Microparticle Deposition on Human Serum Albumin Layers: Unraveling Anomalous Adsorption Mechanism
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Physicochemical Characteristics of Albumin Solutions
3.2. Adsorption Kinetics of HSA on Mica
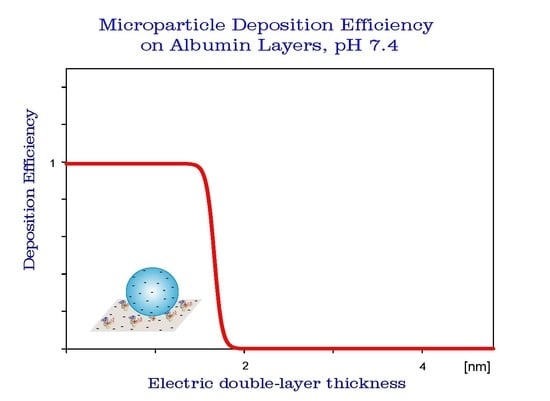
3.3. HSA Layer Characteristics by Colloid Deposition
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adamczyk, Z.; Nattich-Rak, M.; Dąbkowska, M.; Kujda-Kruk, M. Albumin adsorption at solid substrates: A quest for a unified approach. J. Colloid Interface Sci. 2018, 514, 769–790. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.C.; Wong, A. Effect of adsorbed protein on hydroxyapatite zeta potential and Streptococcus mutans adherence. Infect. Immun. 1983, 39, 1285–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogt, A.H.; Dankert, J.; Feijen, J. Adhesion of Staphylococcus epidermidis and taphylococcus saprophyticus to a hydrophobic biomaterial. J. Gen. Microbiol. 1985, 131, 2485–2491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brokke, P.; Dankert, J.; Carballo, J.; Feijen, J. Adherence of Coagulase-Negative Staphylococci onto Polyethylene Catheters in vitro and in vivo: A Study on the Influence of various Plasma Proteins. J. Biomater. Appl. 1991, 5, 204–226. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; McDermott, E.W.; Crown, J. Blood-based biomarkers in breast cancer: From proteins to circulating tumor cells to circulating tumor DNA. Tumor Biol. 2018, 40, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Moidu, N.A.; Rahman, N.S.A.; Syafruddin, S.E.; Low, T.Y.; Mohtar, M.A. Secretion of pro-oncogenic AGR2 protein in cancer. Heliyon 2020, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fanali, G.; Di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef]
- Martin-Rodriguez, A.; Ortega-Vinuesa, J.L.; Hidalgo-Alvarez, R. Electrokinetics of Protein-coated Latex Particles. In Surface Science Series, Volume 106. Interfacial Electrokinetics and Electrophoresis; Delgado, A.V., Ed.; Dekker: New York, NY, USA; Basel, Switzerland, 2002; Volume 22, pp. 641–670. [Google Scholar]
- Peters, T., Jr. All About Albumin: Biochemistry, Genetics and Medical Applications; Academic Press: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Curry, S.; Mandelkow, H.; Brick, P.; Franks, N. Crystal structure of human serum albumin complexed with fatty acid reveals an asymmetric distribution of binding sites. Nat. Genet. 1998, 5, 827–835. [Google Scholar] [CrossRef]
- Protein Data Bank Code 3P7K. Available online: http://www.pdb.org (accessed on 1 June 2012).
- Norde, W. Adsorption of proteins from solution at the solid-liquid interface. Adv. Colloid Interface Sci. 1986, 25, 267–340. [Google Scholar] [CrossRef]
- Akson, M.L.; Hoboken; Edsall, J.T. Advances in Protein Chemistry. Am. J. Med. Sci. 1948, 215, 114. [Google Scholar] [CrossRef] [Green Version]
- Jachimska, B.; Wasilewska, M.; Adamczyk, Z. Characterization of Globular Protein Solutions by Dynamic Light Scattering, Electrophoretic Mobility, and Viscosity Measurements. Langmuir 2008, 24, 6866–6872. [Google Scholar] [CrossRef] [PubMed]
- Gorudko, I.V.; Grigorieva, D.V.; Shamova, E.V.; Kostevich, V.A.; Sokolov, A.V.; Mikhalchik, E.V.; Cherenkevich, S.N.; Arnhold, J.; Panasenko, O.M. Hypohalous acid-modified human serum albumin induces neutrophil NADPH oxidase activation, degranulation, and shape change. Free Radic. Biol. Med. 2014, 68, 326–334. [Google Scholar] [CrossRef]
- Van Dulm, P.; Norde, W. The adsorption of human plasma albumin on solid surfaces, with special attention to the kinetic aspects. J. Colloid Interface Sci. 1983, 91, 248–255. [Google Scholar] [CrossRef]
- Wertz, C.F.; Santore, M.M. Effect of Surface Hydrophobicity on Adsorption and Relaxation Kinetics of Albumin and Fibrinogen: Single-Species and Competitive Behavior. Langmuir 2001, 17, 3006–3016. [Google Scholar] [CrossRef]
