Application of Tungsten-Oxide-Based Electrochromic Devices for Supercapacitors
Abstract
:1. Introduction
2. Tungsten Oxide Electrodes
2.1. Zero Dimensions
2.2. One Dimension
3. Conductive Electrodes
4. Integrated Uses
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cai, G.; Wang, J.; Lee, P.S. Next-Generation multifunctional electrochromic devices. Acc. Chem. Res. 2016, 49, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Zhang, G.; Long, Y.; Ning, H.; Xu, Z.; Qiu, T.; Luo, D.; Li, M.; Yao, R.; Peng, J. Modifying precursor solutions to obtain Screen-Printable inks for tungsten oxides electrochromic film preparation. Coatings 2021, 11, 872. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, G.; Guo, K.; Guo, D.; Shi, M.; Ning, H.; Qiu, T.; Chen, J.; Fu, X.; Yao, R.; et al. Effect of the ammonium tungsten precursor solution with the modification of glycerol on wide band gap WO3 thin film and its electrochromic properties. Micromachines 2020, 11, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, M.; Qiu, T.; Tang, B.; Zhang, G.; Yao, R.; Xu, W.; Chen, J.; Fu, X.; Ning, H.; Peng, J. Temperature-Controlled crystal size of wide band gap nickel oxide and its application in electrochromism. Micromachines 2021, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lu, K.; Zhang, X.; Yuan, W.; Ning, H.; Tao, R.; Liu, X.; Yao, R.; Peng, J. Enhanced transmittance modulation of SiO2-Doped crystalline WO3 films prepared from a polyethylene oxide (PEO) template. Coatings 2018, 8, 228. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Lu, K.; Zhang, X.; Yuan, W.; Shi, M.; Ning, H.; Tao, R.; Liu, X.; Yao, R.; Peng, J. Effects of annealing temperature on optical band gap of sol-gel tungsten trioxide films. Micromachines 2018, 9, 377. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Zhang, J.; Qiu, T.; Ning, H.; Fang, Z.; Zhong, J.; Yang, Y.; Yao, R.; Luo, D.; Peng, J. Fabrication of flexible electrochromic film based on amorphous isopolytungstate by low-temperature inkjet-printed process with a solution crystallization kinetic-controlled strategy. Chem. Eng. J. 2022, 427, 131840. [Google Scholar] [CrossRef]
- Cai, G.; Eh, A.L.; Ji, L.; Lee, P.S. Recent advances in electrochromic smart fenestration. Adv. Sustain. Syst. 2017, 1, 1700074. [Google Scholar] [CrossRef]
- Zhang, G.; Guo, K.; Shen, X.; Ning, H.; Liang, H.; Zhong, J.; Xu, W.; Tang, B.; Yao, R.; Peng, J. Physical Simulation Model of WO3 Electrochromic Films Based on Continuous Electron-Transfer Kinetics and Experimental Verification. ACS Appl. Mater. Interfaces 2021, 13, 4768–4776. [Google Scholar] [CrossRef]
- Lee, E.; Scruton, C.; Peterson, A. Advancement of Electromic Windows; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2006. [Google Scholar]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Properties, requirements and possibilities of smart windows for dynamic daylight and solar energy control in buildings: A state-of-the-art review. Sol. Energy Mater. Sol. Cells 2010, 94, 87–105. [Google Scholar] [CrossRef] [Green Version]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Santato, C.; Odziemkowski, M.; Ulmann, M.; Augustynski, J. Crystallographically Oriented Mesoporous WO3 Films: Synthesis, Characterization, and Applications. J. Am. Chem. Soc. 2001, 123, 10639–10649. [Google Scholar] [CrossRef] [PubMed]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Zhao, D.; Margolese, D.I.; Chmelka, B.F.; Stucky, G.D. Block Copolymer Templating Syntheses of Mesoporous Metal Oxides with Large Ordering Lengths and Semicrystalline Framework. Chem. Mater. 1999, 11, 2813–2826. [Google Scholar] [CrossRef]
- Gangopadhyay, R.; De, A. Conducting Polymer Nanocomposites: A Brief Overview. Chem. Mater. 2000, 12, 608–622. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Song, J.; Pan, L.; Zhang, X.; Wang, L.; Zou, J.-J. Tungsten Oxides for Photocatalysis, Electrochemistry, and Phototherapy. Adv. Mater. 2015, 27, 5309–5327. [Google Scholar] [CrossRef]
