Chemical Preparation Routes and Lowering the Sintering Temperature of Ceramics
Abstract
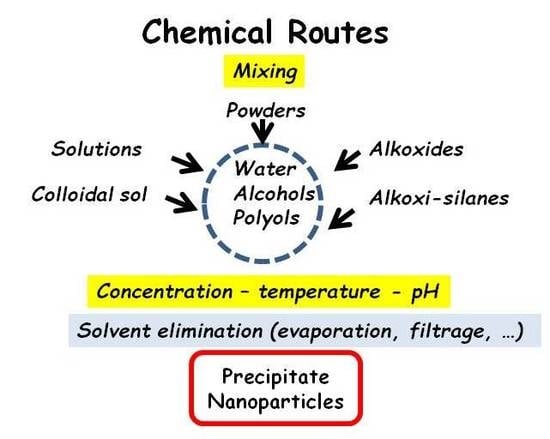
:1. Introduction
2. Production of Fine Powders
2.1. Physical Techniques
2.2. Chemical Routes
2.2.1. Oxides
- (i)
- The elements which establish covalent bonds, called gel or glass former (Si, P, Ge, Al, etc.) [1], form ‘molecular’ moieties—in other words, ‘polymerize’.
- (ii)
- The elements which remain as ‘isolated’ ions; they can remain dissolved in the ‘solvent’ and/or adsorbed on the surface, external surface of the artefact and internal surface of the pores of the ‘precipitate’; for instance, Na+, K+, Li+, Ca2+, etc.
2.2.2. Carbides, Nitrides, etc.
3. Optimization of the Grain–Grain Contact
3.1. Compaction and (Viscous) Liquid Routes
3.2. Additives
3.3. Anisotropy Control
4. Grain–Grain Reaction and Densification
4.1. Liquid-Phase Sintering: Dissolution and Precipitation
4.2. Deprotonation and Phase Transition
4.3. Diffusion
5. Control/Optimization of Structure and Properties
5.1. Multicomponent Non-Stoichiometric Compounds
5.2. Composites (Particulate, Fibers, Metal/Oxides)
5.3. Tools/Requirements for Minimizing the Temperature of Full Densification
5.4. Complex Electrochemical Devices
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Colomban, P. Gel Technology in Ceramics, Glass-Ceramics and Ceramic-Ceramic Composites. Ceram. Int. 1989, 15, 23–50. [Google Scholar] [CrossRef]
- Colomban, P. Nano/micro-structure and Property Control of Single and Multiphase Materials. In Chemical Processing of Ceramics, 2nd ed.; Komarneni, S., Lee, B., Eds.; CRC Press: Boca Raton, FL, USA, 2005; Chapter 12; pp. 303–339. [Google Scholar]
- Colomban, P. Natural nanosized raw materials and Sol-Gel technology: The base of pottery since millennia. In Nanoscience and Cultural Heritage; Dillmann, P., Bellot-Gurlet, L., Nenner, I., Eds.; Springer-Atlantis: Amsterdam, The Netherlands, 2016; pp. 59–73. [Google Scholar]
- Kingery, W.D.; Bowen, H.K.; Uhlmann, D.R. Introduction to Ceramics, 2nd ed.; J. Wiley & Sons: New-York, NY, USA, 1976. [Google Scholar]
- Gerard-Hirne, J.; Rigaud, J.; Cizeron, G.; Peyssou, J.; Godron, Y.; Jouenne, C.A.; Gion, L.; Dumoulin, G.; Pastor, H.; Vassiliev, A.; et al. Le Frittage. Extraits de L’Industrie Céramique, Special Issue; Institut de Céramique Française: Sèvres, France, 1975. [Google Scholar]
- Cooper, E. Ten Thousand Years of Pottery, 4th ed.; University of Pennsylvania Press: Philadelphia, PA, USA, 2000. [Google Scholar]
- Richerson, D.W. The Magic of Ceramics; The American Ceramic Society: Westerville, OH, USA, 2000. [Google Scholar]
- Levin, E.M.; Robin, C.R.; McMurdie, H.F. Phase Diagrams for Ceramists; The American Ceramic Society: Columbus, OH, USA, 1964. [Google Scholar]
- Segal, D. Chemical Synthesis of Advanced Ceramic Materials; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Komarneni, S.; Lee, B. (Eds.) Chemical Processing of Ceramics, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Klein, L.C. Sol-Gel Technology; Noyes Publications: Park Ridge, IL, USA, 1988. [Google Scholar]
- Pierre, A.C. Introduction to Sol-Gel Processing; Kluwer: Boston, MA, USA, 1998. [Google Scholar]
- Pierre, A.C. Introduction to Sol-Gel Processing; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Bouquin, O.; Perthuis, H.; Colomban, P. Low Temperature Sintering and Optimal Physical Properties: A Challenge the NASICON Ceramics Case. J. Mater. Sci. Lett. 1985, 4, 956–959. [Google Scholar] [CrossRef]
- Jouenne, C.-A. Traité de Céramiques et Matériaux Minéraux; Edition Septima: Paris, France, 1980. [Google Scholar]
- Reed, J.S. Principles of Ceramics Processing; Wiley Interscience: New York, NY, USA, 1995. [Google Scholar]
- Perthuis, H.; Velasco, G.; Colomban, P. Na+ and Li+ NASICON Superionic Conductors Thick Films. Jpn. J. Appl. Phys. 1984, 23, 534–543. [Google Scholar] [CrossRef]
- Burke, J.E. Uranium Dioxide Nuclear Fuel. In High-Technology Ceramics. Past, Present, and Future. The Nature of Innovation and Change in Ceramic Technology; Kingery, W.D., Ed.; Ceramics and Civilization Series; The American Ceramic Society: Westerville, OH, USA, 1986; Volume 3, pp. 239–258. [Google Scholar]
- Baden Fuller, A.J. Ferrites at Microwave Frequencies; IEE Electromagnetic Waves Series 23; Peter Peregrinus Ltd. on Behalf of the IEE: London, UK, 1987. [Google Scholar]
- Pujes, J.-P. A Century of Electronics. History of the Thales Group; Thales: Paris, France, 2005. [Google Scholar]
- Weber, J.-M. Un Demi-Siècle D’aéronautique en France. Etudes et Recherches; Tome 1, Les Cahier du CHEAr, CHEAr: Paris, France, 2008. [Google Scholar]
- Ault, N.N. Silicon-Carbide-Its Progression as a Refractory Material. In High-Technology Ceramics. Past, Present, and Future. The Nature of Innovation and Change in Ceramic Technology; Kingery, W.D., Ed.; Ceramics and civilization Series; The American Ceramic Society: Westerville, OH, USA, 1986; Volume 3, pp. 219–230. [Google Scholar]
- Jack, K.H. Silicon Nitride, Sialons, and Related Ceramics. In High-Technology Ceramics. Past, Present, and Future. The Nature of Innovation and Change in Ceramic Technology; Kingery, W.D., Ed.; Ceramics and Civilization Series; The American Ceramic Society: Westerville, OH, USA, 1986; Volume 3, pp. 259–288. [Google Scholar]
- Smith, P.L.; White, J. Basic Refractories and the Emergence of the Steel Industry. In High-Technology Ceramics. Past, Present, and Future. The Nature of Innovation and Change in Ceramic Technology; Kingery, W.D., Ed.; Ceramics and Civilization Series; The American Ceramic Society: Westerville, OH, USA, 1986; Volume 3, pp. 181–209. [Google Scholar]
- Berg, M. Aluminum Oxide Spark Plug Insulators. In High-Technology Ceramics. Past, Present, and Future. The Nature of Innovation and Change in Ceramic Technology; Kingery, W.D., Ed.; Ceramics and Civilization Series; The American Ceramic Society: Westerville, OH, USA, 1986; Volume 3, pp. 211–217. [Google Scholar]
- Cross, L.E.; Newnham, R.E. History of Ferroelectrics. In High-Technology Ceramics. Past, Present, and Future. The Nature of Innovation and Change in Ceramic Technology; Kingery, W.D., Ed.; Ceramics and Civilization Series; The American Ceramic Society: Westerville, OH, USA, 1986; Volume 3, pp. 289–305. [Google Scholar]
- Haertling, G.H. Ferroelectric Ceramics: History and technology. J. Am. Ceram. Soc. 1999, 82, 797–818. [Google Scholar] [CrossRef]
- Stetson, H.W. Multilayer Ceramic Technology. In High-Technology Ceramics. Past, Present, and Future. The Nature of Innovation and Change in Ceramic Technology; Kingery, W.D., Ed.; Ceramics and Civilization Series; The American Ceramic Society: Westerville, OH, USA, 1986; Volume 3, pp. 307–322. [Google Scholar]
