Modeling Incipient Use of Neolithic Cultigens by Taiwanese Foragers: Perspectives from Niche Variation Theory, the Prey Choice Model, and the Ideal Free Distribution
Abstract
:1. Introduction
“…these vast and largely uncharted [transitional] regions are not just uninhabited territory crossed on the way to an anticipated agricultural destination by evolutionary interstates without exits. They are, to the contrary, regions occupied by diverse, vibrant, and successful human societies that have developed stable, long-term economic solutions that combine low-level reliance on domesticates with continued use and management of wild species”[1].
2. Human Behavioral Ecology and Subsistence Strategies of Invasive Dispersals
3. Materials and Methods
3.1. Modelling Taiwan’s Paleolithic Niche Breadth: Paleoenvironment and Subsistence
3.2. Paleolithic Environment and Cultural Adaptations
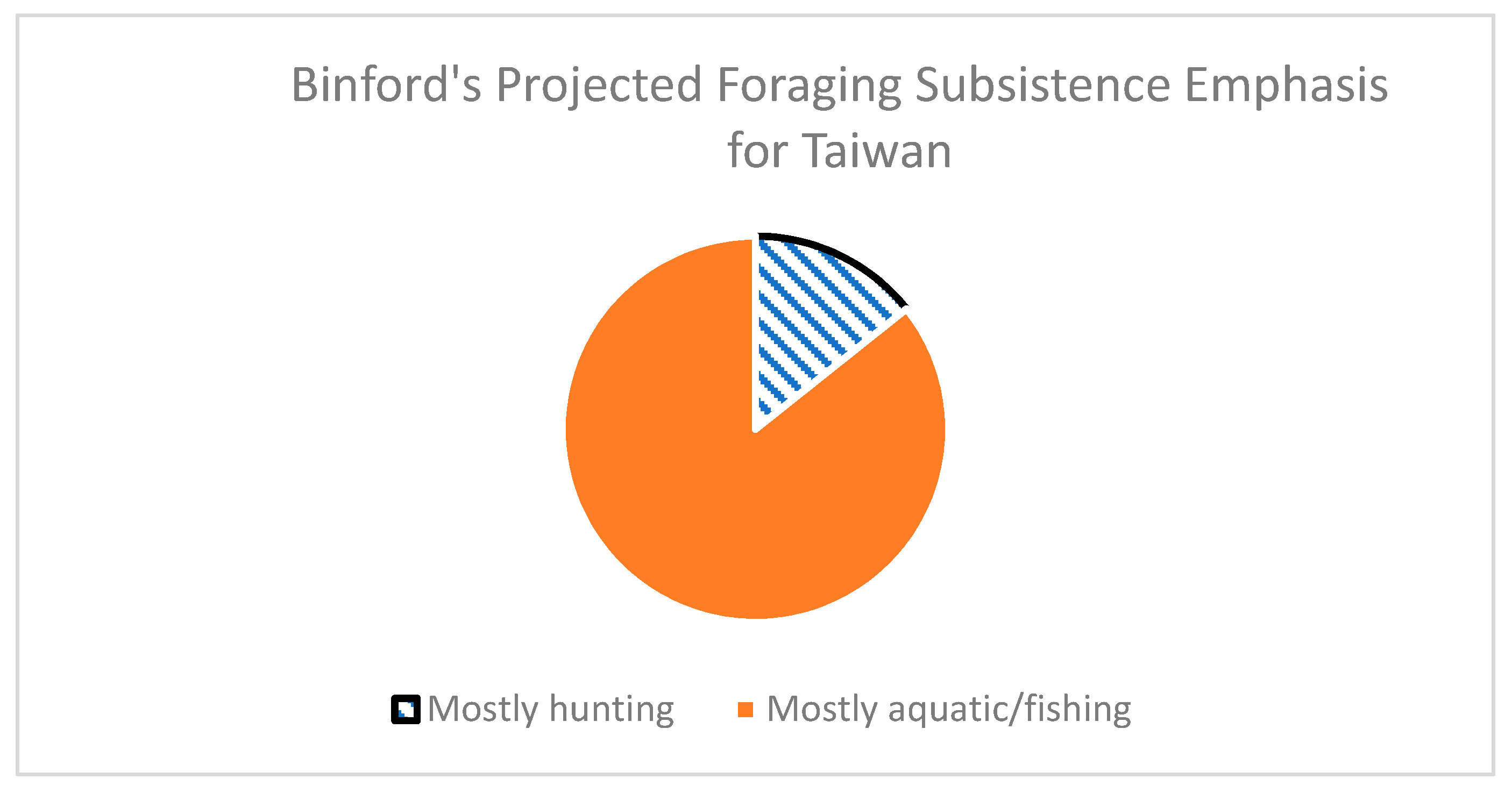
3.3. Reference Information about Foraging from the Binford Hunter-Gatherer Database
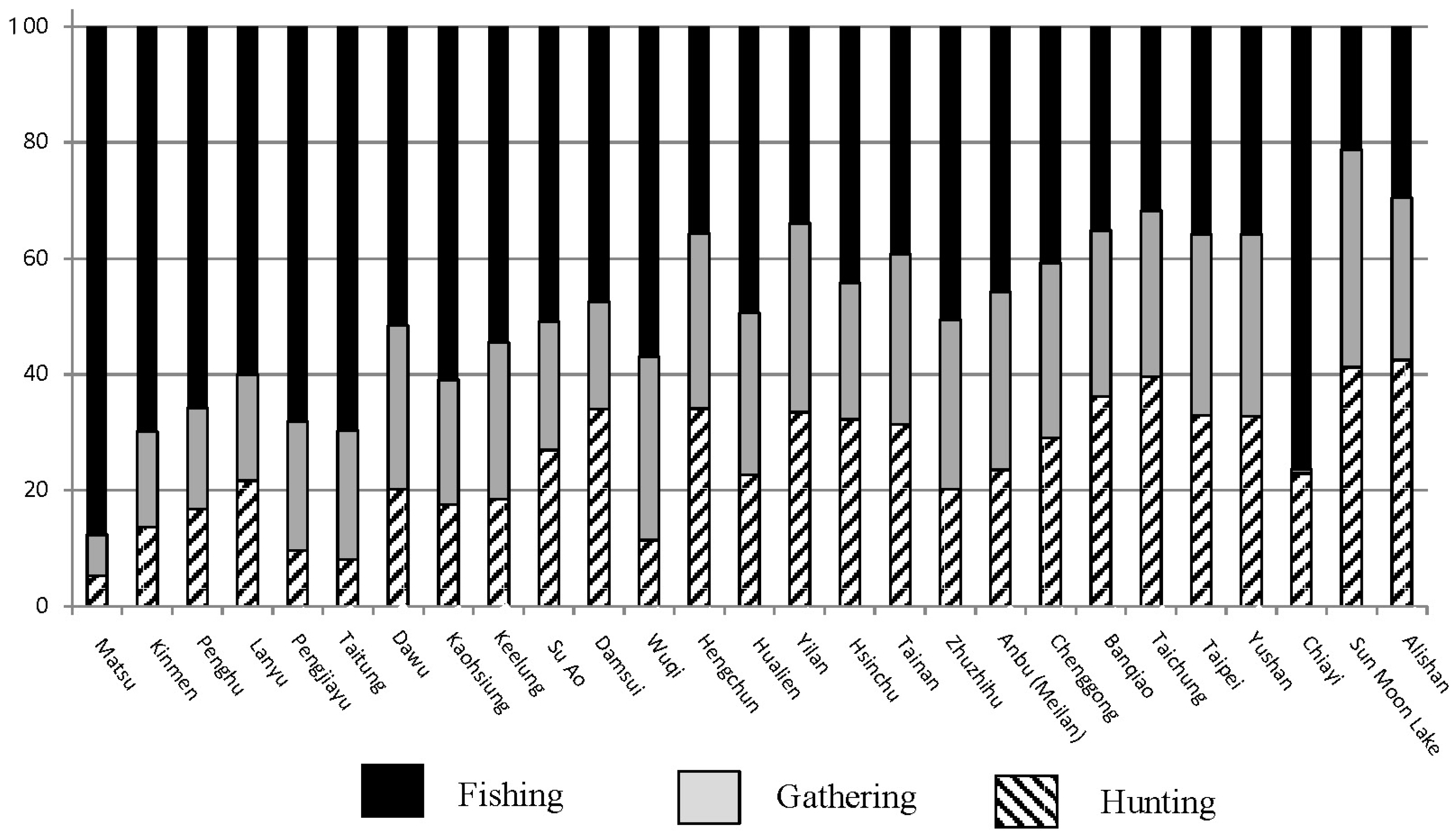
- WHUNTP/WGATHP/WFISHP: Expected percentage of hunting, gathering, and fishing/aquatic resources derived from ethnographically known hunter-gatherer groups who reside in habitats with similar environmental characteristics.