- Meyer, J.; Hamwi, S.; Kröger, M.; Kowalsky, W.; Riedl, T.; Kahn, A. Transition Metal Oxides for Organic Electronics: Energetics, Device Physics and Applications. Adv. Mater. 2012, 24, 5408–5427. [Google Scholar] [CrossRef]
- Niklasson, G.A.; Granqvist, C.G. Electrochromics for smart windows: Thin films of tungsten oxide and nickel oxide, and devices based on these. J. Mater. Chem. 2007, 17, 127–156. [Google Scholar] [CrossRef] [Green Version]
- Gurlo, A. Nanosensors: Towards morphological control of gas sensing activity. SnO2, In2O3, ZnO and WO3 case studies. Nanoscale 2011, 3, 154–165. [Google Scholar] [CrossRef]
- Zheng, M.; Tang, H.; Hu, Q.; Zheng, S.; Li, L.; Xu, J.; Pang, H. Tungsten-Based materials for Lithium-Ion batteries. Adv. Funct. Mater. 2018, 28, 1707500. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Sun, S.; Tang, C.; Jiang, Y.; Wang, D.; Chang, X.; Lei, Y.; Wang, N.; Zhu, Y. Flexible and rechargeable electrochromic aluminium-ion battery based on tungsten oxide film electrode. Sol. Energy Mater. Sol. Cells 2020, 207, 110332. [Google Scholar] [CrossRef]
- Liu, L.; Diao, X.; He, Z.; Yi, Y.; Wang, T.; Wang, M.; Huang, J.; He, X.; Zhong, X.; Du, K. High-performance all-inorganic portable electrochromic Li-ion hybrid supercapacitors toward safe and smart energy storage. Energy Storage Mater. 2020, 33, 258–267. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Zhao, X.; Guo, Q.; Nie, G. Intelligent electrochromic-supercapacitor based on effective energy level matching poly (indole-6-carboxylicacid)/WO3 nanocomposites. New J. Chem. 2020, 44, 20584–20591. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, M.; Zhang, Y.; Cui, J.; Wang, Y.; Shu, X.; Qin, Y.; Tan, H.H.; Liu, J.; Wu, Y. Structure modulated amorphous/crystalline WO3 nanoporous arrays with superior electrochromic energy storage performance. Sol. Energy Mater. Sol. Cells 2020, 212, 110579. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, S.; Bard, A.J. Novel Carbon-Doped TiO2 nanotube arrays with high aspect ratios for efficient solar water splitting. Nano Lett. 2006, 6, 24–28. [Google Scholar] [CrossRef]
- Wetchakun, K.; Samerjai, T.; Tamaekong, N.; Liewhiran, C.; Siriwong, C.; Kruefu, V.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Semiconducting metal oxides as sensors for environmentally hazardous gases. Sens. Actuators B Chem. 2011, 160, 580–591. [Google Scholar] [CrossRef]
- Zheng, H.; Ou, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-Zadeh, K. Nanostructured Tungsten Oxide—Properties, Synthesis, and Applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Saha, A.R.; Nandi, R. Novel active R supercapacitor simulatio applications in low sensitivity multifunction network realisation. Electron. Lett. 1980, 16, 570–571. [Google Scholar] [CrossRef]
- Barghamadi, M.; Kapoor, A.; Wen, C. A review on Li-S batteries as a high efficiency rechargeable lithium battery. J. Electrochem. Soc. 2013, 160, A1256–A1263. [Google Scholar] [CrossRef]
- Frackowiak, E.; Béguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based supercapacitors produced by activation of graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ataalla, M.; Afify, A.S.; Hassan, M.; Abdallah, M.; Milanova, M.; Aboul-Enein, H.Y.; Mohamed, A. Tungsten-based glasses for photochromic, electrochromic, gas sensors, and related applications: A review. J. Non-Cryst. Solids 2018, 491, 43–54. [Google Scholar] [CrossRef]
- Dousti, M.R.; Poirier, G.Y.; de Camargo, A. Structural and spectroscopic characteristics of Eu3+-doped tungsten phosphate glasses. Opt. Mater. 2015, 45, 185–190. [Google Scholar] [CrossRef]
- Xu, M.; Wang, S.; Zhou, S.; Sun, W.; Ding, Z.; Zhang, X.; Zhao, J.; Li, Y. Construction of TiO2@C@Prussian Blue core-shell nanorod arrays for enhanced electrochromic switching speed and cycle stability. J. Alloys Compd. 2022, 908, 164410. [Google Scholar] [CrossRef]