- Hench, L.L.; Ulrich, D.R. (Eds.) Ultrastructure Processing of Ceramics, Glasses and Composites; Wiley: New York, NY, USA, 1984. [Google Scholar]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Hench, L.L. (Ed.) An Introduction to Bioceramics, 2nd ed.; Imperial College Press: London, UK, 2013. [Google Scholar]
- Bill, J.; Aldinger, F. Precursor-derived covalent ceramics. Adv. Mater. 1995, 7, 775–787. [Google Scholar] [CrossRef]
- Riedel, R.; Mera, G.; Hauser, R.; Klonczynski, A. Silicon-based polymer-derived ceramics: Synthesis properties and applications—A review. J. Ceram Soc. Jpn. 2006, 114, 425–444. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.R. Review of Bioactive glass. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef]
- Miguez-Pacheco, V.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef]
- Cox, S.C.; Thornby, J.A.; Gibbons, G.J.; Williams, M.A.; Mallick, K.K. 3d printing of porous hydroxyapatite scaffolds intended for use in bone tissue engineering applications. Mater. Sci. Eng. C 2015, 47, 237–247. [Google Scholar] [CrossRef]
- Chai, W.; Wei, Q.; Yang, M.; Ji, K.; Guo, Y.; Wei, S.; Wang, Y. The printability of three water based polymeric binders and their effects on the properties of 3D printed hydroxyapatite bone scaffold. Ceram. Int. 2020, 46, 6663–6671. [Google Scholar] [CrossRef]
- Geus, J.W. Fast ion transport in solids: Solid state batteries and devices. In Proceedings of the NATO Sponsored Advanced Study Institute on Fast Ion Transport in Solids, Belgirate, Italy, 5–15 September 1972; North Holland-American Elsevier: Amsterdam, The Netherlands, 1973. [Google Scholar]
- Marrony, M. Proton Conducting Ceramics, From Fundamental to Applied Research; Pan Stanford Publishing: Singapore, 2016. [Google Scholar]
- Colomban, P. Proton conductors and their applications: A tentative historical overview of the early researches. Solid State Ionics 2019, 334, 125–144. [Google Scholar] [CrossRef]
- Rabe, T.; Linke, D. Attrition milling of Silicon-Nitride powder under conditions for minimal impurity pickup. Ceram. Int. 1992, 18, 161–166. [Google Scholar] [CrossRef]
- Houivet, D.; El Fallah, J.; Haussonne, J.-M. Dispersion and grinding of oxide powders into an aqueous slurry. J. Am. Ceram. Soc. 2002, 85, 321–328. [Google Scholar] [CrossRef]
- Balakrishna, P.; Murty, B.N.; Ratnam, D.V.; Anuradha, M.; Ganguly, C. Light attrition of uranium dioxide powder. Ceram. Int. 2003, 29, 99–105. [Google Scholar] [CrossRef]
- Mestl, G.; Srinivasan, T.K.K.; Knozinger, H. Mechanically Activated MoO3. 3. Characterization by vibrational Spectroscopy. Langmuir 1995, 11, 3795–3804. [Google Scholar] [CrossRef]
- Michel, D.; Mazerolles, L.; Begin Colin, S. Mechanical alloying of oxides. Ann. Chim. Sci. Mater. 1997, 22, 403–416. [Google Scholar]
- Sengupta, L.C.; Synowcyznski, J.; Chiu, L.H. Investigation of the effect of particle size on the optical and electronic properties of Ba1-xSrxTiO3. Integr. Ferroelectr. 1997, 17, 287–296. [Google Scholar] [CrossRef]
- Kaczmarek, W.A. Phase stability in non-stoichiometric iron-cobalt oxides prepared by mechanochemical activation. In Synthesis and properties of Mechanically Alloyed and Nanocrystalline Materials; Fiorani, D., Magini, M., Eds.; PTS 1 and 2-ISMANAM-96; Mater. Sci. Forum: Bäch, Switzerland, 1997; Volume 235–238, Part 1 and 2; pp. 109–114. [Google Scholar]
- Rickerby, D.G.; Jiang, J.Z.; Lin, R.; Morup, S. Transmission electron microscopy studies of mechanical alloying in the immiscible Fe2O3-SnO2 system. In Mechanically Alloyed, Metastable and Nanocrystalline Materials; Baro, M.D., Surinach, S., Eds.; Mater. Sci. Forum: Bäch, Switzerland, 1998; Volume 269–272, Part 1; pp. 351–356. [Google Scholar]
- Nivoix, V.; Gaffet, E.; Bernard, F.; Gillot, B. Mechanical activation of iron and vanadium oxides in order to obtain nanometric vanadium ferrite similar to a soft chemistry compound. C. R. Acadm. Sci. Ser. II C Chim. 1998, 1, 183–189. [Google Scholar]
- Kong, L.B.; Zhang, T.S.; Ma, J.; Boey, F. Progress in synthesis of ferroelectric ceramic materials via high-energy mechanochemical technique. Progr. Mater. Sci. 2008, 53, 207–322. [Google Scholar] [CrossRef]
- Janot, R.; Gherard, D. Ball-milling in liquid media—Applications to the preparation of anodic materials for lithium-ion batteries. Progr. Mater. Sci. 2005, 50, 1–92. [Google Scholar] [CrossRef]
- Kong, L.B.; Ma, J.; Huang, H.; Zhang, R.F.; Que, W.X. Barium titanate derived from mechanochemically activated powders. J. Alloys Compd. 2002, 337, 226–230. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Progr. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Chi, T.T.K.; Gouadec, G.; Colomban, P.; Wang, G.; Mazerolles, L.; Thanh, D.X.; Liem, N.Q. Off Resonance Raman Spectroscopy of Wurtzite Cds Ground to the nanoscale: Discrimination between structural and size-related effects. J. Raman Spectrosc. 2011, 42, 1007–1015. [Google Scholar] [CrossRef]
- Dislich, H. Sol-Gel 1984–2004. J. Non-Crystall. Solids 1985, 73, 599–612. [Google Scholar] [CrossRef]
- Roy, R. Gel route to homogeneous glass preparation. J. Am. Ceram. Soc. 1969, 52, 344–347. [Google Scholar] [CrossRef]
- Yoldas, B.E. Effect of variation in polymerized oxides on sintering and crystalline transformations. J. Am. Ceram. Soc. 1977, 65, 387–391. [Google Scholar] [CrossRef]
- Sakka, S.; Kamiya, K. Glasses from metal alcoholates. J. Non-Crystall. Solids 1980, 42, 403–422. [Google Scholar] [CrossRef]
- Mazdiyasni, K.S. Powder synthesis from metal-organic precursors. Ceram. Int. 1982, 8, 42–56. [Google Scholar] [CrossRef]
- Johnson, D.W., Jr. Sol-Gel processing of ceramic and glass. Am. Ceram. Soc. Bull. 1985, 64, 1587–1602. [Google Scholar]
- Colomban, P. Chemical Routes and Sol-Gel Processes: The Elaboration of Ultrafine Powders. L’Industrie Céramique 1985, 792, 186–196. [Google Scholar]
- Bradley, D.C.; Mehrotra, R.C.; Gaur, D.P. Metal Alkoxides; Academic Press: London, UK, 1978. [Google Scholar]
- Okamura, H.; Bowen, H.K. Preparation of Alkoxides for the Synthesis of Ceramics. Ceram. Int. 1986, 12, 161–171. [Google Scholar] [CrossRef]
- Snow, G. Fabrication of transparent electronic PLZT ceramics by atmosphere sintering. J. Am. Ceram. Soc. 1973, 56, 91–96. [Google Scholar] [CrossRef]
- Colomban, P. Frittage de céramiques transparentes PLZT. L’Industrie Céramique 1976, 697, 531–535. [Google Scholar]
- Haertling, G.H.; Land, C.E. Hot-Pressed (Pb,La)(Zr,Ti)O3 Ferroelectric Ceramics for Electrooptic Applications. J. Am. Ceram. Soc. 1971, 54, 1–11. [Google Scholar] [CrossRef]
- Thomson, J. Chemical Preparation of PLZT Powders from aqueous Nitrate Solutions. Am. Ceram. Bull. 1973, 52, 368. [Google Scholar]
- Iller, R.K. The Chemistry of Silica; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Davidovits, J. Geopolymers-Inorganic polymeric new materials. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Luley, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J. Understanding the relationship between Geopolymer composition, microstructure and mechanical properties. Colloid. Surf. A 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Rowles, M.; O’Connor, B. Chemical optimisation of the compressive strength of aluminosilicate geopolymers synthetised by sodium silicate activation of metakaolinite. J. Mater. Chem. 2003, 13, 1161–1165. [Google Scholar] [CrossRef]
