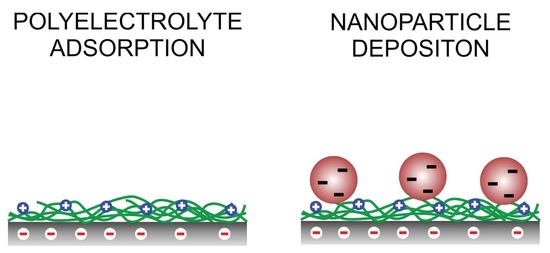
Particle Deposition to Silica Surfaces Functionalized with Cationic Polyelectrolytes
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Topographic Imaging
2.3. Optical Reflectivity
2.4. Electrokinetic Potential
3. Results and Discussion
3.1. Preparation and Characterization of the In Situ Functionalized Substrates
3.2. Preparation and Characterization of the Ex Situ Functionalized Substrates
3.3. Initial Deposition of Nanoparticles
3.4. Saturation Plateau of Nanoparticles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Szilagyi, I.; Trefalt, G.; Tiraferri, A.; Borkovec, M. Polyelectrolyte adsorption, interparticle forces, and colloidal aggregation. Soft Matter 2014, 10, 2479–2502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morga, M.; Adamczyk, Z. Monolayers of cationic polyelectrolytes on mica: Electrokinetic studies. J. Colloid Interface Sci. 2013, 407, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Shafir, A.; Andelman, D. Polyelectrolyte adsorption: Chemical and electrostatic interactions. Phys. Rev. E 2004, 70, 061804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobrynin, A.V.; Rubinstein, M. Theory of polyelectrolytes in solutions and at surfaces. Prog. Polym. Sci. 2005, 30, 1049–1118. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Michna, A.; Szaraniec, M.; Bratek, A.; Barbasz, J. Characterization of poly(ethylene imine) layers on mica by the streaming potential and particle deposition methods. J. Colloid Interface Sci. 2007, 313, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Popa, I.; Maroni, P.; Borkovec, M. Adsorption of poly-L-lysine on silica probed by optical reflectometry. Colloids Surf. A 2010, 360, 20–25. [Google Scholar] [CrossRef]
- Porus, M.; Maroni, P.; Borkovec, M. Structure of adsorbed polyelectrolyte monolayers investigated by combining optical reflectometry and piezoelectric techniques. Langmuir 2012, 28, 5642–5651. [Google Scholar] [CrossRef] [PubMed]
- Kosior, D.; Morga, M.; Maroni, P.; Ciesla, M.; Adamczyk, Z. Formation of poly-L-lysine monolayers on silica: Modeling and experimental studies. J. Phys. Chem. C 2020, 124, 4571–4581. [Google Scholar] [CrossRef]
- Popa, I.; Cahill, B.P.; Maroni, P.; Papastavrou, G.; Borkovec, M. Thin adsorbed films of a strong cationic polyelectrolyte on silica substrates. J. Colloid Interface Sci. 2007, 309, 28–35. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Decher, G.; Schlenoff, J.B. Multilayer Thin Films; Wiley-VCH: Hoboken, NJ, USA, 2002. [Google Scholar]
- Van Tassel, P.R. Polyelectrolyte adsorption and layer-by-layer assembly: Electrochemical control. Curr. Opin. Colloid Interface Sci. 2012, 17, 106–113. [Google Scholar] [CrossRef]
- Kubiak, K.; Adamczyk, Z.; Ocwieja, M. Kinetics of silver nanoparticle deposition at PAH mono layers: Reference QCM results. Langmuir 2015, 31, 2988–2996. [Google Scholar] [CrossRef] [PubMed]
- Michna, A.; Adamczyk, Z.; Ocwieja, M.; Bielanska, E. Kinetics of silver nanoparticle deposition onto poly(ethylene imine) modified mica determined by AFM and SEM measurements. Colloid Surf. A 2011, 377, 261–268. [Google Scholar] [CrossRef]
- Mazurenka, M.; Hamilton, S.M.; Unwin, P.R.; Mackenzie, S.R. In-situ measurement of colloidal gold adsorption on functionalized silica surfaces. J. Phys. Chem. C 2008, 112, 6462–6468. [Google Scholar] [CrossRef]