- SUBSPE: Ordinal classification of projected foraging subsistence specialty. 1 = primarily hunting or dependence on terrestrial animals; 2 = gathering or dependence on terrestrial plants; 3 = primarily fishing or dependence on aquatic prey.
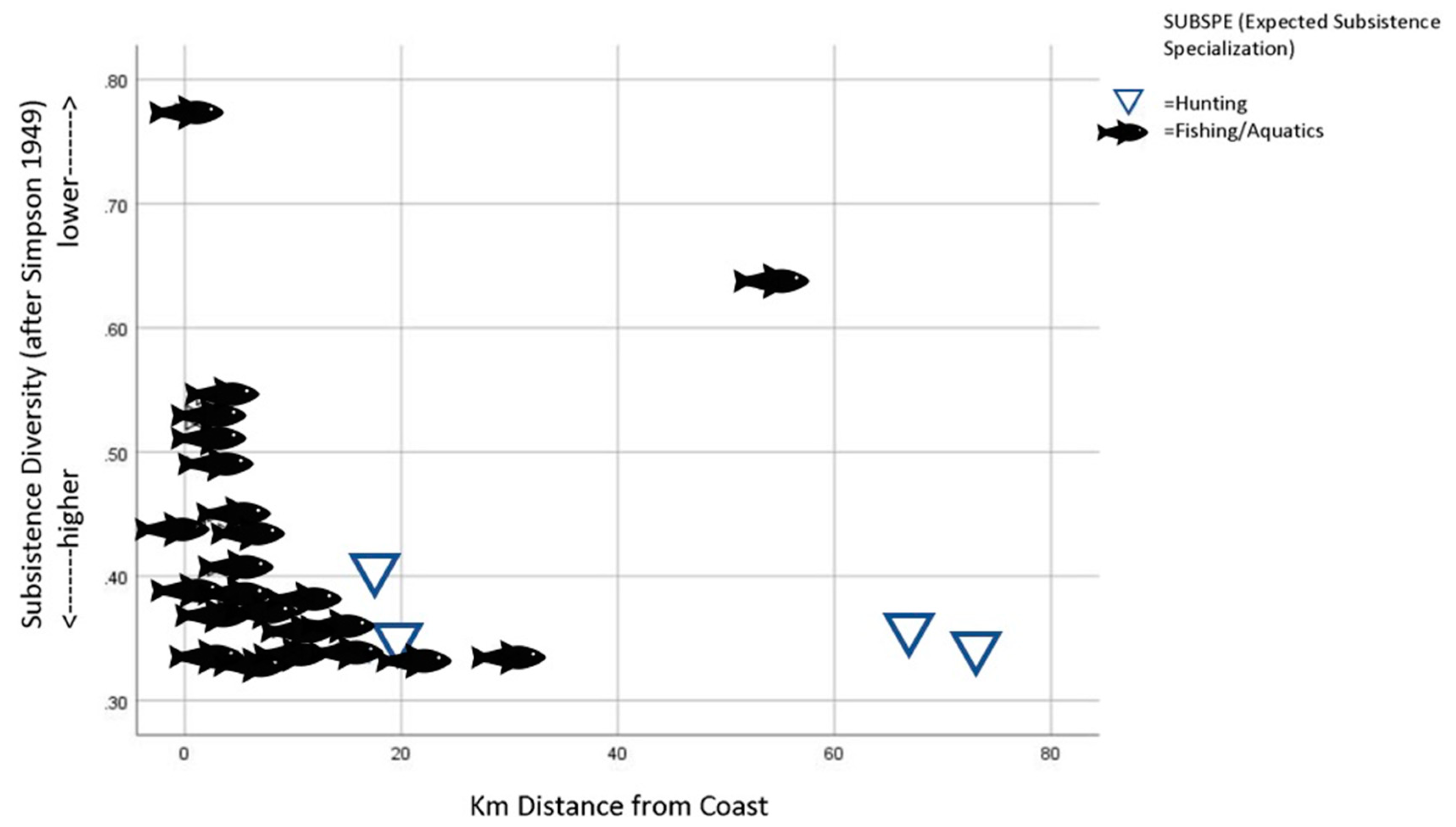
- SUBDIV: An index of foraging diversity or ‘evenness’ that is calculated using Simpson’s diversity index (see below for details of calculation). Expressed as a decimal value between 0 and 1 in which values approaching 1 indicate less evenness or more specialization.
