Ammonium Hydroxide Mediated Hydrothermal Crystallization of Hydroxyapatite Coatings on Titanium Substrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Titanium Substrate Preparation
2.2. Chemical Synthesis
2.3. Characterization
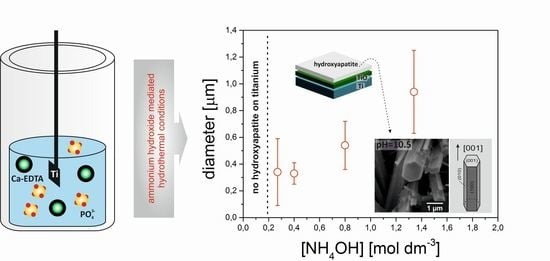
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate deposits: Preparation, properties and biomedical applications. Mater. Sci. Eng. C 2015, 55, 272–326. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, B.; Wagner, H.D. Structural motifs and elastic properties of hierarchical biological tissues—A review. J. Struct. Biol. 2013, 183, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Geng, X.; Liu, G.; Xiao, J.; Li, D.; Zhang, Y.; Zhu, P.; Zhang, C. Deposition, nanostructure and phase composition of suspension plasma-sprayed hydroxyapatite coatings. Ceram. Int. 2016, 42, 8684–8690. [Google Scholar] [CrossRef]
- Fomin, A.; Fomina, M.; Koshuro, V.; Rodionov, I.; Zakharevich, A.; Skaptsov, A. Structure and mechanical properties of hydroxyapatite coatings produced on titanium using plasma spraying with induction preheating. Ceram. Int. 2017, 43, 11189–11196. [Google Scholar] [CrossRef]
- Mayr, H.; Ordung, M.; Ziegler, G. Development of thin electrophoretically deposited hydroxyapatite layers on TiAl6V4 hip prosthesis. J. Mater. Sci. 2006, 41, 8138–8143. [Google Scholar] [CrossRef]
- Nathanael, A.J.; Sabari Arul, N.; Ponpandian, N.; Mangalaraj, D.; Chen, P.C. Nanostructured leaf like hydroxyapatite/TiO2 composite coatings by simple sol-gel method. Thin Solid Films 2010, 518, 7333–7338. [Google Scholar] [CrossRef]
- Ivanova, A.A.; Surmeneva, M.A.; Surmenev, R.A.; Depla, D. Influence of deposition conditions on the composition, texture and microstructure of RF-magnetron sputter-deposited hydroxyapatite thin films. Thin Solid Films 2015, 591, 368–374. [Google Scholar] [CrossRef]
- Suchanek, K.; Bartkowiak, A.; Gdowik, A.; Perzanowski, M.; Kac, S.; Szaraniec, B.; Suchanek, M.; Marszałek, M. Crystalline hydroxyapatite coatings synthesized under hydrothermal conditions on modified titanium substrates. Mater. Sci. Eng. C 2015, 51, 57e63. [Google Scholar] [CrossRef]
- Roeder, R.K.; Converse, G.L.; Leng, H.; Yue, W. Kinetic effects on hydroxyapatite whiskers synthesized by the chelate decomposition method. J. Am. Ceram. Soc. 2006, 89, 2096–2104. [Google Scholar] [CrossRef]
- Zhu, R.; Yu, R.; Yao, J.; Wang, D.; Ke, J. Morphology control of hydroxyapatite through hydrothermal process. J. Alloys Compd. 2008, 457, 555–559. [Google Scholar] [CrossRef]
- Lak, A.; Mazloumi, M.; Mohajerani, M.; Kajbafvala, A.; Zanganeh, S.; Arami, H.; Sadrnezhaad, S.K. Self-assembly of dandelion-like hydroxyapatite nanostructures via hydrothermal method. J. Am. Ceram. Soc. 2008, 91, 3292–3297. [Google Scholar] [CrossRef]
- Xie, R.; Feng, Z.; Li, S.; Xu, B. EDTA-Assisted self-assembly of fluoride-substituted hydroxyapatite coating on enamel substrate. Cryst. Growth Des. 2011, 11, 5206–5214. [Google Scholar] [CrossRef]