- Ding, Y.-X.; Streitmatter, S.; Wright, B.E.; Hlady, V. Spatial Variation of the Charge and Sulfur Oxidation State in a Surface Gradient Affects Plasma Protein Adsorption. Langmuir 2010, 26, 12140–12146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elgersma, A.V.; Zsom, R.L.; Lyklema, J.; Norde, W. Kinetics of single and competitive protein adsorption studied by reflectometry and streaming potential measurements. Colloids Surf. 1992, 65, 17–28. [Google Scholar] [CrossRef]
- Blomberg, E.; Claesson, P.M.; Tilton, R.D. Short-Range Interaction between Adsorbed Layers of Human Serum Albumin. J. Colloid Interface Sci. 1994, 166, 427–436. [Google Scholar] [CrossRef]
- Blomberg, E.; Claesson, P.M.; Fröberg, J.C. Surfaces coated with protein layers: A surface force and ESCA study. Biomaterials 1998, 19, 371–386. [Google Scholar] [CrossRef]
- Sousa, S.R.; Moradas-Ferreira, P.; Saramago, B.; Melo, L.; Barbosa, M.A. Human Serum Albumin Adsorption on TiO2from Single Protein Solutions and from Plasma. Langmuir 2004, 20, 9745–9754. [Google Scholar] [CrossRef]
- Malmsten, M. Ellipsometry Studies of Protein Layers Adsorbed at Hydrophobic Surfaces. J. Colloid Interface Sci. 1994, 166, 333–342. [Google Scholar] [CrossRef]
- Ortega-Vinuesa, J.; Tengvall, P.; Lundström, I. Molecular packing of HSA, IgG, and fibrinogen adsorbed on silicon by AFM imaging. Thin Solid Films 1998, 324, 257–273. [Google Scholar] [CrossRef]
- Kurrat, R.; Ramsden, J.J.; Prenosil, J.E. Kinetic model for serum albumin adsorption: Experimental verification. J. Chem. Soc. Faraday Trans. 1994, 90, 587. [Google Scholar] [CrossRef]
- Kurrat, R.; Prenosil, J.; Ramsden, J. Kinetics of Human and Bovine Serum Albumin Adsorption at Silica–Titania Surfaces. J. Colloid Interface Sci. 1997, 185, 1–8. [Google Scholar] [CrossRef]
- Dąbkowska, M.; Adamczyk, Z. Human Serum Albumin Monolayers on Mica: Electrokinetic Characteristics. Langmuir 2012, 28, 15663–15673. [Google Scholar] [CrossRef] [PubMed]
- Dąbkowska, M.; Adamczyk, Z.; Kujda, M. Mechanism of HSA adsorption on mica determined by streaming potential, AFM and XPS measurements. Colloids Surf. B Biointerfaces 2013, 101, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Pomorska, A.; Adamczyk, Z.; Nattich-Rak, M.; Sadowska, M. Kinetics of human serum albumin adsorption at silica sensor: Unveiling dynamic hydration function. Colloids Surf. B Biointerfaces 2018, 167, 377–384. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Pomorska, A.; Nattich-Rak, M.; Wytrwal-Sarna, M.; Bernasik, A. Protein adsorption mechanisms at rough surfaces: Serum albumin at a gold substrate. J. Colloid Interface Sci. 2018, 530, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Nattich-Rak, M.; Adamczyk, Z.; Kujda, M. Revealing deposition mechanism of colloid particles on human serum albumin monolayers. Colloids Surf. B Biointerfaces 2016, 137, 176–182. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nat. Cell Biol. 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Blum, H.; Beier, H.; Gross, H.J. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 1987, 8, 93–99. [Google Scholar] [CrossRef]
- Sofińska, K.; Adamczyk, Z.; Kujda, M.; Nattich-Rak, M. Recombinant Albumin Monolayers on Latex Particles. Langmuir 2013, 30, 250–258. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Bratek-Skicki, A.; Dąbrowska, P.; Nattich-Rak, M. Mechanisms of Fibrinogen Adsorption on Latex Particles Determined by Zeta Potential and AFM Measurements. Langmuir 2011, 28, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Dąbkowska, M.; Adamczyk, Z. Ionic strength effect in HSA adsorption on mica determined by streaming potential measurements. J. Colloid Interface Sci. 2012, 366, 105–113. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Nattich, M.; Wasilewska, M.; Zaucha, M. Colloid particle and protein deposition—Electrokinetic studies. Adv. Colloid Interface Sci. 2011, 168, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Swan, J.W.; Furst, E.M. A simpler expression for Henry’s function describing the electrophoretic mobility of spherical colloids. J. Colloid Interface Sci. 2012, 388, 92–94. [Google Scholar] [CrossRef]