- Bell, J.L.; Driemeyer, P.E.; Kriven, W.M. Formation of ceramics from meta-kaolin-based Geopolymers. Part I: Cs-based Geopolymer. J. Am. Ceram. Soc. 2009, 92, 1–8. [Google Scholar] [CrossRef]
- Bell, J.L.; Driemeyer, P.E.; Kriven, W.M. Formation of ceramics from meta-kaolin-based Geopolymers. Part II: K-based Geopolymer. J. Am. Ceram. Soc. 2009, 92, 607–615. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S.J. The Role of Inorganic Polymer Technology in the Development of Green Concrete. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Matijevic, E. Monodispersed colloids: Art and Science. Langmuir 1986, 2, 12–20. [Google Scholar] [CrossRef]
- Lisiecki, I.; Pileni, M.P. Synthesis of Well-Defined and Low Size Distribution Cobalt Nanocrystals: The Limited Influence of Reverse Micelles. Langmuir 2003, 19, 9486–9489. [Google Scholar] [CrossRef]
- Costanzo, S.; Simon, G.; Ricciardi, J.; Colomban, P.; Lisiecki, I. Solvent Effect on AOT Surfactant Solvation—A Facile Strategy to Control the Size of Co Nanocrystals and their 2D/3D ordering. J. Phys. Chem. Part C 2016, 120, 22054–22061. [Google Scholar]
- Segal, D. Chemical synthesis of ceramic materials. J. Mater. Chem. 1997, 7, 1297–1305. [Google Scholar] [CrossRef]
- Pechini, M. Method of Preparing Lead and Alkaline-Earth Titanates and Niobates and Coating Method Using the Same to Form a Capacitor. U.S. Patent No. 3 330 697, 11 July 1967. [Google Scholar]
- Touati, F.; Gharbi, N.; Colomban, P. Structural Evolution in Polyolysed Hybrid Organic-Inorganic Alumina Gels. J. Mater. Sci. 2000, 35, 1565–1570. [Google Scholar] [CrossRef]
- Zarzycki, J.; Prassas, M.; Phalippou, J. Synthesis of Glasses from Gels: The Problem of Monoloithic Gels. J. Mater. Sci. 1982, 17, 3371–3379. [Google Scholar] [CrossRef]
- Colomban, P.; Boilot, J.-P. Polymètres inorganiques (xerogels et verres) dans les systèmes M2O-M’O2SiO2-P2O5X2O3. Rev. Chim. Minérale 1985, 22, 235–255. [Google Scholar]
- Colomban, P. Sol-gel control of the micro/nanostructure of functional ceramic-ceramic and metal-ceramic composites. J. Mater. Res. 1998, 13, 803–811. [Google Scholar] [CrossRef]
- Colomban, P. Tailoring and control of the micro/nanostructure of functional (FGM) CMC’s and MMC’s. J. Korean Ceram. Soc. 1999, 5, 55–72. [Google Scholar]
- Karlin, S.; Colomban, P. Phase diagram, short range structure and amorphous phases in the ZrO2-GeO2(-H2O) system. J. Am. Ceram. Soc. 1999, 82, 735–741. [Google Scholar] [CrossRef]
- Boilot, J.-P.; Colomban, P.; Blanchard, N. Formation of Superionic Gels and Glasses by Low Temperature Chemical Polymerization. Solid State Ionics 1983, 9/10, 639–643. [Google Scholar] [CrossRef]
- Vendange, V.; Colomban, P.; Larché, F. Pore size and liquid impregnation of microporous aluminosilicate gels and glasses. Microporous Mater. 1996, 5, 389–400. [Google Scholar] [CrossRef]
- Colomban, P.; Vendange, V. Sintering of alumina and mullite prepared by slow hydrolysis of alkoxides: The role of the protonic species and of pore topology. J. Non-Crystall. Solids 1992, 147/148, 135–140. [Google Scholar] [CrossRef]
- Vendange, V.; Colomban, P. How to tailor the porous structure of alumina and aluminosilicate gels and glasses. J. Mater. Res. 1996, 11, 518–528. [Google Scholar] [CrossRef]
- Vendange, V.; Colomban, P. Determination of the hydroxyl group content in gels and porous “glasses” issued of alkoxide hydrolysis by combined TGA and BET analysis. J. Porous Mater. 1996, 3, 193–200. [Google Scholar] [CrossRef]
- Vendange, V.; Colomban, P. Densification mechanisms of alumina, aluminosilicates and aluminoborosilicates gels, glasses and ceramics. J. Sol-Gel Sci. Technol. 1994, 2, 407–411. [Google Scholar] [CrossRef]
- Perthuis, H.; Colomban, P. Sol-gel Routes Leading to NASICON Ceramics. Ceram. Int. 1986, 12, 39–52. [Google Scholar] [CrossRef]
- Colomban, P. Sol-Gel Synthesis and Densification of NASICON Powders. Adv. Ceram. 1987, 21, 139–154. [Google Scholar]
- Blanchard, N.; Boilot, J.-P.; Colomban, P.; Pouxviel, J.C. New glasses from metal organic precursors – Preparation and Properties. J. Non-Crystall. Solids 1986, 82, 205–209. [Google Scholar] [CrossRef]
- Pouxviel, J.-C.; Boilot, J.-P. Gels from a double alkoxide: (BuO)2-Al-O-Si-(OEt)3. J. Mater. Sci. 1989, 24, 321–327. [Google Scholar] [CrossRef]
- Colomban, P. Structure of Oxide Gels and Glasses by IR and Raman Scattering: I. Aluminas. J. Mater. Sci. 1989, 24, 3002–3010. [Google Scholar] [CrossRef]
- Colomban, P. Structure of Oxide Gels and Glasses by IR and Raman Scattering: II. Mullites. J. Mater. Sci. 1989, 24, 3011–3020. [Google Scholar] [CrossRef]
- Bruneton, E.; Colomban, P. Influence of hydrolysis conditions on crystallization, phase transitions and sintering of zirconias prepared by alkoxide hydrolysis. J. Non-Crystall. Solids 1992, 147/148, 201–205. [Google Scholar]
- Perthuis, H.; Colomban, P. Well Densified NASICON-type Ceramics elaborated using Sol-Gel Process and Sintering at Low Temperatures. Mater. Res. Bull. 1984, 19, 621–631. [Google Scholar] [CrossRef]
- Colomban, P. Sol-Gel Routes and Proton Conductors. In Sol-Gel Processing for Conventional and Alternative Energy; Aparicio, M., Jitianu, A., Klein, L.C., Eds.; Springer Science + Business Media: New York, NY, USA, 2012; Chapter 4; pp. 59–71. [Google Scholar]
- Dunn, B.; Zink, J.I. Optical-Properties of Sol-Gel Glasses doped with organic molecules. J. Mater. Chem. 1991, 1, 903–913. [Google Scholar] [CrossRef]
- Roy, R. Ceramics by the solution-sol-gel route. Science 1987, 238, 1664–1669. [Google Scholar] [CrossRef]
- Colomban, P.; Badot, J.C. Elaboration de céramiques superconductrices ioniques anisotropes par chauffage microonde. Mater. Res. Bull. 1978, 13, 135–139. [Google Scholar] [CrossRef]
- Lenfant, P.; Plas, D.; Ruffo, M.; Boilot, J.-P.; Colomban, P. Céramiques d’alumine β et de ferrite β pour sonde à protons. Mater. Res. Bull. 1980, 15, 1817–1827. [Google Scholar] [CrossRef]
- Boilot, J.P.; Colomban, P. Superionic Conductors from the Sol-Gel Process. In Sol-Gel Technology; Klein, L.C., Ed.; NOYES Publications: Park Ridge, NJ, USA, 1988; Chapter 15; pp. 303–329. [Google Scholar]
- Perthuis, H.; Colomban, P. Li+ Eucriptite Superionic Thick Films. J. Mater. Sci. Lett. 1985, 4, 344–346. [Google Scholar] [CrossRef]
- Bruneton, E.; Bigaré, J.; Michel, D.; Colomban, P. Heterogeneity, nucleation, shrinkage and bloating in sol-gel glass ceramics. The case of LAS compositions. J. Mater Sci. 1997, 32, 3541–3548. [Google Scholar] [CrossRef]