4. Results
4.1. Foraging Subsistence Projections and Preferred Wild Prey in Taiwan
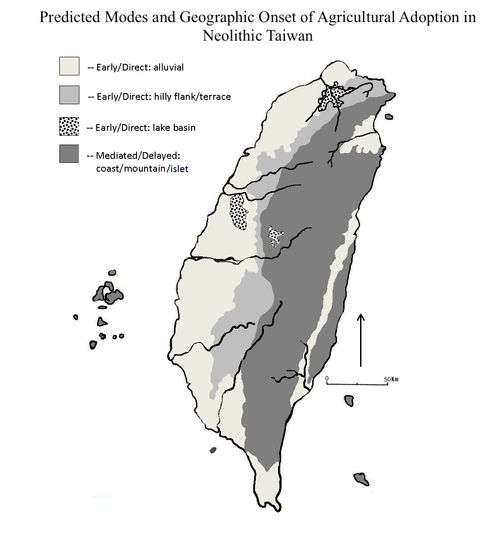
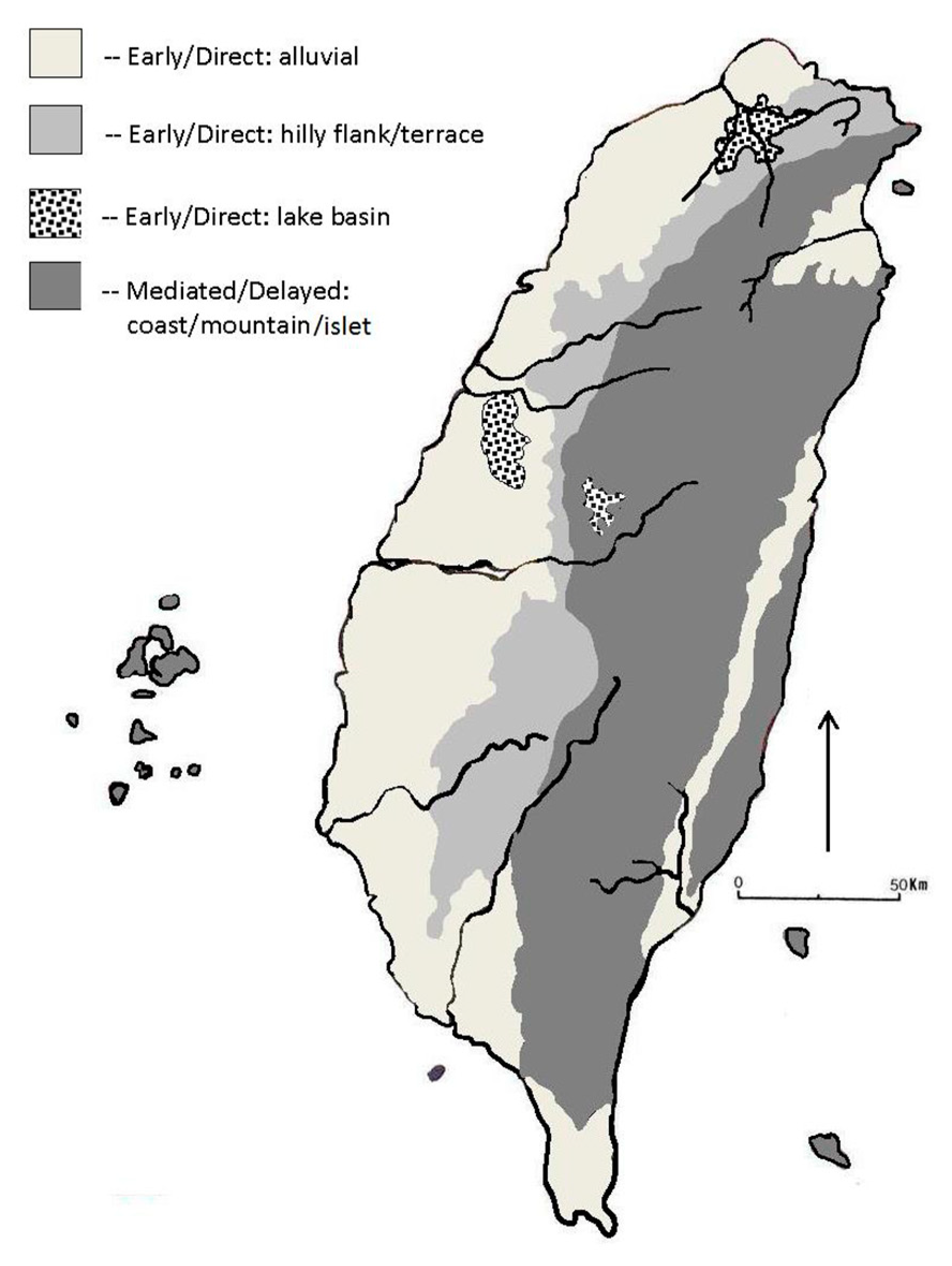
4.2. Transitional Neolithic Paleoenvironments and Archaeology
5. Discussion
- Early Paleolithic foragers migrated to Taiwan from the mainland c. 20,000 years ago, moving into new territory under conditions of ecological release.
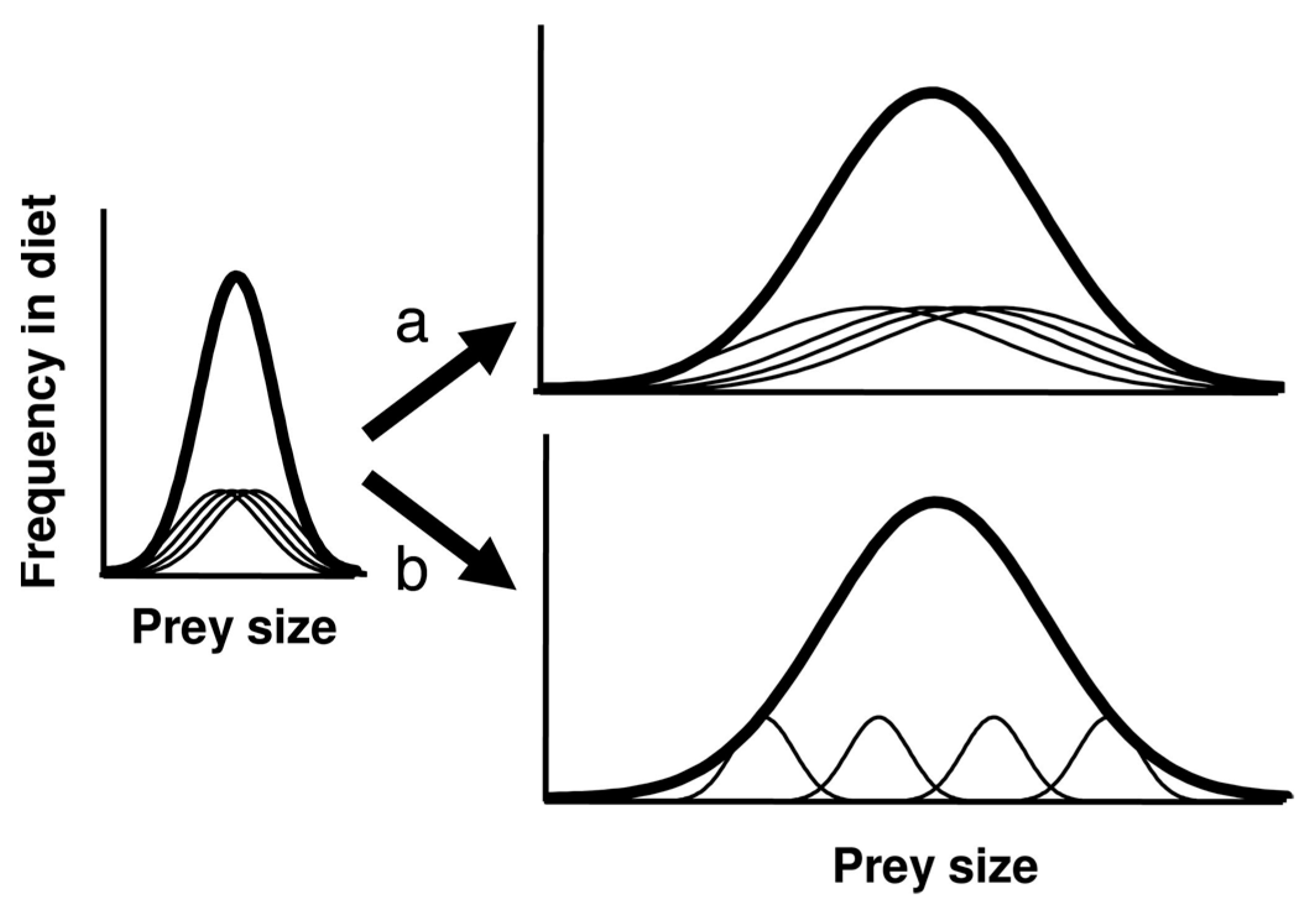
- Niche variation theory predicts an expansion of foraging niche breadth, increasing among-group variation within the niche. By the Persistent Upper Paleolithic, foraging niches divided into two specialized modes: either aquatic resources or mountain hunting.