- Tong, Z.; Tian, Y.; Zhang, H.; Li, X.; Ji, J.; Qu, H.; Li, N.; Zhao, J.; Li, Y. Recent advances in multifunctional electrochromic energy storage devices and photoelectrochromic devices. Sci. China Chem. 2017, 60, 13–37. [Google Scholar] [CrossRef]
- Balducci, A.; Dugas, R.; Taberna, P.L.; Simon, P.; Plée, D.; Mastragostino, M.; Passerini, S. High temperature carbon–carbon supercapacitor using ionic liquid as electrolyte. J. Power Sources 2007, 165, 922–927. [Google Scholar] [CrossRef] [Green Version]
- Largeot, C.; Portet, C.; Chmiola, J.; Taberna, P.; Gogotsi, Y.; Simon, P. Relation between the ion size and pore size for an electric Double-Layer capacitor. J. Am. Chem. Soc. 2008, 130, 2730–2731. [Google Scholar] [CrossRef]
- Wu, W.; Liao, W.; Chen, J.; Wu, J. An efficient route to nanostructured tungsten oxide films with improved electrochromic properties. ChemPhysChem 2010, 11, 3306–3312. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J. Erratum: Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11, 172. [Google Scholar] [CrossRef]
- Lin, Z.C.; Chunhua, C. Electrode Materials for Lithium Ion Battery. Prog. Chem. 2011, 23, 275–283. [Google Scholar]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Wang, Q.; Wei, T.; Fan, Z. Recent advances in design and fabrication of electrochemical supercapacitors with high energy densities. Adv. Energy Mater. 2014, 4, 1300816. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef]
- Burke, A. R&D considerations for the performance and application of electrochemical capacitors. Electrochim. Acta 2007, 53, 1083–1091. [Google Scholar]
- Qu, D. Studies of the activated carbons used in double-layer supercapacitors. J. Power Sources 2002, 109, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Lee, P.S.; Li, C. 3D carbon based nanostructures for advanced supercapacitors. Energy Environ. Sci. 2012, 6, 41–53. [Google Scholar] [CrossRef]
- Niu, J.; Conway, B.E.; Pell, W.G. Comparative studies of self-discharge by potential decay and float-current measurements at C double-layer capacitor and battery electrodes. J. Power Sources 2004, 135, 332–343. [Google Scholar] [CrossRef]
- Yun, T.G.; Kim, D.; Kim, Y.H.; Park, M.; Hyun, S.; Han, S.M. Photoresponsive smart coloration electrochromic supercapacitor. Adv. Mater. 2017, 29, 1606728. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhang, J.; Zhou, Y.; Xie, J.; Zhang, X.; Guan, M.; Pan, B.; Xie, Y. High-performance flexible electrochromic device based on facile semiconductor-to-metal transition realized by WO3·2H2O ultrathin nanosheets. Sci. Rep. 2013, 3, srep01936. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Sun, P.; Chai, Z.; Huang, L.; Cai, X.; Tan, S.; Song, J.; Mai, W. Large-Scale fabrication of pseudocapacitive glass windows that combine electrochromism and energy storage. Angew. Chem. Int. Ed. 2014, 53, 11935–11939. [Google Scholar] [CrossRef]
- Granqvist, C.G.; Arvizu, M.A.; Pehlivan, İ.B.; Qu, H.Y.; Wen, R.T.; Niklasson, G.A. Electrochromic materials and devices for energy efficiency and human comfort in buildings: A critical review. Electrochim. Acta 2018, 259, 1170–1182. [Google Scholar] [CrossRef] [Green Version]
- Bi, Z.; Li, X.; Chen, Y.; He, X.; Xu, X.; Gao, X. Large-Scale Multifunctional Electrochromic-Energy Storage Device Based on Tungsten Trioxide Monohydrate Nanosheets and Prussian White. ACS Appl. Mater. Interfaces 2017, 9, 29872–29880. [Google Scholar] [CrossRef]
- Yun, T.G.; Park, M.; Kim, D.; Kim, D.; Cheong, J.Y.; Bae, J.G.; Han, S.M.; Kim, I. All-Transparent stretchable electrochromic supercapacitor wearable patch device. ACS Nano 2019, 13, 3141–3150. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Hopmann, E.; Elezzabi, A.Y. Nanostructured inorganic electrochromic materials for light applications. Nanophotonics. 2020, 10, 825–850. [Google Scholar] [CrossRef]