- Neira, I.S.; Guitián, F.; Taniguchi, T.; Watanabe, T.; Yoshimura, M. Hydrothermal synthesis of hydroxyapatite whiskers with sharp faceted hexagonal morphology. J. Mater. Sci. 2008, 43, 2171–2178. [Google Scholar] [CrossRef]
- Neira, I.S.; Kole’ko, Y.; Lebedev, O.I.; Van Tendeloo, G.; Gupta, H.S.; Guitian, F.; Yoshimura, M. Effective morphology control of hydroxyapatite crystals via hydrothermal synthesis. Cryst. Growth Des. 2009, 9, 466–474. [Google Scholar] [CrossRef]
- Wu, S.C.; Tsou, H.K.; Hsu, H.C.; Hsu, S.K.; Liou, S.P.; Ho, W.F. A hydrothermal synthesis of eggshell and fruit waste extract to produce nanosized hydroxyapatite. Ceram. Int. 2013, 39, 8183–8188. [Google Scholar] [CrossRef]
- Kimn, I.Y.; Ohtsuki, C. Hydroxyapatite formation from calcium carbonate single crystal under hydrothermal condition: Effects of processing temperature. Ceram. Int. 2016, 42, 1886–1890. [Google Scholar] [CrossRef]
- Yan, L.; Li, Y.; Deng, Z.X.; Zhuang, J.; Sun, X. Surfactant-assisted hydrothermal synthesis of hydroxyapatite nanorods. Int. J. Inorg. Mater. 2001, 3, 633–637. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, D.; Li, D.; Zhu, J.; Li, G.; Xie, J. Controllable synthesis of fluorapatite nanocrystals with various morphologies: Effects of pH value and chelating reagent. J. Alloys Compd. 2009, 485, 396–401. [Google Scholar] [CrossRef]
- Arce, H.; Montero, M.L.; Saenz, A.; Castano, V.M. Effect of pH and temperature on the formation of hydroxyapatite at low temperatures by decomposition of a Ca–EDTA complex. Polyhedron 2004, 23, 1897–1901. [Google Scholar] [CrossRef]
- Taheri, M.M.; Abdul Kadir, M.R.; Shokuhfar, T.; Hamlekhan, A.; Assadian, M.; Shirdar, M.R.; Mirjalili, A. Surfactant-assisted hydrothermal synthesis of Fluoridated Hydroxyapatite nanorods. Ceram. Int. 2015, 41, 9867–9872. [Google Scholar] [CrossRef]
- Tombacz, E. pH-dependent surface charging of metal oxides. Period. Polytech. Chem. Eng. 2009, 53, 77. [Google Scholar] [CrossRef]
- Fujishiro, Y.; Sato, T.; Okuwaki, A. Coating of hydroxyapatite on metal plates using thermal dissociation of calcium-EDTA chelate in phosphate solutions under hydrothermal conditions. J. Mater. Sci. Mater. Med. 1995, 6, 172–176. [Google Scholar] [CrossRef]
- Fujishiro, Y.; Fujimoto, A.; Sato, T.; Okuwaki, A. Coating of hydroxyapatite on titanium plates using thermal dissociation of Calcium-EDTA Chelate Complex in phosphate solutions under hydrothermal conditions. J. Colloid Interface Sci. 1995, 173, 119–127. [Google Scholar] [CrossRef]
- Lu, Z.Z.; Xu, H.Y.; Xin, M.D.; Li, K.W.; Wang, H. Induced growth of (0001)-oriented hydroxyapatite nanorod arrays on ZnO-seeded glass substrate. J. Phys. Chem. C 2010, 114, 820–825. [Google Scholar] [CrossRef]
- Chen, W.; Long, T.; Guo, Y.-J.; Zhu, Z.-A.; Guo, Y.-P. Hydrothermal synthesis of hydroxyapatite coatings with oriented nanorod arrays. RSC Adv. 2014, 4, 185. [Google Scholar] [CrossRef]
- Shen, J.; Qi, Y.; Jin, B.; Wang, X.; Hu, Y.; Jiang, Q. Control of hydroxyapatite coating by self-assembled monolayers on titanium and improvement of osteoblast adhesion. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 124–135. [Google Scholar] [CrossRef]