- The arrival and first dispersal of Neolithic Chinese farmers caused farmer-forager competition for wild resources, especially aquatic, in flat areas near coasts and wetlands. The prey choice model predicts that the niche overlap would lead to reductions in preferred prey and resource depression, whereas the IFD with Allee effects predicts that niche constriction could have increased encounter rates with preferred prey, at least in the beginning.
- Foragers choosing to remain in colonized areas responded adaptively by taking low-ranked prey, then including low-ranked, low-cost cultigens in the diet, expanding the foraging niche temporarily (Figure 10). The PCM predicts this would have occurred rapidly; the IFD predicts a delay due to the beneficial effects of niche construction.
- This phenomenon occurred early and rapidly in coastal areas and neighboring flat regions that were favorable to arriving farmers, then dispersed into more distant mountain uplands and the east coast as demographic packing continued.
- The transition was more gradual in the central mountains and east coast. Foragers who opted to move away from farmer interactions may have encountered forager-forager competition and resource depression in those areas, and demographic growth fueled a cycle that ultimately ended full-time hunting and gathering across Taiwan.
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, B.D. Low-level food production. J. Archaeolog. Res. 2001, 9, 1–43. [Google Scholar] [CrossRef]
- Ikeya, K.; Ogawa, H.; Mitchell, P. (Eds.) Interactions between Hunter-Gatherers and Farmers: From Prehistory to Present; National Museum of Ethnology: Osaka, Japan, 2009; ISBN 9784901906654. [Google Scholar]
- Winterhalder, B.; Kennett, D.H. Behavioral ecology and the transition from hunting and gathering to agriculture. In Behavioral Ecology and the Transition to Agriculture; Kennett, D., Winterhalder, B., Eds.; University of California Press: Berkeley, CA, USA, 2006; pp. 1–21. ISBN 9780520246478. [Google Scholar]
- Hung, H.C.; Carson, M.T. Foragers, fishers and farmers: Origins of the Taiwanese Neolithic. Antiquity 2014, 88, 1115–1131. Available online: https://0-www-cambridge-org.brum.beds.ac.uk/core/journals/antiquity/article/foragers-fishers-and-farmers-origins-of-the-taiwanese-neolithic/7B50C25B6298EB3AEABE1E8EEFD030C8 (accessed on 15 April 2020). [CrossRef]
- Peltier, W.R. On eustatic sea level history: Last Glacial Maximum to Holocene. Quat. Sci. Rev. 2002, 21, 377–396. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/science/article/pii/S0277379101000841 (accessed on 15 April 2020). [CrossRef]
- Rollett, B.V.; Zheng, Z.; Yue, Y. Holocene sea-level change and the emergence of Neolithic seafaring in the Fuzhou Basin (Fujian, China). Quat. Sci. Rev. 2011, 30, 788–797. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/science/article/pii/S0277379111000308 (accessed on 15 April 2020). [CrossRef]
- Bulbeck, D. An integrated perspective on the Austronesian diaspora: The switch from cereal agriculture to maritime foraging in the colonisation of Island Southeast Asia. Aust. Archaeol. 2008, 67, 31–51. Available online: https://0-www-tandfonline-com.brum.beds.ac.uk/doi/abs/10.1080/03122417.2008.11681877 (accessed on 15 April 2020). [CrossRef]
- Bellwood, P. Southeast China and the Prehistory of the Austronesians. In Lost Maritime Cultures: China and the Pacific; Jiao, T.L., Ed.; Bishop Museum Press: Honolulu, HI, USA, 2007; pp. 36–53. ISBN 9781581780635. [Google Scholar]
- Bellwood, P. Formosan prehistory and Austronesian dispersal. In Austronesian Taiwan: Linguistics, History, Ethnology, Prehistory; Blundell, D., Ed.; N. W. Lin Foundation for Culture and Educational Endowment: Berkeley, CA, USA, 2009; pp. 336–364. ISBN 986-8537819. [Google Scholar]
- Blust, R. The Austronesian Languages; Research School of Pacific and Asian Studies: Canberra, Australia, 2009; ISBN 9780858836020. [Google Scholar]
- Chang, K.C. The Neolithic Taiwan Strait. Kaogu 1989, 6, 569. [Google Scholar]
- Chang, K.C. Fengbitou, Tapenkeng, and the Prehistory of Taiwan; Yale University Publications in Anthropology: New Haven, CT, USA, 1969; ISBN 9781444335293. [Google Scholar]
- Chang, K.C.; Goodenough, W.H. Archaeology of southeastern China and its bearing on the Austronesian homeland. In Prehistoric Settlement of the Pacific; Goodenough, W.H., Ed.; American Philosophical Society: Philadelphia, PA, USA, 1996; pp. 28–35. ISBN 9780871698650. [Google Scholar]
- Pawley, A. The Austronesian dispersal: Languages, technologies and people. In Examining the Farming/language Dispersal Hypothesis; Bellwood, P., Renfrew, C., Eds.; MacDonald Institute for Archaeological Research: Cambridge, UK, 2002; pp. 251–273. ISBN 9781902937205. [Google Scholar]
- Tsang, C.H. Recent discoveries at the Tapenkeng Culture Sites in Taiwan: Implications for the Problem of Austronesian Origins. In The Peopling of East Asia: Putting Together Archaeology, Linguistics, and Genetics; Sagart, L., Blench, R., Sanchez-Mazas, A., Eds.; Routledge Curzon: New York, NY, USA, 2005; pp. 63–73. ISBN 9781138862234. [Google Scholar]
- Bellwood, P. Taiwan and the Prehistory of the Austronesian-Speaking Peoples. In Ethnos, Geography and Development: An Interdisciplinary Approach to Human-Environment Relations; Kuan, D.W., Ed.; Shung Ye Museum of Formosan Aborigines: Taipei, Taiwan, 2017; pp. 3–33. ISBN 9789869239639. [Google Scholar]
- Li, K.T. First Farmers and Their Coastal Adaptation in Prehistoric Taiwan. In A Companion to Chinese Archaeology; Underhill, A.P., Ed.; Blackwell: Oxford, UK, 2013; pp. 612–633. ISBN 9781444335294. [Google Scholar]
- Liu, Y.C. Prehistory and Austronesians in Taiwan: An archaeological perspective. In Austronesian Taiwan: Linguistics, History, Ethnology, Prehistory; Blundell, D., Ed.; N. W. Lin Foundation for Culture and Educational Endowment: Berkeley, CA, USA, 2009; pp. 365–400. ISBN 9868537800. [Google Scholar]
- Bolnick, D.I.; Svanbäck, R.; Araújo, M.S.; Persson, L. Comparative support for the niche variation hypothesis that more generalized populations also are more heterogeneous. Proc. Natl. Acad. Sci. USA 2007, 104, 10075–10079. Available online: https://www.pnas.org/content/104/24/10075 (accessed on 15 April 2020). [CrossRef] [Green Version]
- Fix, A.G.; Shepherd, J.C. Migration and Colonization in Human Microevolution; Cambridge University Press: Cambridge, MA, USA, 1999; Volume 24, ISBN 9780521592062. [Google Scholar]
- Binford, L.R. Constructing Frames of Reference: An Analytical Method for Archaeological Theory Building Using Ethnographic and Environmental Data Sets; University of California Press: Berkeley, CA, USA, 2001; ISBN 0520223934. [Google Scholar]
- Svizzero, S. Persistent controversies about the Neolithic Revolution. J. Hist. Archaeol. Anthr. Sci. 2017, 1, 00013. [Google Scholar] [CrossRef]