- Colomban, P.; Lapous, N. New sol-gel matrices of chemically stable composites BAS, NAS and CAS. Compos. Sci. Technol. 1996, 56, 737–746. [Google Scholar] [CrossRef]
- Nagata, K.; Furuno, M. Properties of electrooptic (Pb,La)(Zr,Ti)O3 ceramics fabricated by partial Coprecipitation methods. Jpn. J. Appl. Phys. 1992, 31, 3201–3204. [Google Scholar] [CrossRef]
- Vendange, V.; Colomban, P. Elaboration and thermal stability of alumina, alumino-silicate. Fe, Co, Ni magnetic nanocomposites prepared through a sol-gel route. Mater. Sci. Eng. A 1993, 168, 199–203. [Google Scholar] [CrossRef]
- Vendange, V.; Flavin, E.; Colomban, P. Gyromagnetic microwave resonance of cobalt-aluminoborosilicate nanocomposites. J. Mater. Sci. Lett. 1996, 15, 137–141. [Google Scholar] [CrossRef]
- Mouchon, E.; Colomban, P. Microwave absorbent: Preparation, mechanical properties and RF/microwave conductivity of SiC (and/or mullite) fibres reinforced NASICON matrix composites. J. Mater. Sci. 1996, 31, 323–334. [Google Scholar] [CrossRef]
- Karlin, S.; Colomban, P. Raman study of the chemical and thermal degradation of as received and sol-gel embedded Nicalon and Hi-Nicalon SiC fibres used in ceramic matrix composites. J. Raman Spectrosc. 1997, 28, 219–228. [Google Scholar] [CrossRef]
- Colomban, P.; Wey, M. Sol-gel control of the matrix net-shape sintering in 3D reinforced ceramic matrix composites. J. Eur. Ceram. Soc. 1997, 17, 1475–1483. [Google Scholar] [CrossRef]
- MacDiarmid, A.G. Silanes and their derivatives. Adv. Inorg. Chem. 1961, 3, 207–256. [Google Scholar]
- Tanaka, H.; Kurachi, Y. Synthesis of beta-SiC powder from organic precursor and its sinterability. Ceram. Int. 1988, 14, 109–115. [Google Scholar] [CrossRef]
- Okamura, K.; Matsuzawa, T.; Hasegawa, Y. Gamma-ray irradiation curing on polycarbosilane fibers as the precursor of SiC fibers. J. Mater. Sci. 1985, 4, 55–57. [Google Scholar]
- Colomban, P. SiC, from Amorphous to Nanosized Materials, the Example of SiC Fibres issued of Polymer Precursors. In Silicon Carbide—Materials, Processing and Applications in Electronic Devices; Mukherjee, M., Ed.; INTECHOPEN: London, UK, 2011; Chapter 7; pp. 161–186. Available online: https://www.intechopen.com/books/silicon-carbide-materials-processing-and-applications-in-electronic-devices/sic-from-amorphous-to-nanosized-materials-the-exemple-of-sic-fibres-issued-of-polymer-precursors (accessed on 13 August 2020).
- Durham, S.J.P.; Shanker, K.; Drew, R.A.L. Carbothermal synthesis of silicon-nitride. Effect of reaction conditions. J. Am. Ceram. Soc. 1991, 74, 31–37. [Google Scholar] [CrossRef]
- Kroke, E.; Li, Y.L.; Lecomte, E.; Fasel, C.; Riedel, R. Silazane derived ceramics and related materials. Mater. Sci. Eng. R Rep. 2000, 26, 97–199. [Google Scholar] [CrossRef]
- Cooke, T.F. Inorganic Fibers—A Literature review. J. Am. Ceram. Soc. 1991, 74, 2959–2978. [Google Scholar] [CrossRef]
- Bill, J.; Kamphowe, T.W.; Muller, A.; Wichmann, T.; Zern, A.; Jalowieki, A.; Mayer, J.; Weinmann, M.; Schuhmacher, J.; Muller, K.; et al. Precursor-derived Si-(B-)C-N ceramics: Thermolysis, amorphous state and crystallization. Appl. Organomet. Chem. 2001, 15, 777–793. [Google Scholar] [CrossRef]
- Onbattuvelli, V.; Atre, S. Review of Net Shape Fabrication of thermally Conducting Ceramics. Mater. Manuf. Process 2011, 26, 832–845. [Google Scholar] [CrossRef]
- Corriu, R.J.P. Ceramics and nanostructures from molecular precursors. Angew. Chem. Int. Ed. 2000, 39, 1376–1398. [Google Scholar] [CrossRef]
- Gülgun, M.A.; Nguyen, M.H.; Kriven, W.M. Polymerized Organic-Inorganic Synthesis of Mixed Oxides. J. Am. Ceram. Soc. 1999, 82, 556–560. [Google Scholar] [CrossRef]
- Lee, S.-J.; Kriven, W.M. Crystallization and Densification of Nano-Size Amorphous Cordierite Powder Prepared by a PVA Solution-Polymerization Route. J. Am. Ceram. Soc. 1998, 81, 2605–2612. [Google Scholar] [CrossRef]
- Lee, S.J.; Biegalski, M.D.; Kriven, W.M. Powder synthesis of barium titanate and barium orthotitanate via an ethylene glycol complex polymerization route. J. Mater. Sci. 1999, 14, 3001–3006. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Iimura, M.; Yjima, S. Synthesis of continuous Silicon-Carbide fiber. 2. Conversion of Polycarbosilane fiber into Silicon-Carbide fibers. J. Mater. Sci. 1980, 15, 720–728. [Google Scholar] [CrossRef]
- Burns, G.T.; Taylor, R.B.; Xu, Y.R.; Zangvil, A.; Zank, G.A. High-Temperature Chemistry of the Conversion of Siloxanes to Silicon-Carbide. Chem. Mater. 1992, 4, 1313–1323. [Google Scholar] [CrossRef]
- Bayya, S.S.; Villaloos, G.R.; Hunt, M.P.; Sanghera, J.S.; Sadowski, B.M.; Aggarwal, I.D.; Cinibulk, M.; Carney, C.; Keller, K. Development of transparent polycrystalline Beta-Silicon Carbide. In Material Technologies and Applications to Optics, Structures, Components, and Sub-Systems; Robichaud, J.L., Krodel, M., Goodman, W.A., Eds.; SPIE: Bellingham, MA, USA, 2013; Volume 8837, p. UNSP 88370S. [Google Scholar] [CrossRef]
- Suzuki, T.; Kosacki, I.; Colomban, P.; Anderson, H. Electrical Conductivity and Lattice Defects in Nanocrystalline CeO2 thin films. J. Am. Ceram. Soc. 2001, 84, 2007–2014. [Google Scholar] [CrossRef]
- Kosacki, I.; Petrovsky, V.; Anderson, H.; Colomban, P. Raman Spectroscopy of Nanocrystalline Ceria and Zirconia Thin Films. J. Am. Ceram. Soc. 2002, 85, 2646–2650. [Google Scholar] [CrossRef]
- Naslain, R. Design, preparation and properties of non-oxide CMCs for application in engines and nuclear reactors: An overview. Compos. Sci. Technol. 2004, 64, 155–170. [Google Scholar] [CrossRef]
- Parcianello, G.; Bernardo, E.; Colombo, P. Mullite/Zirconia Nanocomposites from a Preceramic Polymer and Nanosized Fillers. J. Am. Ceram. Soc. 2011, 94, 1357–1362. [Google Scholar] [CrossRef]
- Parcianello, G.; Bernardo, E.; Colombo, P. Low temperature synthesis of zircon from silicone resins and oxide nano-sized particles. J. Eur. Ceram. Soc. 2012, 32, 2819–2824. [Google Scholar] [CrossRef]
- Shin, D.W.; Tanaka, H. Low-temperature Processing of Ceramic Wowen Fabric Ceramic-Matrix Composites. J. Am. Ceram. Soc. 1994, 77, 97–104. [Google Scholar] [CrossRef]
- Havel, M.; Colomban, P. Rayleigh and Raman Image of the Bulk/Surface Surface Nanostructure of SiC based Fibres. Compos. Part B Eng. 2004, 35, 139–147. [Google Scholar] [CrossRef]
- Havel, M.; Baron, D.; Colomban, P. Smart Raman/Rayleigh Imaging of Nanosized SiC Materials Using the Spatial Correlation Model. J. Mater. Sci. 2004, 39, 6183–6190. [Google Scholar] [CrossRef]
- Havel, M.; Baron, D.; Mazerolles, L.; Colomban, P. Phonon confinment in SiC nanocrystal. Comparison of the size determination using TEM and Raman spectroscopy. Appl. Spectrosc. 2007, 61, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.L.; Yang, J.L.; Huang, Y. The transformation mechanism from suspension to green body and the development of colloidal forming. Ceram. Int. 2011, 37, 1435–1451. [Google Scholar] [CrossRef]