- Zhou, B.; Feng, W.; Gao, G.; Wu, G.; Chen, Y.; Li, W. A low cost preparation of WO3 nanospheres film with improved thermal stability of gasochromic and its application in smart windows. Mater. Res. Express 2017, 4, 115702. [Google Scholar] [CrossRef]
- Ahmadraji, T.; Gonzalez-Macia, L.; Ritvonen, T.; Willert, A.; Ylimaula, S.; Donaghy, D.; Tuurala, S.; Suhonen, M.; Smart, D.; Morrin, A.; et al. Biomedical diagnostics enabled by integrated organic and printed electronics. Anal. Chem. 2017, 89, 7447–7454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, G.; Cui, M.; Kumar, V.; Darmawan, P.; Wang, J.; Wang, X.; Lee-Sie Eh, A.; Qian, K.; Lee, P.S. Ultra-large optical modulation of electrochromic porous WO3 film and the local monitoring of redox activity. Chem. Sci. 2016, 7, 1373–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Oh, H.; Bae, J.H.; Kim, H.; Moon, H.C.; Kim, S.H. Electrostatic-Force-Assisted dispensing printing of electrochromic gels for Low-Voltage displays. ACS Appl. Mater. Interfaces 2017, 9, 18994–19000. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Chan, C.; He, J. One-step inkjet printing of tungsten oxide-poly (3,4-ethylenedioxythiophene): Polystyrene sulphonate hybrid film and its applications in electrochromic devices. Thin Solid Film 2016, 603, 276–282. [Google Scholar] [CrossRef]
- Shen, J.; Xie, L.; Mao, J.; Zheng, L. A passive UHF-RFID tag with inkjet-printed electrochromic paper display. IEEE 2013, 2013, 118–123. [Google Scholar]
- Yu, J.; Holdcroft, S. Synthesis, Solid-Phase reaction, and patterning of Acid-Labile 3,4-Ethylenedioxythiophene-Based conjugated polymers. Chem. Mater. 2002, 14, 3705–3714. [Google Scholar] [CrossRef]
- Cong, S.; Tian, Y.; Li, Q.; Zhao, Z.; Geng, F. Single-Crystalline tungsten oxide quantum dots for fast pseudocapacitor and electrochromic applications. Adv. Mater. 2014, 26, 4260–4267. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Xu, Y.; Chen, T.; Liu, M.; Niu, F.; Wei, S.; Liu, J. Simultaneous synthesis of WO3−x quantum dots and Bundle-Like nanowires using a One-Pot Template-Free solvothermal strategy and their versatile applications. Small 2017, 13, 1603689. [Google Scholar]
- Inamdar, A.I.; Kim, J.; Jo, Y.; Woo, H.; Cho, S.; Pawar, S.M.; Lee, S.; Gunjakar, J.L.; Cho, Y.; Hou, B.; et al. Highly efficient electro-optically tunable smart-supercapacitors using an oxygen-excess nanograin tungsten oxide thin film. Sol. Energy Mater. Sol. Cells 2017, 166, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Kleperis, J.J.; Cikmach, P.D.; Lusis, A.R. Colour centres in amorphous tungsten trioxide thin films. Physica status solidi. Appl. Res. 1984, 83, 291–297. [Google Scholar]
- Gu, H.; Guo, C.; Zhang, S.; Bi, L.; Li, T.; Sun, T.; Liu, S. Highly efficient, Near-Infrared and visible light modulated electrochromic devices based on polyoxometalates and W18O49 nanowires. ACS Nano 2018, 12, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Moshofsky, B.; Mokari, T. Length and diameter control of ultrathin nanowires of substoichiometric tungsten oxide with insights into the growth mechanism. Chem. Mater. 2013, 25, 1384–1391. [Google Scholar] [CrossRef]
- Liao, C.; Chen, F.; Kai, J. WO3−x nanowires based electrochromic devices. Sol. Energy Mater. Sol. Cells 2006, 90, 1147–1155. [Google Scholar] [CrossRef]
- Navarro, J.R.G.; Mayence, A.; Andrade, J.; Lerouge, F.; Chaput, F.; Oleynikov, P.; Bergström, L.; Parola, S.; Pawlicka, A. WO3 nanorods created by Self-Assembly of highly crystalline nanowires under hydrothermal conditions. Langmuir 2014, 30, 10487–10492. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, S.; Zhang, T.; Yao, Q.; Fisher, A.; Lee, J.Y. Monoclinic oxygen-deficient tungsten oxide nanowires for dynamic and independent control of near-infrared and visible light transmittance. Mater. Horiz. 2018, 5, 291–297. [Google Scholar] [CrossRef]