- Yu, P. Ethnoarchaeology of foraging and the case of the vanishing agriculturalists in the Amazon Basin. J. Anthr. Archaeol. 2015, 38, 59–66. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/science/article/pii/S0278416514000683 (accessed on 15 April 2020). [CrossRef]
- Bird, D.W.; O’Connell, J.F. Behavioral ecology and archaeology. J. Archaeol. Res. 2006, 14, 143–188. [Google Scholar] [CrossRef]
- Bird, D.W.; O’Connell, J.F. Human behavioral ecology. In Archaeological Theory Today; Hodder, I., Ed.; Polity Press: Cambridge, MA, USA, 2012; pp. 37–61. ISBN 9780745653075. [Google Scholar]
- Broughton, J.M.; Cannon, M.D. (Eds.) Evolutionary Ecology and Archaeology; University of Utah Press: Salt Lake City, UT, USA, 2010; ISBN 9780874809350. [Google Scholar]
- Codding, B.F.; Bird, D.W. Behavioral ecology and the future of archaeological science. J. Archaeol. Sci. 2015, 56, 9–20. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/science/article/pii/S0305440315000655?casa_token=yVm483vDuhgAAAAA:tPRWrRljxrWNR0_FAJj0Bx03La8Z_ickOJAQI_53Skf_p7T3chXy8ogpRHbpOmcHromv9ZhaPw (accessed on 5 July 2020). [CrossRef]
- Lupo, K.D. Evolutionary foraging models in zooarchaeological analysis: Recent applications and future challenges. J. Archaeol. Res. 2007, 15, 143–189. Available online: https://0-link-springer-com.brum.beds.ac.uk/article/10.1007/s10814-007-9011-1 (accessed on 5 July 2020). [CrossRef]
- O’Connell, J.F. Ethnoarchaeology needs a general theory of behavior. J. Archaeol. Res. 1995, 3, 205–255. [Google Scholar] [CrossRef]
- Johnson, A.L. Exploring adaptive variation among hunter-gatherers with Binford’s frames of reference. J. Archaeol. Res. 2014, 22, 1–42. Available online: https://0-link-springer-com.brum.beds.ac.uk/article/10.1007/s10814-013-9068-y (accessed on 15 April 2020). [CrossRef]
- Fretwell, S.D.; Lucas, H.L. On territorial behavior and other factors influencing habitat distribution in birds. Acta Biotheor. 1969, 19, 16–36. Available online: https://0-link-springer-com.brum.beds.ac.uk/article/10.1007/BF01601953 (accessed on 5 July 2020). [CrossRef]
- Kennett, D.J.; Anderson, A.; Winterhalder, B. The Ideal Free Distribution, food production, and the colonization of Oceania. In Behavioral Ecology and the Transition to Agriculture; Kennett, D.J., Winterhalder, B., Eds.; University of California Press: Berkeley, CA, USA, 2009; pp. 265–288. ISBN 9780520246478. [Google Scholar]
- Allee, W.C.; Bowen, E. Studies in animal aggregations: Mass protection against colloidal silver among goldfishes. J. Exp. Zool. 1932, 61, 185–207. [Google Scholar] [CrossRef]
- Codding, B.F.; Parker, A.K.; Jones, T.L. Territorial behavior among Western North American foragers: Allee effects, within group cooperation, and between group conflict. Quat. Int. 2019, 518, 31–40. Available online: https://www.researchgate.net/publication/321270258_Territorial_behavior_among_Western_North_American_foragers_Allee_effects_within_group_cooperation_and_between_group_conflict (accessed on 18 July 2020). [CrossRef]
- Bliege Bird, R.B.; McGuire, C.; Bird, D.W.; Price, M.H.; Zeanah, D.; Nimmo, D.G. Fire mosaics and habitat choice in nomadic foragers. Proc. Natl. Acad. Sci. USA 2020, 117, 12904–12914. Available online: https://www.pnas.org/content/117/23/12904.short (accessed on 5 July 2020). [CrossRef]
- Bliege Bird, R.B.; Bird, D.W.; Fernandez, L.E.; Taylor, N.; Taylor, W.; Nimmo, D. Aboriginal burning promotes fine-scale pyrodiversity and native predators in Australia’s Western Desert. Biol. Conserv. 2018, 219, 110–118. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/science/article/abs/pii/S0006320717317962 (accessed on 18 July 2020). [CrossRef]
- Winterhalder, B.; Kennett, D.J.; Grote, M.N.; Bartruff, J. Ideal free settlement of California’s northern Channel Islands. J. Anthropol. Archaeol. 2010, 29, 469–490. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/science/article/abs/pii/S0278416510000425 (accessed on 5 July 2020). [CrossRef] [Green Version]
- Yu, P. Tempo and mode of Neolithic crop adoption by Paleolithic hunter-gatherers of Taiwan: Ethnoarchaeological and behavioral ecology perspectives. In Hunter-Gatherers in Asia: From Prehistory to Present; Ikeya, K., Nishiaki, Y., Eds.; Senri Ethnological Studies (SES)/National Museum of Ethnography: Osaka, Japan, 2021. (in press) [Google Scholar]
- Elton, C.S. The Ecology of Invasions by Plants and Animals; University of Chicago Press: Chicago, IL, USA, 1958; ISBN 9780521592062. [Google Scholar]
- MacArthur, R.H.; Pianka, E.R. On optimal use of a patchy environment. Am. Nat. 1966, 100, 603–609. Available online: https://0-www-jstor-org.brum.beds.ac.uk/stable/2459298?seq=1 (accessed on 5 July 2020). [CrossRef] [Green Version]
- Stephens, D.W.; Krebs, J.R. Foraging Theory; Princeton University Press: Princeton, NJ, USA, 1987; Volume 1, ISBN 9780691084428. [Google Scholar]
- Gremillion, K.J. Seed processing and the origins of food production in eastern North America. Am. Antiq. 2004, 69, 215–233. Available online: https://0-www-jstor-org.brum.beds.ac.uk/stable/4128417?seq=1#metadata_info_tab_contents (accessed on 10 June 2018). [CrossRef]
- Gremillion, K.J.; Piperno, D.R. Human behavioral ecology, phenotypic (developmental) plasticity, and agricultural origins: Insights from the emerging evolutionary synthesis. Curr. Anthropol. 2009, 50, 615–619. Available online: https://0-www-journals-uchicago-edu.brum.beds.ac.uk/doi/abs/10.1086/605360 (accessed on 15 April 2020). [CrossRef] [PubMed] [Green Version]
- Miller, D.S. From Colonization to Domestication: Population, Environment, and the Origins of Agriculture in Eastern North America; University of Utah Press: Salt Lake City, UT, USA, 2018; ISBN 1607816164. [Google Scholar]
- Pearsall, D.M. Investigating the Transition to Agriculture. Curr. Anthropol. 2009, 50, 609–613. Available online: https://0-www-jstor-org.brum.beds.ac.uk/stable/10.1086/605406#metadata_info_tab_contents (accessed on 15 April 2020). [CrossRef]
- Winterhalder, B.; Kennett, D.H. Four neglected concepts with a role to play in explaining the origins of agriculture. Curr. Anthropol. 2009, 50, 645–648. Available online: https://0-www-jstor-org.brum.beds.ac.uk/stable/10.1086/605355?seq=1#metadata_info_tab_contents (accessed on 15 April 2020). [CrossRef] [PubMed] [Green Version]
- Jackson, M.C.; Britton, J.R. Stable isotope analyses indicate trophic niche overlap of invasive Pseudorasbora parva and sympatric cyprinid fishes. Ecol. Freshw. Fish 2013, 22, 654–657. Available online: https://0-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/pdf/10.1111/eff.12063 (accessed on 15 April 2020). [CrossRef]
- Hamilton, S.; Johnston, R.F. Evolution in the House Sparrow; VI. variability and niche width. Auk 1978, 95, 313–323. Available online: https://0-academic-oup-com.brum.beds.ac.uk/auk/article-abstract/95/2/313/5208845?redirectedFrom=fulltext (accessed on 15 April 2020).