- Santore, M.M.; Kozlova, N. Micrometer scale adhesion on nanometer-scale patchy surfaces: Adhesion rates, adhesion thresholds, and curvature-based selectivity. Langmuir 2007, 23, 4782–4791. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, Z.; Bratek, A.; Szelag, E.; Bastrzyk, A.; Michna, A.; Barbasz, J. Colloid particle deposition on heterogeneous surfaces produced by polyelectrolyte adsorption. Colloid Surf. A 2009, 343, 111–117. [Google Scholar] [CrossRef]
- Serizawa, T.; Takeshita, H.; Akashi, M. Electrostatic adsorption of polystyrene nanospheres onto the surface of an ultrathin polymer film prepared by using an alternate adsorption technique. Langmuir 1998, 14, 4088–4094. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Zembala, A.; Michna, A. Polyelectrolyte adsorption layers studied by streaming potential and particle deposition. J. Colloid Interface Sci. 2006, 303, 353–364. [Google Scholar] [CrossRef]
- O’Brien, R.W.; White, L.R. Electrophoretic mobility of a spherical colloidal particle. J. Chem. Soc. Farad. Trans. II 1978, 74, 1607–1626. [Google Scholar] [CrossRef]
- Evans, D.F.; Wennerstrom, H. The Colloidal Domain; John Wiley: New York, NY, USA, 1999. [Google Scholar]
- Azzam, R.M.A.; Bashara, N.M. Ellipsometry and Polarized Light; North Holland: Amsterdam, The Netherlands, 1977. [Google Scholar]
- Schinke, C.; Peest, P.C.; Schmidt, J.; Brendel, R.; Bothe, K.; Vogt, M.R.; Kroger, I.; Winter, S.; Schirmacher, A.; Lim, S.; et al. Uncertainty analysis for the coefficient of band-to-band absorption of crystalline silicon. AIP Adv. 2015, 5, 067168. [Google Scholar] [CrossRef] [Green Version]
- Malitson, I.H. Interspecimen comparison of refractive index of fused silica. J. Optical Soc. Am. 1965, 55, 1205. [Google Scholar] [CrossRef]
- Dijt, J.C.; Cohen Stuart, M.A.; Fleer, G.J. Reflectometry as a tool for adsorption studies. Adv. Colloid Interface Sci. 1994, 50, 79–101. [Google Scholar] [CrossRef]
- Bohmer, M.R.; van der Zeeuw, E.A.; Koper, G.J.M. Kinetics of particle adsorption in stagnation point flow studied by optical reflectometry. J. Colloid Interface Sci. 1998, 197, 242–250. [Google Scholar] [CrossRef]
- Koper, G.J.M. Optical properties of colloidal films. Colloids Surf. A 2000, 165, 39–57. [Google Scholar] [CrossRef]
- Porus, M.; Maroni, P.; Borkovec, M. Highly-sensitive reflectometry setup capable of probing the electrical double layer on silica. Sens. Actuators B 2010, 151, 250–255. [Google Scholar] [CrossRef]
- Werner, C.; Korber, H.; Zimmermann, R.; Dukhin, S.; Jacobasch, H.J. Extended electrokinetic characterization of flat solid surfaces. J. Colloid Interface Sci. 1998, 208, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Zimmermann, R.; Kratzmüller, T. Streaming potential and streaming current measurements at planar solid/liquid interfaces for simultaneous determination of zeta potential and surface conductivity. Colloids Surf. A 2001, 192, 205–213. [Google Scholar] [CrossRef]
- Donath, E.; Voigt, A. Streaming current and streaming potential on structured surfaces. J. Colloid Interface Sci. 1986, 109, 122–139. [Google Scholar] [CrossRef]
- Roessler, S.; Zimmermann, R.; Scharnweber, D.; Werner, C.; Worch, H. Characterization of oxide layers on Ti6Al4V and titanium by streaming potential and streaming current measurements. Colloid Surf. B 2002, 26, 387–395. [Google Scholar] [CrossRef]
- Schudel, M.; Behrens, S.H.; Holthoff, H.; Kretzschmar, R.; Borkovec, M. Absolute aggregation rate constants of hematite particles in aqueous suspensions: A comparison of two different surface morphologies. J. Colloid Interface Sci. 1997, 196, 241–253. [Google Scholar] [CrossRef]
- Barringer, E.A.; Bowen, H.K. High-purity, monodisperse TiO2 powders by hydrolysis of titanium tetraethoxide 2. Aqueous interfacial electrochemistry and dispersion stability. Langmuir 1985, 1, 420–428. [Google Scholar] [CrossRef]