- Shen, K.; Sheng, K.; Wang, Z.; Zheng, J.; Xu, C. Cobalt ions doped tungsten oxide nanowires achieved vertically aligned nanostructure with enhanced electrochromic properties. Appl. Surf. Sci. 2020, 501, 144003. [Google Scholar] [CrossRef]
- Bai, Z.; Wen, S.; Xv, H.; Cheng, X.; Li, M. Preparation and properties of all solid-state electrochromic devices based on tungsten oxide nanocomposites. Mater. Res. Express 2021, 8, 095703. [Google Scholar] [CrossRef]
- Wei, W.; Li, Z.; Guo, Z.; Li, Y.; Hou, F.; Guo, W.; Wei, A. An electrochromic window based on hierarchical amorphous WO3/SnO2 nanoflake arrays with boosted NIR modulation. Appl. Surf. Sci. 2022, 571, 151277. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; He, W.; Chen, H.; Tang, X.; Chen, Y.; Chen, Y. Enhanced electrochromic properties of nanostructured WO3 film by combination of chemical and physical methods. Coatings 2021, 11, 959. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Q.; Wang, H.; Li, Y. Red, Green, Blue (RGB) Electrochromic Fibers for the New Smart Color Change Fabrics. ACS Appl. Mater. Interfaces 2014, 6, 13043–13050. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.; Kim, J.W.; Sung, Y.; Kim, W.B. Electrochromic properties of tungsten oxide nanowires fabricated by electrospinning method. Sol. Energy Mater. Sol. Cells 2009, 93, 2062–2068. [Google Scholar] [CrossRef]
- Zhao, Z.; Miyauchi, M. Nanoporous-Walled tungsten oxide nanotubes as highly active Visible-Light-Driven photocatalysts. Angew. Chem. Int. Ed. 2008, 47, 7051–7055. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, K.; Alivisatos, A.P. Tunable localized surface plasmon resonances in tungsten oxide nanocrystals. J. Am. Chem. Soc. 2012, 134, 3995–3998. [Google Scholar] [CrossRef]
- Reddy, B.N.; Kumar, P.N.; Deepa, M. A poly (3,4-ethylenedioxypyrrole)-Au@3-based electrochromic pseudocapacitor. ChemPhysChem 2015, 16, 377–389. [Google Scholar] [CrossRef]
- Mohan, L.; Avani, A.V.; Kathirvel, P.; Marnadu, R.; Packiaraj, R.; Joshua, J.R.; Nallamuthu, N.; Shkir, M.; Saravanakumar, S. Investigation on structural, morphological and electrochemical properties of Mn doped WO3 nanoparticles synthesized by co-precipitation method for supercapacitor applications. J. Alloys Compd. 2021, 882, 160670. [Google Scholar] [CrossRef]
- Chang, C.; Chiang, Y.; Cheng, M.; Lin, S.; Jian, W.; Chen, J.; Cheng, Y.; Ma, Y.; Tsukagoshi, K. Fabrication of WO3 electrochromic devices using electro-exploding wire techniques and spray coating. Sol. Energy Mater. Sol. Cells 2021, 223, 110960. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Sheng, S.; He, Z.; Gao, J.; Yu, S. Manipulating Nanowire Assemblies toward Multicolor Transparent Electrochromic Device. Nano Lett. 2021, 21, 9203–9209. [Google Scholar] [CrossRef]
- Jo, M.; Kim, K.; Ahn, H. P-doped carbon quantum dot graft-functionalized amorphous WO3 for stable and flexible electrochromic energy-storage devices. Chem. Eng. J. 2022, 445, 136826. [Google Scholar] [CrossRef]
- Jain, R.K.; Khanna, A.; Gautam, Y.K.; Singh, B.P. Sputter deposited crystalline V2O5, WO3 and WO3/V2O5 multi-layers for optical and electrochemical applications. Appl. Surf. Sci. 2021, 536, 147804. [Google Scholar] [CrossRef]
- Liu, X.; Wang, G.; Wang, J.; Gong, X.; Chang, J.; Jin, X.; Zhang, X.; Wang, J.; Hao, J.; Liu, B. Electrochromic and capacitive properties of WO3 nanowires prepared by One-Step water bath method. Coatings 2022, 12, 595. [Google Scholar] [CrossRef]
- Li, W.; Zhang, J.; Zheng, Y.; Cui, Y. High performance electrochromic energy storage devices based on Mo-doped crystalline/amorphous WO3 core-shell structures. Sol. Energy Mater. Sol. Cells 2022, 235, 111488. [Google Scholar] [CrossRef]
- Prasad, A.K.; Park, J.; Kang, S.; Ahn, K. Electrochemically co-deposited WO3-V2O5 composites for electrochromic energy storage applications. Electrochim. Acta 2022, 422, 140340. [Google Scholar] [CrossRef]