- Chaim, R.; Levin, M.; Shlayer, A.; Estournes, C. Sintering and densification of nanocrystalline ceramic oxide powders: A review 2. Adv. Appl. Ceram. 2008, 107, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Li, W.; Wang, H.Z.; Zhou, J.X.; Chao, Z.J.; Zai, Q.Z. Fabrication of nano Y-TZP materials by superhigh pressure compaction. J. Eur. Ceram. Soc. 2001, 21, 135–138. [Google Scholar] [CrossRef]
- Henderson, R.J.; Chandler, H.W.; Akisanya, A.R.; Barber, H.; Moriarty, B. Finite element modeling of cold isostatic pressing. J. Eur. Ceram. Soc. 2000, 20, 1121–1128. [Google Scholar] [CrossRef]
- Guillon, O.; Roedel, J.; Bordia, R.K. Effect of green-state processing on the sintering stress and viscosity of alumina compacts. J. Am. Ceram. Soc. 2007, 90, 1637–1640. [Google Scholar] [CrossRef]
- Ananthakumar, S.; Manohar, P.; Warrier, K.G.K. Effect of boehnite and organic binders on extrusion of alumina. Ceram. Int. 2004, 30, 837–842. [Google Scholar] [CrossRef]
- Nampi, P.P.; Kume, S.; Hotta, Y.; Watari, K.; Ito, M.; Toda, H.; Matsutani, A. The effect of polyvinyl alcohol as a binder and stearic acid as an internal lubricant in the formation, and subsequent sintering of spray-dried alumina. Ceram. Int. 2011, 37, 3445–3450. [Google Scholar] [CrossRef]
- Lagrange, J.L.; Colomban, P. Double particle reinforcement of Ceramic-matrix composites prepared by a Sol-Gel route. Compos. Sci. Technol. 1998, 58, 653–658. [Google Scholar] [CrossRef]
- Colomban, P. Sol-gel route to functional and hierarchical ceramic matrix composites. In Proceedings of the 3rd International Conference on Intelligent Materials and 3rd European Conference on Smart Structures and Materials ICIM’96, ECSSM’96, Lyon’96, Lyon, France, 3–5 June 1996; Gobin, P.F., Tatibouet, J., Eds.; SPIE: Bellingham, MA, USA, 1996; Volume 2779, pp. 813–818. [Google Scholar]
- Xue, J.X.; Liu, J.X.; Xie, B.H.; Zhang, G.J. Pressure-induced preferential grain growth, texture development and anisotropic properties of hot pressed hexagonal boron nitride ceramics. Scr. Mater. 2011, 65, 966–969. [Google Scholar] [CrossRef]
- Shui, A.; Zhang, Y.; Uchida, N.; Uematsu, K. Origin of shape deformation during sintering of alumina compacts. J. Ceram. Soc. Jpn. 1998, 106, 873–876. [Google Scholar] [CrossRef] [Green Version]
- Chantaramee, N.; Tanaka, S.; Kato, Z.; Uchida, N.; Uematsu, K. Characterization of particles packing in alumina green tape. J. Eur. Ceram. Soc. 2009, 29, 943–948. [Google Scholar] [CrossRef]
- Imran, Z.M.; Tanaka, S.; Uematsu, K. Effect of polyacrilic acid (PAA) binder system on particle orientation during dry-pressing. Powder Technol. 2009, 196, 133–138. [Google Scholar] [CrossRef]
- Mandal, H. New developments in alpha-SiAlON ceramics. J. Eur. Ceram. Soc. 1999, 19, 2349–2357. [Google Scholar] [CrossRef]
- Chai, Q.Z.; Yang, D.; Zhao, X.M.; Chao, X.L.; Yang, Z.P. Lead-free (K,Na)NbO3-based ceramics with high optical transparency and large energy storage. J. Eur. Ceram. Soc. 2018, 101, 2321–2329. [Google Scholar] [CrossRef]
- Ikesue, A. Ce:YAG ceramic scintillator for electron beam detector. J. Ceram. Soc. Jpn. 2000, 108, 1020–1023. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.; Miyazawa, K.; Kuwabara, M. Low temperature synthesis of BaTiO3 ceramics by sol-gel process and its electro-optic properties. In Key Engineering Materials; Murata, M., Koumoto, K., Takenatka, T., Fujitsu, S., Eds.; Trans Tech Publication: Bäch, Switzerland, 2002; Volume 214–215, pp. 43–48. [Google Scholar]
- Carty, W.M.; Senapatti, U. Porcelain-raw materials, processing, phase evolution, and mechanical behaviour. J. Am. Ceram. Soc. 1998, 81, 3–20. [Google Scholar] [CrossRef]
- Sciau, P.; Noé, L.; Colomban, P. Metal nanoparticles in contemporary potters’ master pieces: Lustre and red “pigeon blood” pottery: Models to understand the ancient technology. Ceram. Int. 2016, 42, 15349–15357. [Google Scholar] [CrossRef]
- Matsubara, M.; Yamaguchi, T.; Kikuta, K.; Hirano, S. Sinterability and piezoelectric properties of (K,Na)NbO3 ceramics with novel sintering aid. Jpn. J. Appl. Phys. 2004, 43, 7159–7163. [Google Scholar] [CrossRef]
- Nicholas, J.D.; De Jonghe, L.C. Prediction and evaluation of sintering aids for Cerium Gadolinium Oxide. Solid State Ionics 2007, 178, 1187–1194. [Google Scholar] [CrossRef]
- Kleebe, H.J.; Hoffmann, M.J.; Ruhle, M. Influence of secondary phase chemistry on grain-boundary film thickness in silicon-nitride. Zeitschr. Metallkunde 1992, 83, 610–617. [Google Scholar]
- Cheng, H.F.; Lin, T.F.; Hu, C.T.; Lin, I.N. Effect os sintering aids on microstructures and PTCR characteristics of (Sr0.2Ba0.8)TiO3 ceramics. J. Am. Ceram. Soc. 1993, 76, 827–832. [Google Scholar] [CrossRef]
- Radford, K.C.; Bratton, R.J. Zirconia Electrolyte Cells. 1. Sintering studies. J. Mater. Sci. 1979, 14, 59–65. [Google Scholar] [CrossRef]
- Gomez, E.; Echeberria, J.; Iturriza, I.; Castro, F. Liquid phase sintering of SiC with additions of Y2O3, Al2O3 and SiO2. J. Eur. Ceram. Soc. 2004, 24, 2895–2903. [Google Scholar] [CrossRef]
- Greskovich, C.; Prochazka, S. Stability of Si3N4 and liquid phase(s) sintering. J. Am. Ceram. Soc. 1981, 64, C96–C97. [Google Scholar] [CrossRef]
- Huang, C.L.; Weng, M.H.; Lion, C.T.; Wu, C.C. Low temperature sintering and microwave dielectric properties of Ba2Ti9O20 ceramics using glass additions. Mater. Res. Bull. 2000, 35, 2445–2456. [Google Scholar] [CrossRef]
- Tong, J.H.; Clark, D.; Bernau, L.; Subramaniyan, A.; O’Hayre, R. Proton-conducting yttrium-doped barium cerate ceramics synthesized by a cos-effective solid-state reactive sintering method. Solid State Ionics 2010, 181, 1486–1498. [Google Scholar] [CrossRef]
- Slodczyk, A.; Lacroix, O.; Colomban, P. Water Pressure Enhanced Sintering of Alkaline-Earth Perovskite ceramics. Ceram. Int. 2015, 41, 11528–11533. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.Y.; Lee, C.; Jeong, Y.H.; Chung, K.H.; Lee, D.; Paik, D. Microstructural and piezoelectric properties of low temperature sintering PMN-PZT ceramics with the amount of Li2CO3 addition. Mater. Chem. Phys. 2005, 90, 386–390. [Google Scholar] [CrossRef]
- Mori, M.; Hiei, Y.; Sammes, N.M. Sintering behaviour and mechanism of Sr-doped lanthanum chromites with A site excess composition in air. Solid State Ionics 1999, 123, 103–111. [Google Scholar] [CrossRef]
- Gil, V.; Tartaj, J.; Moure, C.; Duran, P. Sintering, microstructural development, and electrical properties of gadolinia-doped ceria electrolyte with bismuth oxide as a sintering aid. J. Eur. Ceram. Soc. 2006, 26, 3161–3171. [Google Scholar] [CrossRef]
- Yao, K.; He, X.J.; Xu, Y.; Chen, M.M. Screen-printed piezoelectric ceramic thick films with sintering additives introduced through a liquid-phase approach. Sens. Actuators A Phys. 2005, 118, 342–348. [Google Scholar] [CrossRef]
- Pollet, M.; Marinel, S. Low temperature sintering of CaZrO3 using lithium fluoride addition. J. Eur. Ceram. Soc. 2003, 23, 1925–1933. [Google Scholar] [CrossRef]