- Smoluchowski, M. Drei Vortreag ueber diffusion, Brownische Molekularbewegung und Koagulation von Kolloidteilchen. Phys. Zeit 1916, 17, 557–571. [Google Scholar]
- Adamczyk, Z. Particles at Interfaces: Interactions, Deposition, Structure; Elsevier B.V.: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Adamczyk, Z.; Sadlej, K.; Wajnryb, E.; Nattich-Rak, M.; Ekiel-Jeżewska, M.; Bławzdziewicz, J. Streaming potential studies of colloid, polyelectrolyte and protein deposition. Adv. Colloid Interface Sci. 2010, 153, 1–29. [Google Scholar] [CrossRef]
- Ekiel-Jeżewska, M.L.; Adamczyk, Z.; Bławzdziewicz, J. Streaming Current and Effective ζ-Potential for Particle-Covered Surfaces with Random Particle Distributions. J. Phys. Chem. C 2019, 123, 3517–3531. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Docoslis, A.; Wu, W.; Giese, R.F. Influence of macroscopic and microscopic interactions on kinetic rate constants I. Role of the extended DLVO theory in determining the kinetic adsorption constant of proteins in aqueous media, using von Smoluchowski’s approach. Colloids Surf. B Biointerfaces 1999, 14, 99–104. [Google Scholar] [CrossRef]
- Van Oss, C.J. Chapter Three—The Extended DLVO Theory. Interface Sci. Technol. 2008, 16, 31–48. [Google Scholar]
- Kubiak-Ossowska, K.; Tokarczyk, K.; Jachimska, B.; Mulheran, P.A. Bovine Serum Albumin Adsorption at a Silica Surface Explored by Simulation and Experiment. J. Phys. Chem. B 2017, 121, 3975–3986. [Google Scholar] [CrossRef] [Green Version]
- Nattich-Rak, M.; Adamczyk, Z.; Wasilewska, M.; Sadowska, M. Revealing fibrinogen monolayer conformations at different pHs: Electrokinetic and colloid deposition studies. J. Colloid Interface Sci. 2015, 449, 62–71. [Google Scholar] [CrossRef] [PubMed]









 ) 0.04 M; (4) (
) 0.04 M; (4) (  ) 0.03 M; (5) (■) 0.02 M. The solid lines are fits of experimental data. Part (a), pH 5.5; part (b), pH 7.4 (PBS).
) 0.03 M; (5) (■) 0.02 M. The solid lines are fits of experimental data. Part (a), pH 5.5; part (b), pH 7.4 (PBS).
 ) 0.04 M; (4) (
) 0.04 M; (4) (  ) 0.03 M; (5) (■) 0.02 M. The solid lines are fits of experimental data. Part (a), pH 5.5; part (b), pH 7.4 (PBS).
) 0.03 M; (5) (■) 0.02 M. The solid lines are fits of experimental data. Part (a), pH 5.5; part (b), pH 7.4 (PBS).

 ) pH 7.4, 2 (▲) pH 5.5, 3 (●) pH 3.5 (previous data from Ref. [31]). The solid lines are fits of experimental data.
) pH 7.4, 2 (▲) pH 5.5, 3 (●) pH 3.5 (previous data from Ref. [31]). The solid lines are fits of experimental data.
 ) pH 7.4, 2 (▲) pH 5.5, 3 (●) pH 3.5 (previous data from Ref. [31]). The solid lines are fits of experimental data.
) pH 7.4, 2 (▲) pH 5.5, 3 (●) pH 3.5 (previous data from Ref. [31]). The solid lines are fits of experimental data.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nattich-Rak, M.; Dąbkowska, M.; Adamczyk, Z. Microparticle Deposition on Human Serum Albumin Layers: Unraveling Anomalous Adsorption Mechanism. Colloids Interfaces 2020, 4, 51. https://0-doi-org.brum.beds.ac.uk/10.3390/colloids4040051
Nattich-Rak M, Dąbkowska M, Adamczyk Z. Microparticle Deposition on Human Serum Albumin Layers: Unraveling Anomalous Adsorption Mechanism. Colloids and Interfaces. 2020; 4(4):51. https://0-doi-org.brum.beds.ac.uk/10.3390/colloids4040051
Chicago/Turabian StyleNattich-Rak, Małgorzata, Maria Dąbkowska, and Zbigniew Adamczyk. 2020. "Microparticle Deposition on Human Serum Albumin Layers: Unraveling Anomalous Adsorption Mechanism" Colloids and Interfaces 4, no. 4: 51. https://0-doi-org.brum.beds.ac.uk/10.3390/colloids4040051