- Bauer, D.; Buchhammer, H.; Fuchs, A.; Jaeger, W.; Killmann, E.; Lunkwitz, K.; Rehmet, R.; Schwarz, S. Stability of colloidal silica, sikron and polystyrene latex influenced by the adsorption of polycations of different charge density. Colloids Surf. A 1999, 156, 291–305. [Google Scholar] [CrossRef]
- Elgersma, A.V.; Zsom, R.L.J.; Lyklema, J.; Norde, W. Kinetics of single and competitive protein adsorption studied by reflectometry and streaming potential measurements. Colloids Surf. 1992, 65, 17–28. [Google Scholar] [CrossRef]
- Freudenberg, U.; Zimmermann, R.; Schmidt, K.; Behrens, S.H.; Werner, C. Charging and swelling of cellulose films. J. Colloid Interface Sci. 2007, 309, 360–365. [Google Scholar] [CrossRef]
- Meszaros, R.; Thompson, L.; Bos, M.; de Groot, P. Adsorption and electrokinetic properties of polyethylenimine on silica surfaces. Langmuir 2002, 18, 6164–6169. [Google Scholar] [CrossRef]
- Dabros, T.; van de Ven, T.G.M. A direct method for studying particle deposition onto solid surfaces. Colloid Polym. Sci. 1983, 261, 694–707. [Google Scholar] [CrossRef]
- Dijt, J.C.; Cohen Stuart, M.A.; Hofman, J.E.; Fleer, G.J. Kinetics of polymer adsorption in stagnation point flow. Colloids Surf. 1990, 51, 141–158. [Google Scholar] [CrossRef]
- Kleimann, J.; Lecoultre, G.; Papastavrou, G.; Jeanneret, S.; Galletto, P.; Koper, G.J.M.; Borkovec, M. Deposition of nanosized latex particles onto silica and cellulose surfaces studied by optical reflectometry. J. Colloid Interface Sci. 2006, 303, 460–471. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Warszynski, P. Role of electrostatic interactions in particle adsorption. Adv. Colloid Interface Sci. 1996, 63, 41–149. [Google Scholar] [CrossRef]
- Semmler, M.; Mann, E.K.; Ricka, J.; Borkovec, M. Diffusional deposition of charged latex particles on water-solid interfaces at low ionic strength. Langmuir 1998, 14, 5127–5132. [Google Scholar] [CrossRef]
- Cahill, B.P.; Papastavrou, G.; Koper, G.J.M.; Borkovec, M. Adsorption of poly(amido amine) (PAMAM) dendrimers on silica: Importance of electrostatic three-body attraction. Langmuir 2008, 24, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Feder, J. Random sequential adsorption. J. Theor. Biol. 1980, 87, 237–254. [Google Scholar] [CrossRef]
- Schaaf, P.; Voegel, J.C.; Senger, B. From random sequential adsorption to ballistic deposition: A general view of irreversible deposition processes. J. Phys. Chem. B 2000, 104, 2204–2214. [Google Scholar] [CrossRef]
- Miklavic, S.J. Mean-field potential for heterogeneous electrical double layers with application to the surface pressure of charged monolayers. J. Colloid Interface Sci. 1995, 171, 446–455. [Google Scholar] [CrossRef]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosior, D.; Maroni, P.; Borkovec, M. Particle Deposition to Silica Surfaces Functionalized with Cationic Polyelectrolytes. Colloids Interfaces 2021, 5, 26. https://0-doi-org.brum.beds.ac.uk/10.3390/colloids5020026
Kosior D, Maroni P, Borkovec M. Particle Deposition to Silica Surfaces Functionalized with Cationic Polyelectrolytes. Colloids and Interfaces. 2021; 5(2):26. https://0-doi-org.brum.beds.ac.uk/10.3390/colloids5020026
Chicago/Turabian StyleKosior, Dominik, Plinio Maroni, and Michal Borkovec. 2021. "Particle Deposition to Silica Surfaces Functionalized with Cationic Polyelectrolytes" Colloids and Interfaces 5, no. 2: 26. https://0-doi-org.brum.beds.ac.uk/10.3390/colloids5020026