- Stevenson, A.J.; Kupp, E.R.; Messing, G.L. Low temperature, transient liquid phase sintering of B2O3-SiO2-doped Nd:YAG transparent ceramics. J. Mater. Sci. 2011, 26, 1151–1158. [Google Scholar] [CrossRef]
- Riedel, R.; Passing, G.; Schonfelder, H.; Brook, R.J. Synthesis of dense silicon-based ceramics at low-temperatures. Nature 1992, 355, 714–717. [Google Scholar] [CrossRef]
- Zhang, S.C.; Hilmas, G.E.; Fahrenholtz, W.G. Pressureless densification of zirconium diboride with boron carbide addition. J. Am. Ceram. Soc. 2006, 89, 1544–1550. [Google Scholar] [CrossRef]
- Presser, V.; Nickel, K.G. Silica on silicon carbide. Crit. Rev. Solid State Mater. Sci. 2008, 33, 1–99. [Google Scholar] [CrossRef]
- Grande, T.; Sommerset, H.; Hagen, E.; Wiik, K.; Einarsrud, M.A. Effect of weight loss on liquid-phase-sintered silicon carbide. J. Am. Ceram. Soc. 1997, 80, 1047–1052. [Google Scholar] [CrossRef]
- Negita, K. Effective sintering aids for silicon-carbide ceramics—Reactivities of Silicon Carbide with various additives. J. Am. Ceram. Soc. 1986, 69, C308–C310. [Google Scholar] [CrossRef]
- Colomban, P.; Gouadec, G. The ideal ceramic fiber/oxide matrix composite: How to conciliate antagonist physical and chemical requirements? Ann. Chim. Sci. Matér. 2005, 30, 673–688. [Google Scholar] [CrossRef]
- Colomban, P. Proton and Protonic Species: The Hidden Face of Solid State Chemistry. How to Measure H-Content in Materials? Fuel Cells 2013, 13, 6–18. [Google Scholar] [CrossRef]
- Slodczyk, A.; Colomban, P. Probing the Nanodomain Origin and Phase Transition Mechanisms in (un)poled PMN-PT single Crystals and textured Ceramics. Materials 2010, 3, 5007–5029. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B. Narrow-band electrons in transition-metal oxides. Czech. J. Phys. B 1967, 17, 304–336. [Google Scholar] [CrossRef]
- Goodenough, J.B. Metallic oxides. Progr. Solid State Chem. 1971, 5, 145–399. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Zhou, J.-S. Localized to Itinerant Electronic Transitions in Transition-Metal Oxides with the Perovskite Structure. Chem. Mater. 1998, 10, 2980–2993. [Google Scholar] [CrossRef]
- Rao, C.N.R. Structural aspects of superconducting cuprates. Acta Crystallog. B Structur. Sci. 1995, 51, 604–618. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, P.; Rao, C.N.R. Electron transport properties of transition metal oxide systems with the K2NiF4 structure. Mater. Res. Bull. 1973, 8, 405–412. [Google Scholar] [CrossRef]
- Arul, N.S.; Nithya, V.D. Revolution of Perovskite: Synthesis, Properties and Applications; Springer Nature: Singapore, 2020. [Google Scholar]
- Haghenmuller, P.; Van Gool, W. (Eds.) Solid Electrolytes. General Principles, Characterization, Materials Applications, 1st ed.; Academic Press: New York, NY, USA, 1978. [Google Scholar]
- Mahan, G.D.; Roth, W.L. Superionic Conductors; Springer: Boston, MA, USA, 1976. [Google Scholar]
- Boilot, J.-P.; Collin, G.; Colomban, P.; Comes, R. X-ray-scattering study of the fact-ion conductor beta‘’-alumina. Phys. Rev. B 1980, 22, 5912–5923. [Google Scholar] [CrossRef]
- Colomban, P.; Lucazeau, G. Vibrational study and conduction mechanismes in beta-alumina. I. Stoichiometric beta alumina. J. Chem. Phys. 1980, 72, 1213–1224. [Google Scholar] [CrossRef]
- Collongues, R.; Gourier, D.; Kahn, A.; Boilot, J.-P.; Colomban, P.; Wicker, A. β Alumina, a Typical Solid Electrolyte: Latest Developments in Fundamental Approach and in Battery Utilization. J. Phys. Chem. Solids 1984, 45, 981–1013. [Google Scholar] [CrossRef]
- Xu, L.Q.; Li, J.Y.; Liu, C.; Zou, G.Q.; Hou, H.S.; Ji, X.B. Research progress in inorganic solid-state electrolytes for sodium-ion batteries. Acta Pysico-Chim. Sin. 2020, 36, UNSP 1905013. [Google Scholar]
- Lenain, G.E.; McKinstry, H.A.; Limaye, S.Y.; Woodward, A. Low-thermal expansion of alkali-zirconium phosphate. Mater. Res. Bull. 1984, 19, 1451–1456. [Google Scholar] [CrossRef]
- Limaye, S.Y.; Agrawal, D.K.; Roy, R.; Mehrotra, Y. Synthesis, sintering and thermal-expansion of Ca1-xSrxZr4P6O24—An ultra-low thermal-expansion ceramic system. J. Mater. Sci. 1991, 26, 93–98. [Google Scholar] [CrossRef]
- Colomban, P.; Mouchon, E. Phase transition in thermal history and expansion of NASICON solid solution and lithium derivative ceramics and of SiC (mullite) fibers-NASICON composites. Solid State Ionics 1994, 73, 209–220. [Google Scholar] [CrossRef]
- Woodcock, D.A.; Lightfoot, P.; Ritter, C. Mechanism of low thermal expansion in the cation-ordered Nasicon structure. Chem. Comm. 1998, 1, 107–108. [Google Scholar] [CrossRef]
- Pet’kov, V.I.; Asabina, E.A. Thermophysical properties of NZP ceramics (A review). Glass Ceram. 2004, 61, 233–239. [Google Scholar] [CrossRef]
- Blinov, V.A. Mechanism of nucleated crystallisation of glasses in lithia-alumina-silica and cordierite systems. J. Mater. Sci. 1969, 4, 461–468. [Google Scholar] [CrossRef]
- Yang, J.S.; Sakka, S.; Yoko, T.; Kozuka, H. Preparation of lithium aluminosilicate glass-ceramic monolith from metal oxide solution. 2. Conversion of gel to glass-ceramic monoliths and their properties. J. Mater. Sci. 1991, 26, 1827–1833. [Google Scholar] [CrossRef]
- Kikuchi, N.; Sei, T.; Tsuchiya, T.; Hayashi, S.; Hayamizu, K. Preparation of cordierite ceramics by the sol-gel process and their properties. J. Ceram. Soc. Jpn. 1993, 101, 824–829. [Google Scholar] [CrossRef] [Green Version]
- Low, I.M.; Suhermann, P.M.; Phillips, P.N. Synthesis and properties of spodumene-modified mullite ceramics formed by sol-gel processing. J. Mater. Sci. Lett. 1997, 16, 982–984. [Google Scholar] [CrossRef]
- Khosrovani, N.; Sleight, A.W. Strong anisotropic thermal expansion in oxides. Int. J. Inorg. Mater. 1999, 1, 3–10. [Google Scholar] [CrossRef]
- Ren, M.G.; Du, J.C. Structural origin of the thermal and diffusion behaviors of lithium aluminosilicate crystal polymorphs anf glasses. J. Am. Ceram. Soc. 2016, 99, 2823–2833. [Google Scholar] [CrossRef]
- Ogiwara, T.; Noda, Y.; Kimura, O. Low-Temperature Sintering of beta-Spodumene Ceramics Using Li2O-GeO2 as a Sintering Additive. J. Am. Ceram. Soc. 2013, 96, 2577–2582. [Google Scholar] [CrossRef]
- Xu, H.W.; Heaney, P.J.; Yates, D.M.; Von Dreele, R.B.; Bourke, M.A. Structural mechanisms underlying near-zero thermal expansion in beta-eucryptite: A combined synchrotron x-ray and neutron Rietveld analysis. J. Mater. Res. 1999, 14, 3138–3151. [Google Scholar] [CrossRef]
- Mittal, R.; Gupta, M.K.; Chaplot, S.L. Phonons and anomalous thermal expansion behaviour in crystalline solids. Progr. Mater. Sci. 2018, 92, 360–445. [Google Scholar] [CrossRef] [Green Version]