- Soule, M.; Stewart, B.R. The “niche-variation” hypothesis: A test and alternatives. Am. Nat. 1970, 104, 85–97. Available online: https://0-www-jstor-org.brum.beds.ac.uk/stable/2459075?seq=1#metadata_info_tab_contents (accessed on 15 April 2020). [CrossRef]
- Werner, T.K.; Sherry, T.W. Behavioral feeding specialization in Pinaroloxias inornata, the “Darwin’s finch” of Cocos Island, Costa Rica. Proc. Natl. Acad. Sci. USA 1987, 84, 5506–5510. Available online: https://www.pnas.org/content/84/15/5506 (accessed on 15 April 2020). [CrossRef] [Green Version]
- Van Valen, L. Morphological variation and width of ecological niche. Am. Nat. 1965, 99, 377–390. Available online: https://0-www-jstor-org.brum.beds.ac.uk/stable/2459179?seq=1#metadata_info_tab_contents (accessed on 15 April 2020). [CrossRef]
- Snyder, W.E.; Evans, E.W. Ecological effects of invasive arthropod generalist predators. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 95–122. Available online: https://0-www-annualreviews-org.brum.beds.ac.uk/doi/full/10.1146/annurev.ecolsys.37.091305.110107 (accessed on 15 April 2020). [CrossRef] [Green Version]
- Olsson, K.; Stenroth, P.; Nyström, P.; Graneli, W. Invasions and niche width: Does niche width of an introduced crayfish differ from a native crayfish? Freshw. Biol. 2009, 54, 1731–1740. Available online: https://portal.research.lu.se/portal/en/publications/invasions-and-niche-width-does-niche-width-of-an-introduced-crayfish-differ-from-a-native-crayfish(e78fca3d-d88c-40fc-9628-292b33506e33).html (accessed on 15 April 2020). [CrossRef]
- Porter, S.D.; Savignano, D.A. Invasion of polygyne fire ants decimates native ants and disrupts arthropod community. Ecology 1990, 71, 2095–2106. Available online: https://0-esajournals-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/10.2307/1938623 (accessed on 15 April 2020). [CrossRef]
- Hutchinson, G.E. Concluding Remarks. Cold Spring Harb. Symp. Quant. Biol. 1957, 22, 415–421. Available online: http://symposium.cshlp.org/content/22/415 (accessed on 1 September 2020). [CrossRef]
- Bolnick, D.I.; Ingram, T.; Stutz, W.E.; Snowberg, L.K.; Lau, O.L.; Paull, J.S. Ecological release from interspecific competition leads to decoupled changes in population and individual niche width. Proc. R. Soc. B Biol. Sci. 2010, 277, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Svanbäck, R.; Bolnick, D.I. Intraspecific competition affects the strength of individual specialization: An optimal diet theory method. Evol. Ecol. Res. 2005, 7, 993–1012. [Google Scholar] [CrossRef]
- Araújo, M.S.; Bolnick, D.I.; Layman, C.A. The ecological causes of individual specialisation. Ecol. Lett. 2011, 14, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Penk, M.; Irvine, K.; Donohue, I. Ecosystem-level effects of a globally-spreading invertebrate invader are not moderated by a functionally similar native. J. Anim. Ecol. 2015, 84, 1628–1636. [Google Scholar] [CrossRef]
- Smith, B.D. A comparison of niche construction theory and diet breadth models as explanatory frameworks for the initial domestication of plants and animals. J. Archaeol. Res. 2015, 23, 215–262. Available online: https://0-link-springer-com.brum.beds.ac.uk/article/10.1007/s10814-015-9081-4 (accessed on 15 April 2020). [CrossRef]
- Boserup, E. The Conditions of Agricultural Growth: The Economics of Agrarian Change under Population Pressure; Transaction Publishers: New Brunswick, NJ, USA, 2011; ISBN 9781138537187. [Google Scholar]
- Zeanah, D.W. Foraging models, niche construction, and the Eastern Agricultural Complex. Am. Antiq. 2017, 82, 3–24. Available online: https://0-www-cambridge-org.brum.beds.ac.uk/core/journals/american-antiquity/article/foraging-models-niche-construction-and-the-eastern-agricultural-complex/7481D608E03F7864C6C6FB5990F1B9F1 (accessed on 15 April 2020). [CrossRef] [Green Version]
- David, N.; Kramer, C. Ethnoarchaeology in Action; Cambridge University Press: New York, NY, USA, 2001; ISBN 9780521667791. [Google Scholar]
- Gifford-Gonzales, D. Ethnoarchaeology—Looking back, looking forward. SAA Archaeol. Rec. 2010, 10, 22–25. Available online: http://digital.ipcprintservices.com/publication/?i=30669&article_id=308302&view=articleBrowser (accessed on 15 April 2020).
- Yu, P. Ethnoarchaeology as a strategy for building frames of reference for research problems. In Hunter Gatherer and Mid-Range Societies, Encyclopedia of Global Archaeology; Prentiss, A., Ed.; Springer Publishing: New York, NY, USA, 2014; ISBN 9781441904263. [Google Scholar]
- Song, Y.C.; Xu, G.S. A Scheme of Vegetation Classification of Taiwan, China. Acta Bot. Sin. 2003, 45, 883–895. Available online: https://europepmc.org/article/cba/364797 (accessed on 15 April 2020).