- Li, K.; Shao, Y.; Liu, S.; Zhang, Q.; Wang, H.; Li, Y.; Kaner, R.B. Aluminum-Ion-Intercalation supercapacitors with ultrahigh areal capacitance and highly enhanced cycling stability: Power supply for flexible electrochromic devices. Small 2017, 13, 1700380. [Google Scholar] [CrossRef]
- Amatucci, G.G.; Badway, F.; Pasquier, A.D.U.; Zheng, T. An asymmetric hybrid nonaqueous energy storage cell. J. Electrochem. Soc. 2001, 148, A930–A939. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Y.; Li, H.; Liu, J.; Yu, S. Large area Co-Assembly of nanowires for flexible transparent smart windows. J. Am. Chem. Soc. 2017, 139, 9921–9926. [Google Scholar] [CrossRef]
- Azam, A.; Kim, J.; Park, J.; Novak, T.G.; Tiwari, A.P.; Song, S.H.; Kim, B.; Jeon, S. Two-Dimensional WO3 nanosheets chemically converted from layered WS2 for High-Performance electrochromic devices. Nano Lett. 2018, 18, 5646–5651. [Google Scholar] [CrossRef]
- Wang, S.; Xu, H.; Zhao, J.; Li, Y. Two-dimensional WO3 nanosheets for high-performance electrochromic supercapacitors. Inorg. Chem. Front. 2022, 9, 514–523. [Google Scholar] [CrossRef]
- Wang, S.; Xu, H.; Hao, T.; Xu, M.; Xue, J.; Zhao, J.; Li, Y. 3D conifer-like WO3 branched nanowire arrays electrode for boosting electrochromic-supercapacitor performance. Appl. Surf. Sci. 2022, 577, 151889. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Q.; Zhang, W.; Wang, Y.; Chen, W. Preparation of all-solid-state supercapacitor integrated with energy level indicating functionality. Synth. Met. 2016, 220, 494–501. [Google Scholar] [CrossRef]
- Bi, Z.; Li, X.; Chen, Y.; Xu, X.; Zhang, S.; Zhu, Q. Bi-functional flexible electrodes based on tungsten trioxide/zinc oxide nanocomposites for electrochromic and energy storage applications. Electrochim. Acta 2017, 227, 61–68. [Google Scholar] [CrossRef]
- Periasamy, P.; Krishnakumar, T.; Sathish, M.; Chavali, M.; Siril, P.F.; Devarajan, V.P. Structural and electrochemical studies of tungsten oxide (WO3) nanostructures prepared by microwave assisted wet-chemical technique for supercapacitor. J. Mater. Sci. Mater. Electron. 2018, 29, 6157–6166. [Google Scholar] [CrossRef]
- Gayathri, P.T.G.; Shaiju, S.S.; Remya, R.; Deb, B. Hydrated tungsten oxide nanosheet electrodes for broadband electrochromism and energy storage. Mater. Today Energy 2018, 10, 380–387. [Google Scholar]
- Guo, Q.; Zhao, X.; Li, Z.; Wang, B.; Wang, D.; Nie, G. High performance multicolor intelligent supercapacitor and its quantitative monitoring of energy storage level by electrochromic parameters. ACS Appl. Energy Mater. 2020, 3, 2727–2736. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Leftheriotis, G.; Huang, H.; Xia, Y.; Liang, C.; Gan, Y.; Zhang, W. Integrated photo-chargeable electrochromic energy-storage devices. Electrochim. Acta 2020, 345, 136235. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Z.; Guo, Q.; Yang, X.; Nie, G. High performance organic-inorganic hybrid material with multi-color change and high energy storage capacity for intelligent supercapacitor application. J. Alloys Compd. 2021, 855, 157480. [Google Scholar] [CrossRef]
- Zhu, G.; Sun, Y.; Li, M.; Tao, C.; Zhang, X.; Yang, H.; Guo, L.; Lin, B. Ionic crosslinked polymer as protective layer in electrochromic supercapacitors for improved electrochemical stability and ion transmission performance. Electrochim. Acta. 2021, 365, 137373. [Google Scholar] [CrossRef]
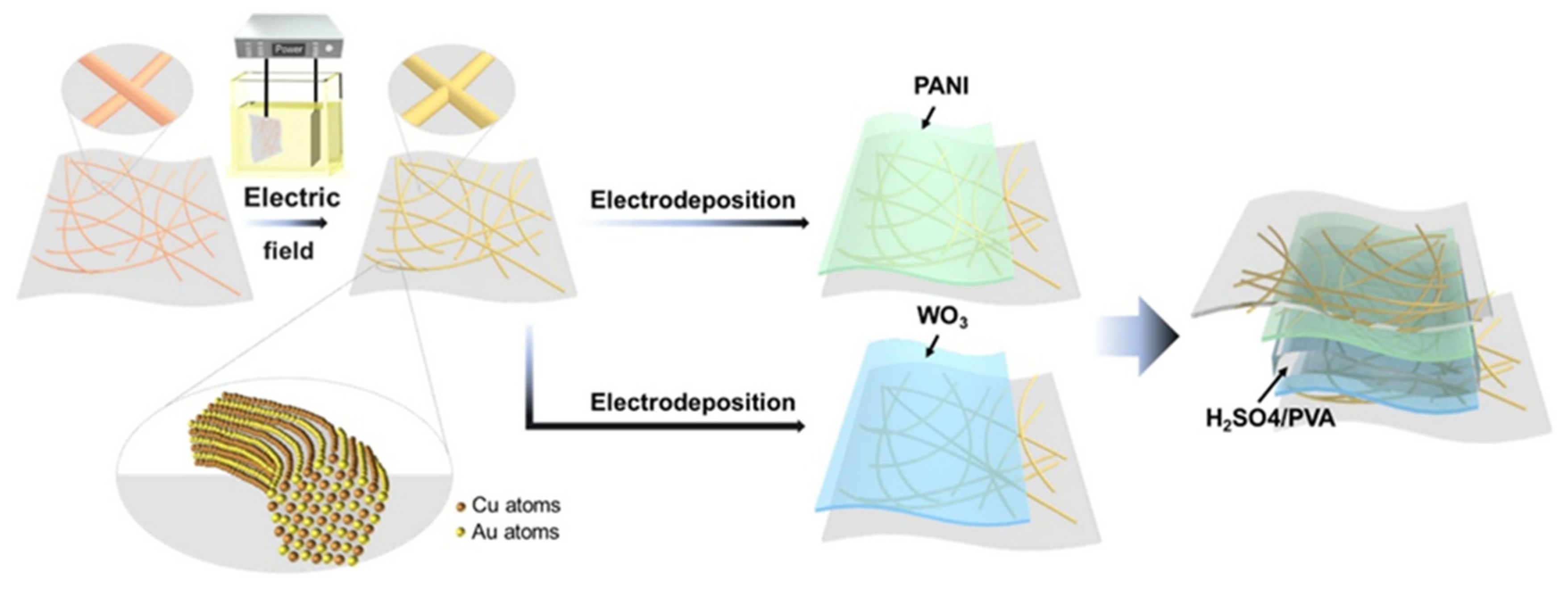
- Zhang, H.; Tian, Y.; Wang, S.; Feng, J.; Hang, C.; Wang, C.; Ma, J.; Hu, X.; Zheng, Z.; Dong, H. Robust Cu-Au alloy nanowires flexible transparent electrode for asymmetric electrochromic energy storage device. Chem. Eng. J. 2021, 426, 131438. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, Y.; Wang, S.; Huang, Y.; Wen, J.; Hang, C.; Zheng, Z.; Wang, C. Highly stable flexible transparent electrode via rapid electrodeposition coating of Ag-Au alloy on copper nanowires for bifunctional electrochromic and supercapacitor device. Chem. Eng. J. 2020, 399, 125075. [Google Scholar] [CrossRef]