- Lichtenstein, A.I.; Jones, R.O.; de Gironcoli, S.; Baroni, S. Anisotropic thermal expansion in silicates: A density functional study of beta-eucryptite and related materials. Phys. Rev. B 2000, 62, 11487–11493. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Courret, H.; Romain, F.; Gouadec, G.; Michel, D. Sol-gel-prepared pure and lithium-doped hexacelsian polymorphs: An infrared, Raman, and thermal expansion study of the beta-phase stabilization by frozen short-range disorder. J. Am. Ceram. Soc. 2000, 83, 2974–2982. [Google Scholar] [CrossRef]
- Colomban, P. Orientational Disorder, Glass-Crystal Transition and Superionic Conductivity in NASICON. Solid State Ionics 1986, 21, 97–115. [Google Scholar] [CrossRef]
- Colomban, P. Raman Study of the Polymer-Superionic NASICON Transformation. Dynamics, Static inorganic orientational Disorder and Superionic Conductivity. J. Mol. Struct. 1986, 143, 191–194. [Google Scholar] [CrossRef]
- Collin, G.; Comes, R.; Boilot, J.-P.; Colomban, P. Disorder of tetrahedra in Nasicon-type structure—I.: Na3Sc2(PO4)3 Structures and Ion-Ion correlations. J. Phys. Chem. Solids 1986, 47, 845–854. [Google Scholar] [CrossRef]
- Heitjans, P.; Masoud, M.; Feldhoff, A.; Wilkening, M. NMR and impedance studies of nanocrystalline and amorphous ion conductors: Lithium niobate as a model system. Faraday Discuss. 2007, 134, 67–82. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.F.; Fang, H.Z.; Yang, F.; Liu, Z.K.; Wang, Y. Amorphous LiLaTiO3 as Solid Electrolyte Material. J. Electrochem. Soc. 2014, 161, A473–A479. [Google Scholar] [CrossRef]
- Soraru, G.D.; Dallapiccola, E.; D’Andrea, G. Mechanical characterization of sol-gel-derived silicon oxycarbide glasses. J. Am. Ceram. Soc. 1996, 79, 2074–2080. [Google Scholar] [CrossRef]
- Boulton, J.M.; Jones, K.; Emblem, H.G. Gels, Filaments and fibers from alkoxisilanes and aluminum chlorhydrate polyols complexes. J. Mater. Sci. 1989, 24, 979–990. [Google Scholar] [CrossRef]
- Yoko, T.; Kamiya, K.; Tanaka, K. Preparation of multiple oxide BaTiO3 fibers by the Sol-Gel method. J. Mater. Sci. 1990, 25, 3922–3929. [Google Scholar] [CrossRef]
- Tucker, D.S.; Sparks, J.S.; Esker, D.C. Production of continuous mullite fibers via Sol-Gel Processing. Am. Ceram. Bull. 1990, 69, 1971–1974. [Google Scholar]
- Richards, E.A.; Goodbrake, C.J.; Sowman, H.G. Reactions and microstructure development in mullite fibers. J. Am. Ceram. Soc. 1991, 74, 2404–2409. [Google Scholar] [CrossRef]
- Yuh, J.; Ninno, J.C.; Sigmund, W.A. Synthesis of barium titanate (BaTiO3) nanofibers via electrospinning. Mater. Lett. 2005, 59, 3645–3647. [Google Scholar] [CrossRef]
- Mouchon, E.; Colomban, P. Oxide ceramic-matrix fiber woven fabric composites exhibiting dissipative fracture behavior. Composites 1995, 26, 175–182. [Google Scholar] [CrossRef]
- Sporn, D.; Grossmann, J.; Kaiser, A.; Jahn, R.; Berger, A. Sol-gel processing of nanostructured ceramic and ceramic/metal composite materials. Nanostructured Mater. 1995, 6, 329–332. [Google Scholar] [CrossRef]
- Colomban, P.; Bruneton, E.; Lagrange, J.L.; Mouchon, E. Sol-gel mullite matrix-SiC and -mullite 2D woven fabric composites with or without zirconia containing interphase: Elaboration and properties. J. Eur. Ceram Soc. 1996, 16, 301–314. [Google Scholar] [CrossRef]
- Okada, K.; Yasohama, S.; Hayashi, S.; Yasumori, A. Sol-gel synthesis of mullite long fibres from water solvent systems. J. Eur. Ceram. Soc. 1998, 18, 1879–1884. [Google Scholar] [CrossRef]
- Son, K.C. Preparation of mullite fibers by the sol-gel method. J. Sol-Gel Sci. Technol. 1998, 13, 1017–1021. [Google Scholar]
- Zhang, R.B.; Hou, X.B.; Ye, C.S.; Wang, B.L. Enhanced mechanical and thermal properties of anisotropic fibrous porous mullite-zirconia composites produced using sol-gel impregnation. J. Alloys Compd. 2017, 699, 511–516. [Google Scholar] [CrossRef]
- Vendange, V. Des membranes Micro- et Mesoporeuses aux Nanocomposites Magnetiques. Doctorate Thesis, Universté Pierre-et-Marie-Curie (Paris 6), 1994. Available online: http://www.theses.fr/1994PA066282 (accessed on 13 August 2020).
- Colomban, P.; Vendange, V. Sol-gel routes towards magnetic nanocomposites with tailored microwave absorption. In V-Nanophase and Nanocomposite Materials II, Proceedings of the MRS Fall Meeting, Boston, MA, USA, 1–6 December 1996; Komarnemi, S., Parker, J.C., Wollenberger, H.J., Eds.; MRS: Warrendale, PA, USA, 1997; Volume 457, pp. 451–462. [Google Scholar]
- Colomban, P. Glazes and Enamels. In Encyclopedia of Glass Science, Technology, History, and Culture, 1st ed.; Richet, P., Ed.; The Journal of the American Ceramic Society; Wiley & Sons, Inc.: New York, NY, USA, 2020; Chapter 10.6. [Google Scholar]
- Epler, R.A.; Epler, D.R. Glazes and Glass Coatings; The American Ceramic Society: Westerville, OH, USA, 2000. [Google Scholar]
- Niepce, J.C.; Hugentobler, D. Capacitors. In Concise Encyclopedia of Advanced Ceramic Materials; Pergamon Press: Oxford, UK, 1991; pp. 53–57. [Google Scholar]
- Berger, M.-H.; Bunsell, A.R. Fine Ceramic Fibers; Marcel Dekker Inc.: New York, NY, USA, 1999. [Google Scholar]
- Bunsell, A.R. (Ed.) Handbook of Tensile Properties of Textiles and Technical Fibres, 2nd ed.; The Textile Institute, Woodhead Publishing—Elsevier: Kidlington, UK, 2018. [Google Scholar]
- Boilot, J.-P.; Collin, G.; Colomban, P. Crystal Structure of the true NASICON: Na3Zr2Si2PO12. Mater. Res. Bull. 1987, 22, 669–676. [Google Scholar] [CrossRef]
- Colomban, P. Multi-level ceramic composites: The advantage of sol-Gel routes-Composites céramiques multiniveaux ou l’intérêt des méthodes sol-gel. In Proceedings of the 8e Journées Nationales sur les Composites (JNC’8), Palaiseau, France, 16–18 Novembre 1992; Allix, O., Favre, J.P., Ladevèze, P., Eds.; AMAC: Paris, France, 1992; pp. 73–84. [Google Scholar]
- Subasri, R.; Mathews, T.; Sreedharan, O.M.; Raghunathan, V.S. Microwave processing of sodium beta alumina. Solid State Ionics 2003, 158, 199–204. [Google Scholar] [CrossRef]
- Subasri, R.; Nafe, H. Texture in Na-beta-Al2O3 due to microwave processing. Mater. Chem. Phys. 2008, 112, 16–19. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, X.P.; Wei, X.-L.; Yanh, H.; Shen, X.D. Synthesis of Na-β″-Al2O3 electrolytes by microwave sintering precursors derived from the sol-gel method. J. Alloys Compd. 2010, 497, 295–299. [Google Scholar] [CrossRef]
- Tak, S.K. Impedance study of Beta-Alumina by Microwave Sintering. AIP Conf. Proc. 2013, 1536, 1211–1212. [Google Scholar] [CrossRef]
- Benavente, R.; Salvador, M.D.; Penaranda-Foix, F.L.; Garcia-Moreno, O.; Borrell, A. High thermal stability of microwave sintered low-epsilon(ε) beta-eucryptite materials. Ceram. Int. 2015, 41, 13817–13822. [Google Scholar] [CrossRef]
- Colomban, P.; Mouchon, E.; Badot, J.C.; Belhadj-Tahar, N.; Fourier-Lamer, A. Radiofrequency and microwave conductivity in NASICON superionic conductor. Solid State Ionics 1992, 53–56, 813–824. [Google Scholar] [CrossRef]