- Lee, C.Y.; Liew, P.M. Late Quaternary vegetation and climate changes inferred from a pollen record of Dongyuan Lake in southern Taiwan. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 287, 58–66. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/science/article/pii/S0031018210000167?casa_token=3oPH7oCmZY8AAAAA:sbce5Q82gnmOkAUpF9GWZFF-YE2S-XwRbanPffYAc6cEC9J-DQgcq6xbiK9G6unkjBbAIXOnqg (accessed on 5 July 2020). [CrossRef]
- Tsang, C.H.; Li, K.T. Archaeological Heritage in the Tainan Science Park of Taiwan; National Museum of Prehistory: Taitung City, Taiwan, 2018; ISBN 9789860474138. [Google Scholar]
- Chen, C.T.A.; Ruo, R.; Paid, S.C.; Liu, C.T.; Wong, G.T.F. Exchange of water masses between the East China Sea and the Kuroshio off northeastern Taiwan. Cont. Shelf Res. 1995, 15, 19–39. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/science/article/pii/0278434393E0001O (accessed on 22 April 2020). [CrossRef]
- Lin, J.X.; Dai, L.P.; Yuan, W.; Min, L. Quaternary marine transgressions in eastern China. J. Palaeogeogr. 2012, 1, 105–125. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/science/article/pii/S209538361530119X (accessed on 22 April 2020).
- McCullough, D.R.; Takatsuki, S.; Kaji, K. (Eds.) Sika deer: Biology and Management of Native and Introduced Populations; Springer Science & Business Media: Berlin, Germany, 2008. [Google Scholar]
- Lien, C.M. Chang-pin Culture of Taiwan and Characteristics of Its Lithic Industry. In Emergence and Diversity of Modern Human Behavior in Paleolithic Asia; Kaifu, Y., Izuho, M., Goebel, T., Sato, H., Ono, A., Eds.; Texas A & M Press: College Station, TX, USA, 2014; pp. 239–248. ISBN 9781623492762. [Google Scholar]
- Sung, W.S. The archaeology of Taiwan. In Taiwan of China; Chen, C.L., Ed.; Zhongyang Wenwu Gongyingshe: Taipei, Taiwan, 1980; pp. 93–220. (In Chinese) [Google Scholar]
- Sung, W.S. Changpinian: A Newly Discovered Pre-Ceramic Culture from the Agglomerate Caves on the East Coast of Taiwan; Newsletter of Ethnological Society of Taiwan: Taipei, Taiwan, 1969; Volume 9, pp. 1–9. [Google Scholar]
- Tsang, C.H.; Chen, W.S.; Li, K.T. A Progress Report on First Year Results of the Ba Xian Dong Archaeological Survey Research Project; Academia Sinica: Taipei, Taiwan, 2009. (In Chinese) [Google Scholar]
- Tsang, C.H.; Chen, W.S.; Li, K.T. (Eds.) Tai Dong Chang Bin Ba Xian Dong Yi Zhi Diao Cha Yan Jiu Ji Hua (Di Er Nian); Academia Sinica: Taipei, Taiwan, 2011. (In Chinese) [Google Scholar]
- Tsang, C.H. Lun Changbin Wenhua de Niandai yu Leiyuan. In Baxiandong Guodeng Yizhi Baohu yu Yanjiu Guoji Xueshu Yantaohui Luwenji; Wenhuabu Wenhua Zichanju, Taidongxian Zhengfu, Zhongyang Yanjiuyan, Lishi Yuyan yankuisuo Zhixing: Taipei, Taiwan, 2013; pp. 1–23. (In Chinese) [Google Scholar]
- Kuo, S.C. New Frontiers in the Neolithic Archaeology of Taiwan (5600–1800 BP); Springer: New York, NY, USA, 2019; ISBN 9789813292628. [Google Scholar]
- Chen, W.C. The Early Occupation of Taiwan. In The Handbook of East and Southeast Asian Archaeology; Habu, J., Lape, P.V., Olsen, J.W., Eds.; Springer: New York, NY, USA, 2017; pp. 277–291. ISBN 9781493965212. [Google Scholar]
- Liu, Y.; Guo, S.; Lu, R. Taimin Diqu Kaogu Yihi Pucha Yanjiu Jihua (Diqiqi); Taipeixian, Jilongshi, Taipeishi, Neizhengbu Weituo, Zhongyang Yanjiuyan, Lishi Yuyan yankuisuo Zhixing: Taipei, Taiwan, 2004. [Google Scholar]
- Liu, Y. 2011 Zhuminzhi Kaogupian. In Taiwan Quanzhi; Guoshiguan Taiwan Wenxianguan: Nantou, Taiwan, 2011; Volume 3. [Google Scholar]
- Liu, Y.; Chen, J.; Zheng, H.; Li, J. Taizhongxian Kaogu Yizhi Pucha yu Yanjiu Jihua Yanjiu Baogao; Taizhongixian Wenhuaju Weituo, Zhongyan Yanjiuyuan Renwen Shehui Kexue Zongxin, Kaoguxue Yanjiu Zhuanti Zhongxin Zhixing: Taichung, Taiwan, 2007. [Google Scholar]
- Liu, Y. Disanzhang: Shiqian Yizhi. In Taizhong Xianzhi (Juanyi) Tudizhi; Taizhongxian Zhengfu: Taichung, Taiwan, 1989; pp. 773–849. [Google Scholar]
- Li, K.T. 1985 Duiyu Taiwan Kaogu Yanjiu de Ryogan Renshi; Taiwan Wenxian: Taipei, Taiwan, 1985; Volume 36, pp. 15–23. [Google Scholar]
- Huang, S.; Chen, Y.; Yan, X. Kending Guojia Gongyuan Kaogu Minzhu Diaocha Baogao; Neizhengbu Yingjianshu Kending Guojia Gongyuan Guanlichu Weituo, Guoli Taiwan Daxue Renlei Xuexi Zhixing: Taipei, Taiwan, 1987. [Google Scholar]
- Li, K.T.; Liu, Y.; Chang, C. Eluanbi Gongyuan Kaogu Diaocha Baogao; Jiaotongbu Guangguangju Kending Fenjing Tedingqu Guanlingchu Weituo, Guoli Taiwan Daxue Renlei Xuexi Zhixing: Taipei, Taiwan, 1983. [Google Scholar]
- Huang, S.; Chen, Y. Donghe Diqu Yizhi Shijue di Shiqian Wenhua Chongjian; Xingzhengyuan Wenhua Jianshe Weiyuanhui Weituo, Guoli Taiwan Daxue Renlei Xuexi Zhixing: Taipei, Taiwan, 1990. [Google Scholar]
- Binford, L.R.; Johnson, A.L. Program for Calculating Environmental and Hunter-Gatherer Frames of Reference (ENVCALC2.1). Updated Java Version. August 2014. Available online: http://ajohnson.sites.truman.edu/data-and-program/ (accessed on 25 June 2018).
- Lin, C.; Taiwan Forestry Research Institute, Taipei; Yu, P.; Boise State University, Boise, ID, USA. Personal communication, 2017.