- Yao, P.; Xie, S.; Ye, M.; Yu, R.; Liu, Q.; Yan, D.; Cai, W.; Guo, W.; Liu, X.Y. Smart electrochromic supercapacitors based on highly stable transparent conductive graphene/CuS network electrodes. RSC Adv. 2017, 7, 29088–29095. [Google Scholar] [CrossRef] [Green Version]
- Ginting, R.T.; Ovhal, M.M.; Kang, J. A novel design of hybrid transparent electrodes for high performance and ultra-flexible bifunctional electrochromic-supercapacitors. Nano Energy 2018, 53, 650–657. [Google Scholar] [CrossRef]
- Adib, M.R.; Kondalkar, V.V.; Lee, K. Development of highly sensitive ethane gas sensor based on 3D WO3 nanocone structure integrated with low-powered in-plane microheater and temperature sensor. Adv. Mater. Technol. 2020, 5, 2000009. [Google Scholar] [CrossRef]
- Chang, X.; Hu, R.; Sun, S.; Liu, J.; Lei, Y.; Liu, T.; Dong, L.; Yin, Y. Sunlight-charged electrochromic battery based on hybrid film of tungsten oxide and polyaniline. Appl. Surf. Sci. 2018, 441, 105–112. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, B.; Huang, H.; Gan, Y.; Xia, Y.; Liang, C.; Zhang, W.; Zhang, J. A Solar-Driven flexible electrochromic supercapacitor. Materials 2020, 13, 1206. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Cai, G.; Liu, X.; Tang, D. Pressure-Based biosensor integrated with a flexible pressure sensor and an electrochromic device for visual detection. Anal. Chem. 2021, 93, 2916–2925. [Google Scholar] [CrossRef]
- An, X.; Yu, J.C.; Wang, Y.; Hu, Y.; Yu, X.; Zhang, G. WO3 nanorods/graphene nanocomposites for high-efficiency visible-light-driven photocatalysis and NO2 gas sensing. J. Mater. Chem. 2012, 22, 8525. [Google Scholar] [CrossRef]
- Poongodi, S.; Kumar, P.S.; Mangalaraj, D.; Ponpandian, N.; Meena, P.; Masuda, Y.; Lee, C. Electrodeposition of WO3 nanostructured thin films for electrochromic and H2S gas sensor applications. J. Alloys Compd. 2017, 719, 71–81. [Google Scholar] [CrossRef]
- Lee, K.; Fang, Y.; Lee, W.; Ho, J.; Chen, K.; Liao, K. Novel electrochromic devices (ECD) of tungsten oxide (WO3) thin film integrated with amorphous silicon germanium photodetector for hydrogen sensor. Sens. Actuators B Chem. 2000, 69, 96–99. [Google Scholar] [CrossRef]
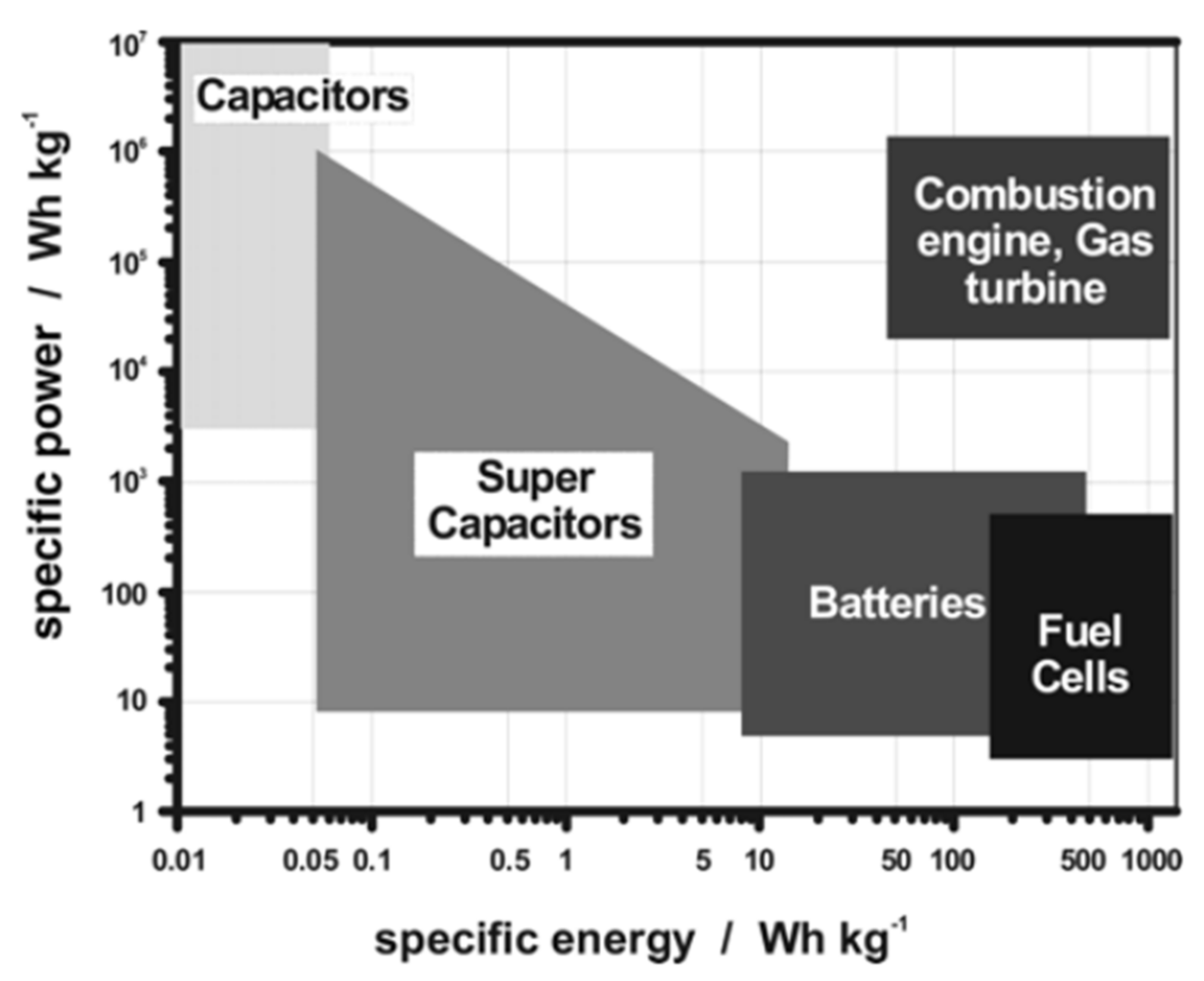

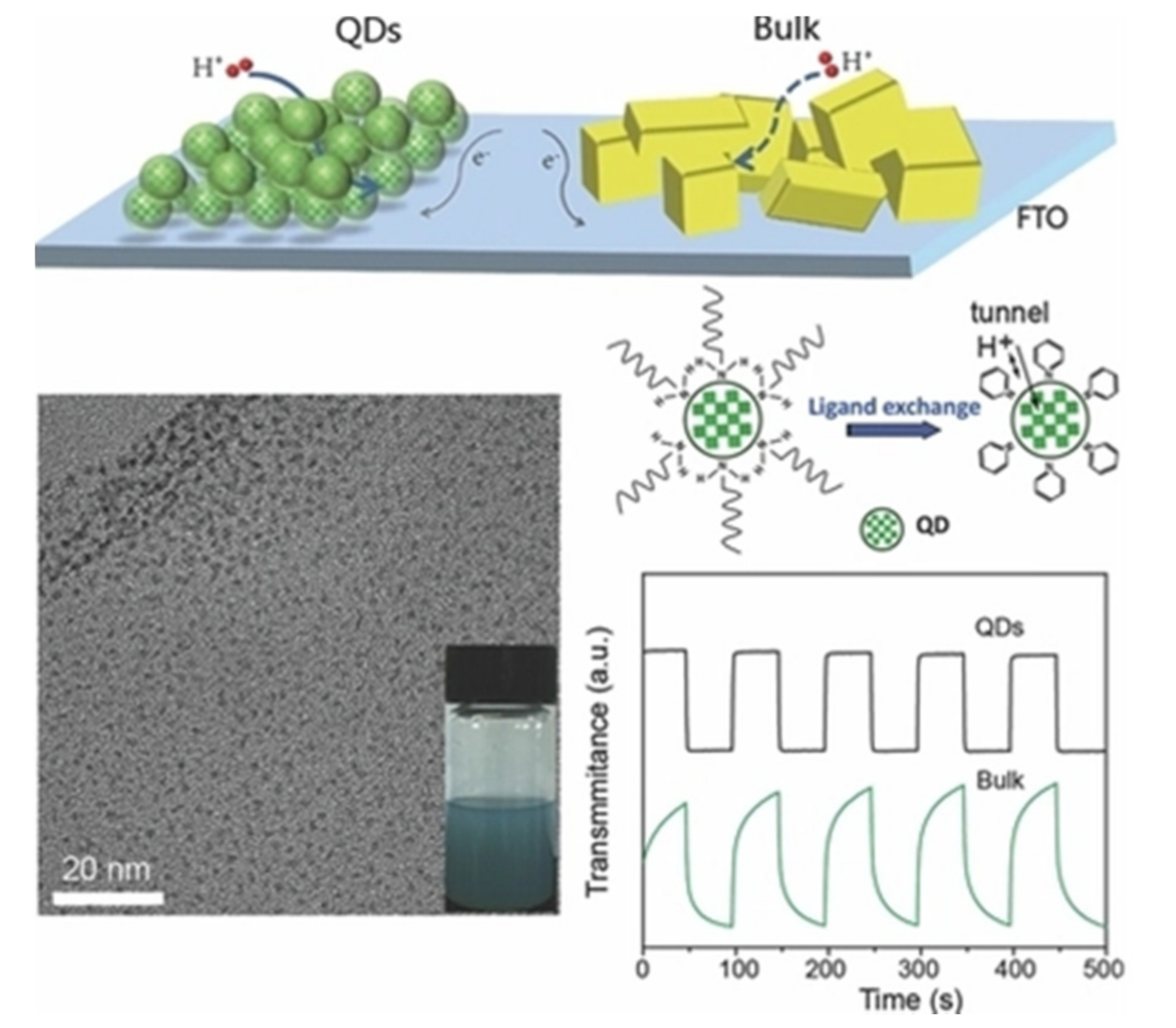
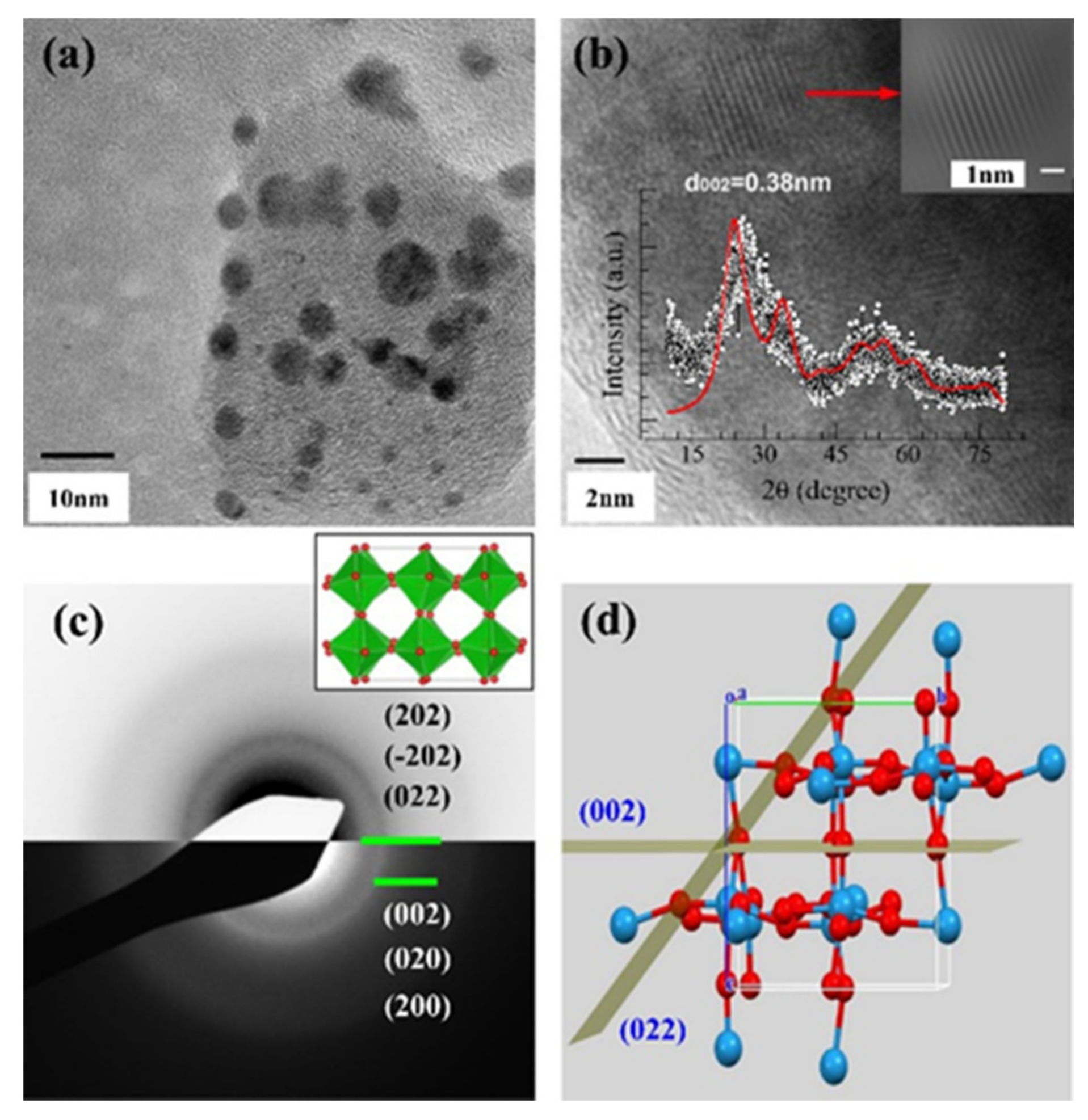

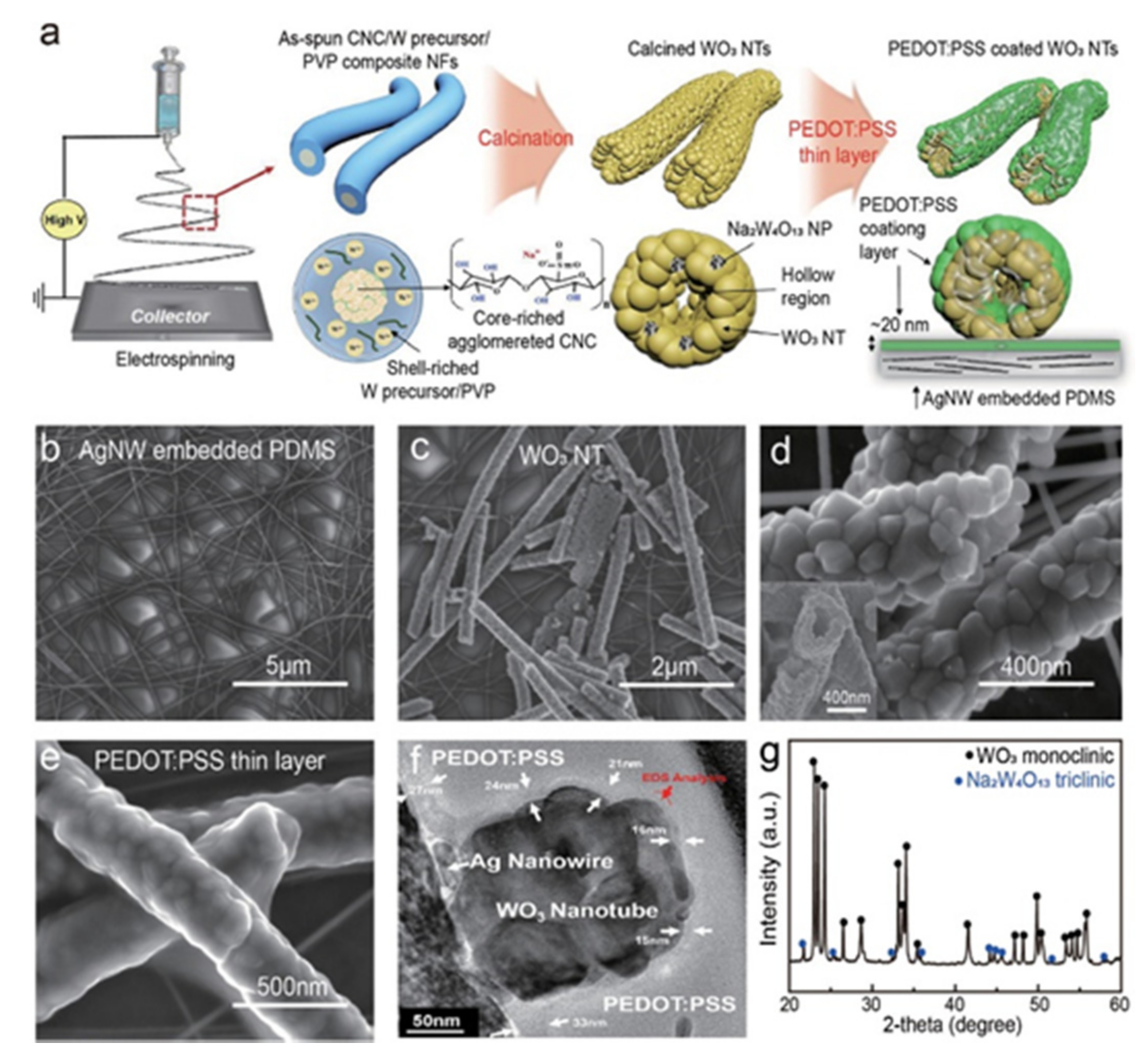

| Time | Film Fabrication Technology | Working Voltage | Specific Capacitance | Coloring Efficiency | Light Modulation Parameters | Base Plate | Article Content |
|---|---|---|---|---|---|---|---|
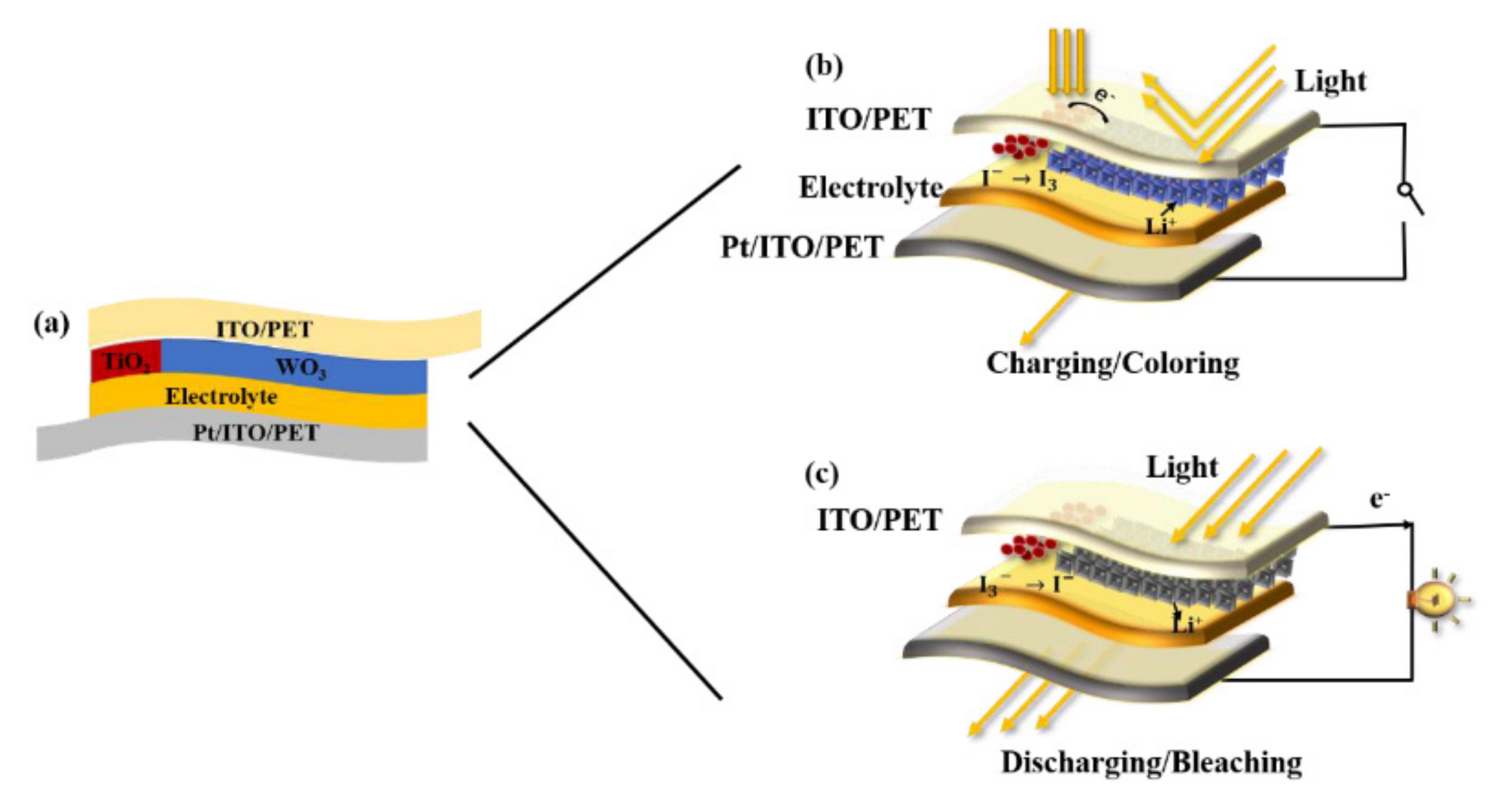
| 2016 [101] | Solvent-based thermal deposition | −1 V–1 V | 20% | Rigid | Using polyaniline (PANI) as the anode, WO3 as the cathode, and polyvinylpyrrolidone (PVP)-LiClO4 as the solid electrolyte, an all-solid-state supercapacitor with electrochromic function was successfully prepared, in which the color change represents the change in energy levels during charging and discharging. | ||
| 2017 [55] | Spraying | 2.5 V | 406 F/g | 64.8 cm2 C−1 | 40% | Flexible | Light-responsive electrochromic supercapacitor with cellulose nanofiber/silver nanowire/reduced graphene oxide/WO3 composite electrode, integrated with a solar sensor to achieve intelligent color change of the device. |
| 2017 [95] | Hydrothermal method | 1.8 V | 459 F/cm3 | 0.22 a.u | Flexible | W18O49 nanowire and carbon nanotube composite electrodes were prepared, using Al-based electrolytes, confirming the promise of Al-based applications in flexible electronic devices. | |
