant−1) | NN (plant−1) | NFW (mg plant−1) | NFW/NN (mg Nodule−1) |
|---|---|---|---|---|---|---|
| 0 | 649 a | 217 a | 2.99 a | 49 a | 366 a | 7.47 a |
| 20 | 533 b | 217 a | 2.46 b | 47 a | 342 b | 7.28 a |
| 50 | 459 c | 184 b | 2.49 b | 37 a | 273 c | 7.38 ab |
| 100 | 316 d | 132 c | 2.40 b | 28 b | 175 d | 6.25 b |
| Paraquat Added to Mineral Solution (µM) | Nshoot (mg plant−1) | Nroot (mg plant−1) | Nnodules (mg plant−1) |
|---|---|---|---|
| 0 | 23.29 a | 4.62 a | 2.4 a |
| 20 | 11.93 b | 4.47 b | 1.82 b |
| 50 | 12.18 b | 3.39 c | 2.15 b |
| 100 | 9.39 c | 2.42 d | 1.12 c |
| Paraquat Added to Mineral Solution (µM) | Lb [mg (g NFW−1)] | Lipid Peroxidation [nmol MDA (g NFW−1)] |
|---|---|---|
| 0 | 7.02 a | 64.2 c |
| 20 | 4.13 b | 74.8 b |
| 50 | 4.16 b | 75.2 b |
| 100 | 3.58 c | 85.4 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tortosa, G.; Parejo, S.; Cabrera, J.J.; Bedmar, E.J.; Mesa, S. Oxidative Stress Produced by Paraquat Reduces Nitrogen Fixation in Soybean-Bradyrhizobium diazoefficiens Symbiosis by Decreasing Nodule Functionality. Nitrogen 2021, 2, 30-40. https://0-doi-org.brum.beds.ac.uk/10.3390/nitrogen2010003
Tortosa G, Parejo S, Cabrera JJ, Bedmar EJ, Mesa S. Oxidative Stress Produced by Paraquat Reduces Nitrogen Fixation in Soybean-Bradyrhizobium diazoefficiens Symbiosis by Decreasing Nodule Functionality. Nitrogen. 2021; 2(1):30-40. https://0-doi-org.brum.beds.ac.uk/10.3390/nitrogen2010003
Chicago/Turabian StyleTortosa, Germán, Sergio Parejo, Juan J. Cabrera, Eulogio J. Bedmar, and Socorro Mesa. 2021. "Oxidative Stress Produced by Paraquat Reduces Nitrogen Fixation in Soybean-Bradyrhizobium diazoefficiens Symbiosis by Decreasing Nodule Functionality" Nitrogen 2, no. 1: 30-40. https://0-doi-org.brum.beds.ac.uk/10.3390/nitrogen2010003