- Li, H.; Mei, L.; Liu, H.; Liu, Y.; Liao, L.; Kumar, R.V. Growth mechanism of surfactant-free size-controlled luminescent hydroxyapatite nanocrystallites. Cryst. Growth Des. 2017, 17, 2809–2815. [Google Scholar] [CrossRef]
- Kokubo, T.; Pattanayak, D.K.; Yamaguchi, S.; Takadama, H.; Matsushita, T.; Kawai, T.; Takemoto, M.; Fujibayashi, S.; Nakamura, T. Positively charged bioactive Ti metal prepared by simple chemical and heat treatment. J. R. Soc. Interface 2010, 7, 503–513. [Google Scholar] [CrossRef]
- Pattanayak, D.K.; Yamaguchi, S.; Matsushita, T.; Nakamura, T.; Kokubo, T. Apatite forming ability of titanium in terms of pH of the exposed solution. J. R. Soc. Interface 2012, 9, 2145–2155. [Google Scholar] [CrossRef]
- Kosmulski, M. Compilation of PZC and IEP of sparingly soluble metal oxides and hydroxides from literature. Adv. Colloid. Interface Sci. 2009, 152, 14–25. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Hardcastle, F.D.; Ishihara, H.; Sharma, R.; Biris, A.S. Photoelectroactivity and Raman spectroscopy of anodized titania (TiO2) photoactive water-splitting catalysts as a function of oxygen-annealing temperature. J. Mater. Chem. 2011, 21, 6337–6345. [Google Scholar] [CrossRef]
- Tsuda, H.; Arends, J. Orientational micro-Raman spectroscopy on hydroxyapatite single crystals and human enamel crystallites. J. Dent. Res. 1994, 73, 1703–1710. [Google Scholar] [CrossRef]
- Heimann, R.B.; Ntsoane, T.P.; Pineda-Vargas, C.A.; Przybylowicz, W.J.; Topić, M. Biomimetic formation of hydroxyapatite investigated by analytical techniques with high resolution. J. Mater. Sci. Mater. Med. 2008, 19, 3295–3302. [Google Scholar] [CrossRef]




| Sample | NH4OH Concentration [mol·dm−3] | pH | HAp Layer Thickness Mean Value (SD) * (n = 6) [µm] | Ca/P Molar Ratio |
|---|---|---|---|---|
| CaPx = 0.2 | 0.2 | 6.0 | — | — |
| CaPx = 0.27 | 0.27 | 7.2 | 16.5(2.4) | 1.51(0.10) |
| CaPx = 0.4 | 0.4 | 9.0 | 16.9(3.1) | 1.57(0.10) |
| CaPx = 0.8 | 0.8 | 10.0 | 29.5(1.3) | 1.69(0.11) |
| CaPx=1.34 | 1.34 | 10.5 | 48.2(5.2) | 1.74(0.11) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suchanek, K.; Perzanowski, M.; Lekki, J.; Strąg, M.; Marszałek, M. Ammonium Hydroxide Mediated Hydrothermal Crystallization of Hydroxyapatite Coatings on Titanium Substrate. Ceramics 2019, 2, 180-189. https://0-doi-org.brum.beds.ac.uk/10.3390/ceramics2010016
Suchanek K, Perzanowski M, Lekki J, Strąg M, Marszałek M. Ammonium Hydroxide Mediated Hydrothermal Crystallization of Hydroxyapatite Coatings on Titanium Substrate. Ceramics. 2019; 2(1):180-189. https://0-doi-org.brum.beds.ac.uk/10.3390/ceramics2010016
Chicago/Turabian StyleSuchanek, Katarzyna, Marcin Perzanowski, Janusz Lekki, Martyna Strąg, and Marta Marszałek. 2019. "Ammonium Hydroxide Mediated Hydrothermal Crystallization of Hydroxyapatite Coatings on Titanium Substrate" Ceramics 2, no. 1: 180-189. https://0-doi-org.brum.beds.ac.uk/10.3390/ceramics2010016