| 2017 [102] | Hydrothermal method; pulsed laser deposition | 15.24 mF/cm2 | 80.6 cm2/C | 68.2% | Flexible | A new flexible electrochromic supercapacitor designed by preparing tungsten trioxide/zinc oxide (WO3/ZnO) nanocomposites on a flexible substrate, which can visually monitor the energy storage level through fast and reversible color change. | |
| 2018 [103] | Wet chemical method | 264 F/g | Tungsten oxide (WO3) nanostructures were synthesized using a wet chemical method and treated using microwave irradiation. The effects of microwaves on the structure, morphology, and performance of WO3 supercapacitors are discussed. | ||||
| 2018 [104] | Solution method | ±2.5 V | 173 F/cm3 | 63% | Rigid | An electrochromic multifunctional smart glass was assembled by using WO3 crystal nanosheets (a single purely phase-active layer was used for visual and near-infrared (NIR) modulation). The device can be restored to degradation over a period of one year. | |
| 2019 [60] | Electrostatic spinning | 1.5 V | 471 F/g | 83.9 cm2/C | 40% | Flexible | Poly(dimethyl siloxane) (PDMS), double-stacked WO3 nanotube electrodes embedded with Au/Ag core–shell nanowires were prepared, and the devices could maintain high performance under stretching and extrusion, which is instructive for work on flexible and stretchable electrochromic supercapacitors. |
| 2020 [105] | Hydrothermal method | 1.0 V | 10.11 mF/cm2 | 608 cm2/C | 40% | Flexible | Construction of P51CA/WO3 composites with PEDOT to form an ECSC. The relationship between the electrochromic coefficient of the material and the electrochromic coefficient of the device was established. |
| 2020 [106] | Magnetron sputtering | ±1.5 V | 147 F/g | 40% to 50% | Rigid | Preparation of mesoporous WO3 films for combination with solar cells to produce solar-rechargeable electrochromic supercapacitors for self-powered devices | |
| 2021 [107] | Hydrothermal method | −0.5 V to 1.0 V | 89.2 mF/cm2 | 45% | Asymmetric electrostatic discharge (ESD) based on P5ICN/WO3 was successfully achieved by electrochemical polymerization of WO3-poly(5-cyanoindole) (P5ICN/WO3) hybrids formed by aggregation of nanoclusters, which exhibited good electrochromic and supercapacitive properties. | ||
| 2021 [108] | Hydrothermal method | −1.7–1.4 V | 21.8 mF/cm2 | 191 cm2/C | 45.8% | Flexible | Three protective layers with different negative ions (i.e., Br, Tf, TFSI) were synthesized as WO3 electrodes. The effects of different counterions and treatment conditions on the cyclic stability and electrochemical properties were investigated. The results show that the introduction of the crosslinked protective layers not only greatly improved the electrochemical stability of the composites, but also led to a significant improvement in the electrochemical properties of the composites, i.e., the phenomenon of activation. |
| 2022 [7] | Spray-film printing | 1.5 V–4.5 V | 102.3 F/cm3 | 93.6 cm2/C | 46.2% | Flexible | Improved flexible amorphous tungsten oxide films by controlling the alkalinity of the solution to inhibit spontaneous crystallization, nucleation, and crystal growth. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Yan, H.; Ning, H.; Li, X.; Zhong, J.; Fu, X.; Qiu, T.; Luo, D.; Yao, R.; Peng, J. Application of Tungsten-Oxide-Based Electrochromic Devices for Supercapacitors. Appl. Syst. Innov. 2022, 5, 60. https://0-doi-org.brum.beds.ac.uk/10.3390/asi5040060
Li M, Yan H, Ning H, Li X, Zhong J, Fu X, Qiu T, Luo D, Yao R, Peng J. Application of Tungsten-Oxide-Based Electrochromic Devices for Supercapacitors. Applied System Innovation. 2022; 5(4):60. https://0-doi-org.brum.beds.ac.uk/10.3390/asi5040060
Chicago/Turabian StyleLi, Muyun, Haoyang Yan, Honglong Ning, Xinglin Li, Jinyao Zhong, Xiao Fu, Tian Qiu, Dongxiang Luo, Rihui Yao, and Junbiao Peng. 2022. "Application of Tungsten-Oxide-Based Electrochromic Devices for Supercapacitors" Applied System Innovation 5, no. 4: 60. https://0-doi-org.brum.beds.ac.uk/10.3390/asi5040060