- Butal, A.; Tung, G.S.; Miaraw, P. The Ethnobotany of Amis in Eastern Formosa; Jen Teh Yen, Council of Agriculture Executive Yuan: Taipei, Taiwan, 2009; ISBN 9789860223156. [Google Scholar]
- Lu, T.H.; Ke, Y.N.; Lin, S.F.; Lu, S.Y. Plants Used by Paiwan in Taiwan; Report prepared for Taiwan Forestry Bureau, Council of Agriculture, Executive Yuan: Taipei, Taiwan, 2011. [Google Scholar]
- Simpson, E.H. The Measurement of Diversity. Nature 1949, 163, 688. Available online: https://0-www-nature-com.brum.beds.ac.uk/articles/163688a0 (accessed on 15 April 2020). [CrossRef]
- Chu, C.Y. Report on the restoration, excavation, construction, and supervision of the Talungtung Site (Dalongdong Yizhi Qiangjiu Fajue Ji Shigong Jiankan Jihuau Chengguo Baogao); Tree Valley Foundation, Taipei City Government Department of Cultural Affairs: Taipei, Taiwan, 2012. (In Chinese) [Google Scholar]
- Huang, S.C. Report on Archaeological Investigation at Pa-chia Village, Kui-jun Township; Bulletin of the Department of Archaeology and Anthropology: Tainan, Taiwan, 1974; pp. 62–68. (In Chinese) [Google Scholar]
- Tsang, C.H. Archaeology of the Penghu Islands; Academia Sinica: Taipei, Taiwan, 1992; ISBN 9789576710483. (In Chinese) [Google Scholar]
- National Museum of Natural Science. Report on the Salvage Excavation Project at the Construction Site at the “Anho Road Site”; Report commissioned by Ching He Construction Co., Ltd.; National Museum of Natural Science: Taichung City, Taiwan, 2016. (In Chinese) [Google Scholar]
- Tsang, C.H. (Ed.) Final Report on the Tainan Science Park Archaeological Rescue and Monitoring Project; Taiwan National Museum of Prehistory: Taitung City, Taiwan, 2006. (In Chinese) [Google Scholar]
- Tsang, C.H. Final Report on the Archaeological Project of Restoring Areas of the Taoyeh Site Excluded from the Preservation Area, Tainan Science Park; Report prepared for the Southern Taiwan Science Bureau; Institute of History and Philology, Academia Sinica: Taipei, Taiwan, 2004. (In Chinese) [Google Scholar]
- Chen, S.Q.; Yu, P. Early ‘Neolithics’ of China: Variation and Evolutionary Implications. J. Anthropol. Res. 2017, 73, 149–180. Available online: https://0-www-journals-uchicago-edu.brum.beds.ac.uk/doi/abs/10.1086/692104?af=R (accessed on 22 April 2020). [CrossRef] [Green Version]
- Liu, L.; Field, J.; Fullagar, R.; Zhao, C.; Chen, X.; Yu, J. A functional analysis of grinding stones from an early Holocene site at Donghulin, North China. J. Archaeol. Sci. 2010, 37, 2630–2639. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/science/article/pii/S0305440310001858 (accessed on 22 April 2020). [CrossRef]
- Sagart, L.; Hsu, T.F.; Tsai, Y.C.; Wu, C.C.; Huang, L.T.; Chen, Y.C.; Chen, Y.F.; Tseng, Y.C.; Lin, H.Y.; Hsing, Y.I.C. A northern Chinese origin of Austronesian agriculture: New evidence on traditional Formosan cereals. Rice 2018, 11, 57–73. Available online: https://thericejournal.springeropen.com/articles/10.1186/s12284-018-0247-9 (accessed on 15 April 2020). [CrossRef]
- Jiao, T.L. Toward an alternative perspective on the foraging and low-level food production on the coast of China. Quat. Int. 2016, 419, 54–61. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/science/article/pii/S1040618215006606 (accessed on 15 April 2020). [CrossRef]
- Zhang, C.; Hung, H.C. Later hunter-gatherers in southern China, 18,000–3000 BC. Antiquity 2012, 86, 11–29. Available online: https://0-www-cambridge-org.brum.beds.ac.uk/core/journals/antiquity/article/later-huntergatherers-in-southern-china-18-0003000-bc/AE598987DF32F318EB18B4CA16B814E0 (accessed on 15 April 2020).




| Prey Type Rank Order | Habitat Type |
|---|---|
| 1. Shellfish, nearshore fish including migratory catadromous species, waterfowl | Littoral/estuary/delta wetland/lake basin |
| 2. artiodactyls (deer, sambar, muntjac, serow), perissodactyls (boar) | Coastal plain, forested mountains, valleys |
| 3. Deep water fish, turtles, marine mammals | Pelagic/offshore, islets |
| 4. Arboreal and burrowing prey (macaque, pangolin, flying squirrel, and birds) | Forested valleys, piedmont and uplands |
| 5. Resident river fish, catadromous species, turtles | Riverine/inland |
| 6. Wild plants (geophytes, ferns, fungus, fruit, algae) | Forested valleys, piedmont and uplands |
| Prey Type Rank Order | Habitat Type |
|---|---|
| 1. Shellfish, nearshore fish including migratory catadromous species, waterfowl | Littoral/estuary/delta wetland/lake basin |
| 2. artiodactyls (deer, sambar, muntjac, serow), perissodactyls (boar) | Coastal plain, forested mountains, valleys |
| 3. Cultigens and edible weeds | Coastal plain, valleys |
| 4. Arboreal and burrowing prey (macaque, pangolin, flying squirrel, and birds) | Forested valleys, piedmont and uplands |
| 5. Resident river fish, catadromous species, turtles | Riverine/inland |
| 6. Deep water fish, turtles, marine mammals | Pelagic/offshore, islets |
| 7. Wild plants (geophytes, ferns, fungus, fruit, algae) | Forested valleys, piedmont and uplands |
| Model/Prediction | Categories of Evidence (Incipient NEOLITHIC, c. 6000–5000 BP) |
|---|---|
| 1. Prey Choice Model/Resource Depression | Subsistence: Decreases in frequency, diversity, age, and size of high ranked prey species relative to lower ranked prey. Technology: Increased processing tools and features such as ovens and graters. Settlement: Temporal and geographic overlap between Paleolithic and Neolithic sites in flatlands near the coast. Low frequency of earliest Neolithic sites in mountain uplands, islets, and east coast. |
| 2. IFD/Allee Effects/Niche Construction and Resource Benefits | Subsistence: Initially, increases in frequency, diversity, age, and size of highly ranked prey types relative to lower ranked prey. Could decrease over time after population rises. Technology: Few processing tools and features such as ovens and graters; more procurement tools (e.g., fishing and hunting equipment). Settlement: Temporal and geographic overlap between Paleolithic and Neolithic sites in flatlands near the coast; initial increase in Paleolithic sites close to Neolithic settlements, then replacement by agricultural sites. Low frequency of earliest Neolithic sites in mountain uplands, islets, and east coast. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, P.-L. Modeling Incipient Use of Neolithic Cultigens by Taiwanese Foragers: Perspectives from Niche Variation Theory, the Prey Choice Model, and the Ideal Free Distribution. Quaternary 2020, 3, 26. https://0-doi-org.brum.beds.ac.uk/10.3390/quat3030026
Yu P-L. Modeling Incipient Use of Neolithic Cultigens by Taiwanese Foragers: Perspectives from Niche Variation Theory, the Prey Choice Model, and the Ideal Free Distribution. Quaternary. 2020; 3(3):26. https://0-doi-org.brum.beds.ac.uk/10.3390/quat3030026
Chicago/Turabian StyleYu, Pei-Lin. 2020. "Modeling Incipient Use of Neolithic Cultigens by Taiwanese Foragers: Perspectives from Niche Variation Theory, the Prey Choice Model, and the Ideal Free Distribution" Quaternary 3, no. 3: 26. https://0-doi-org.brum.beds.ac.uk/10.3390/quat3030026