- Colomban, P.; Badot, J.-C. Frequency dependent conductivity and microwave relaxations in protonic conductors. Solid State Ionics 1993, 61, 55–62. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Giddey, S.; Munnings, A. Kulkarni. Review of Progress in High Temperature Solid Oxide Fuel Cells. J. Aust. Ceram. Soc. 2014, 50, 23–37. [Google Scholar]
- Colomban, P. (Ed.) Proton Conductors; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Knauth, P.; Di Vona, M.L. Solid State Proton Conductors: Properties and Applications in Fuel Cell; Wiley: New York, NY, USA, 2012. [Google Scholar]
- Preininger, M.; Wurm, J.; Subotic, V.; Schauperl, R.; Hochenauer, C. Performance characterization of a solid oxide cell stack with chromium-based interconnects (CFY). Int. J. Hydrogen Energy 2017, 42, 28653–28664. [Google Scholar] [CrossRef]
- Kleiminger, L.; Li, T.; Li, K.; Kelsall, G.H. Syngas (CO-H2) production using high temperature micro-tubular solid oxide electrolysers. Electrochim. Acta 2015, 179, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Ji, S.; Chang, I.; Lee, Y.H.; Park, J.; Lee, M.H.; Cha, S.W. Fabrication of low-temperature solid oxide fuel cells with a nanothin protective layer by atomic layer deposition. Nanoscale Res. Lett. 2013, 8, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Bernay, C.; Ringuede, A.; Colomban, P.; Lincot, D.; Cassir, M. Yttria-doped Zirconia Thin Films Deposited by Atomic Layer Deposition ALD—A Structural, Morphological and Electrical Characterization. J. Phys. Chem. Solids 2003, 64, 1761–1770. [Google Scholar] [CrossRef]
- Cheong, K.Y.; Impellizzeri, G.; Fraga, M.A. Emerging Materials for Energy Conversion and Storage; Elsevier: Amsterdam, NL, USA, 2018. [Google Scholar]
- Zhang, J.; Jung, Y.-G. (Eds.) Advanced Ceramic and Metallic Coating and Thin Film Materials for Energy and Environmental Applications; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Schnell, J.P.; Velasco, G.; Colomban, P. Thin Films of β-Alumina-like Structure. Solid State Ionics 1981, 5, 291–294. [Google Scholar] [CrossRef]
- Schnell, J.P.; Velasco, G.; Croset, M.; Dubreuil, D.; Dieumegard, D.; Colomban, P. Hydrogenated Alumina-like Thin Film. Solid State Ionics 1983, 9/10, 1465–1468. [Google Scholar] [CrossRef]
- Nenov, T.; Yordanov, S.P. Ceramic Sensors: Technology and Applications; Technomic Publishing Co. Inc.: Lancaster, PA, USA, 1996. [Google Scholar]
- Cretin, M.; Fabry, P. Characterizations of conductive ceramics for ionic lithium potentiometric sensors. Ann. Chim. Sci. Matériaux 1995, 20, 433–438. [Google Scholar]
- Rabinovich, L.; Gun, J.; Tsionsky, M.; Lev, O. Fuel-cell type ceramic-carbon oxygen sensors. J. Sol-Gel Sci. Technol. 1997, 8, 1077–1081. [Google Scholar] [CrossRef]
- Rabinovich, L.; Lev, O. Sol-gel derived composite ceramic carbon electrodes. Electroanalysis 2001, 13, 265–275. [Google Scholar] [CrossRef]
- Sakka, S. (Ed.) Sol-gel Science and Technology; Volume 3—Applications of Sol-Gel Technology; Kluwer Academic: Hingham, MA, USA, 2003. [Google Scholar]
- Uma, T.; Nogami, M. Development of H2/O2 fuel cell based on proton conducting P2O5-SiO2-PMA glasses as electrolyte. In Advanced Materials Research; Nogami, M., Jin, R., Yang, W., Eds.; Trans Tech Publications: Bäch, Switzerland, 2006; Volume 11–12, pp. 149–155. [Google Scholar]
- Yun, J.W.; Yoon, S.P.; Park, S.; Han, J.; Nam, S.W.; Lim, T.H.; Kim, J.S. Modifying the cathodes of intermediate-temperature solid oxide fuel cells with a Ce0.8Sm0.2O2 sol-gel coating. Int. J. Hydrogen Energy 2009, 34, 9213–9219. [Google Scholar] [CrossRef]
- Yun, J.W.; Han, J.; Yoon, S.P.; Park, S.; Kim, H.S.; Nam, S.W. Ce0.8Gd0.2O2 modification on La0.6Sr0.4Co0.2Fe0.8O3 cathode for improving a cell performance in intermediate temperature solid oxide fuel cells. J. Ind. Eng. Chem. 2011, 17, 439–444. [Google Scholar] [CrossRef]
- Choi, H.-J.; Na, Y.H.; Seo, D.W.; Woo, S.K.; Kim, S.D. Densification of gadolinia-doped ceria diffusion barriers for SOECs and IT-SOFCs by a sol-gel process. Ceram. Int. 2016, 42 Pt A, 545–550. [Google Scholar] [CrossRef]
- Zhou, F.; Song, X.W.; Zhou, X.M.; Gao, J.Q.; Bao, J.X.; Tian, Z.; An, S.L. Limiting-current oxygen sensor with LaNi0.6Fe0.4O3-delta a dense diffusion barrier and Ce0.8Gd0.15Ca0.05O2-delta electrolyte. Ceram. Int. 2019, 45, 12060–12065. [Google Scholar] [CrossRef]
- Park, H.K.; Smyrl, W.H.; Ward, M.D. V2O5 Xerogel films as intercalation host for Lithium. 1. Insertion stoichiometry, site concentration, and specific energy. J. Electrochem. Soc. 1995, 142, 1068–1073. [Google Scholar] [CrossRef]






| Composition | Reagents | References |
|---|---|---|
| Silica | Ludox® Alkoxides | Iller [68], Roy [102] Phalippou et al. [81] Dunn & Zink [101] |
| Alumina | Alkoxide | Colomban [96] |
| Aluminates (β alumina) Ytrium aluminate | Alkoxide + Na/K ions PVA/EG routes | Colomban [100,103,104,105] Gülgun et al. [125] |
| Aluminosilicates Cordierite Leucite | Alkoxide mixture or Al-O-Si alkoxide Pechini resin/PVA “Geopolymers” | Yoldas [57] Colomban [97] Lee and Kriven [126] Bell et al. [72,73] |
| Na/Li aluminosilicates | See above + Na/Li/K/Ba/Ca ions | Perthuis et al. [17,106], Bruneton et al. [107] Colomban and Lapous [108] |
| Zirconia/Ceria | Alkoxide | Mazdiyasni [59] Bruneton and Colomban [98] Kosacki et al. (2002) [131,132] |
| Zirconates/Titanates BaTiO3 | Alkoxides + ions Ethylene glycol (EG) | Snow [64], Colomban [2,65], Nagata et al. [109] Lee et al. [127] |
| NASICON Na/Li zircon-phospho-silicates | Alkoxides + ions | Perthuis and Colomban [99] Bouquin et al. [14] |
| Composites reinforced with nanoparticles, fibers, textiles | Alkoxides + salts | Vendange and Colomban [110], Vendange et al. [111] Mouchon and Colomban [112], Karlin and Colomban [113], Colomban and Wey [114] |
| Silicon carbides * | Silanes Polycarbosilanes | McDiarmid [115], Corriu [124] Tanaka and Kurachi [116], Okamura et al. [117] Colomban [118] Hasegawa et al. [128] Burns et al. [129] Naslain [133] Bayya et al. [130] Shin and Tanaka [136], |
| Silicon nitrides * Oxides from polymer precursors (zircon, mullite, zirconia, etc.) | Polysilazanes Polyorganoboro- Silazanes, etc. Polysilsequioxanes | Durham et al. [119], Kroke et al. [120], Bill and Aldinger [32], Riedel et al. [33], Cooke [121], Bill et al. [122], Onbattuvelli and Atre [123] Parcianello et al. [135] Parcianello et al. [134] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colomban, P. Chemical Preparation Routes and Lowering the Sintering Temperature of Ceramics. Ceramics 2020, 3, 312-339. https://0-doi-org.brum.beds.ac.uk/10.3390/ceramics3030029
Colomban P. Chemical Preparation Routes and Lowering the Sintering Temperature of Ceramics. Ceramics. 2020; 3(3):312-339. https://0-doi-org.brum.beds.ac.uk/10.3390/ceramics3030029
Chicago/Turabian StyleColomban, Philippe. 2020. "Chemical Preparation Routes and Lowering the Sintering Temperature of Ceramics" Ceramics 3, no. 3: 312-339. https://0-doi-org.brum.beds.ac.uk/10.3390/ceramics3030029