Advances in Methods for Recovery of Ferrous, Alumina, and Silica Nanoparticles from Fly Ash Waste
Abstract
:1. Introduction
2. Properties and Applications of Fly Ash
2.1. Morphological Properties of Fly Ash
2.2. Elemental Properties of Fly Ash
2.3. Chemical Properties of Fly Ash
2.4. Physical Properties of Fly Ash
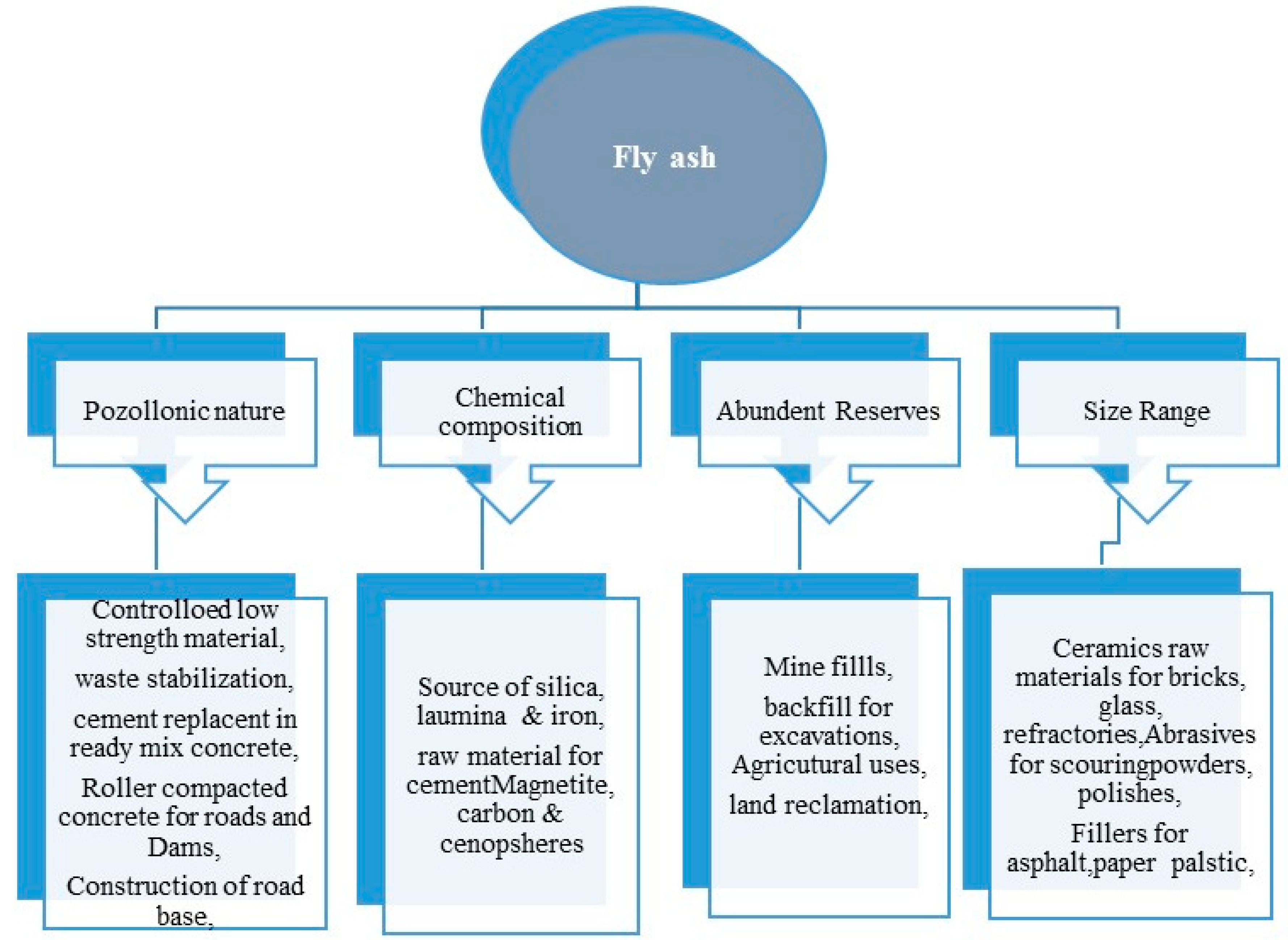
2.5. Applications of Fly Ash
3. Fly Ash Production and Utilization at a Glance in India
4. Fly Ash as a Source of Ferrous, Alumina and Silica
5. Ferrous Particles: Properties and Advances in Their Recovery Process from Fly Ash
5.1. Properties of Ferrous Particles Extracted from Fly Ash
5.2. Advances in the Recovery of Ferrous Particles from Fly Ash
6. Extraction and Synthesis of Alumina Nanoparticles from Fly Ash
6.1. Alkali-Based Extraction
6.2. Acid-Based Extraction
6.3. Acid-Alkali Based Extraction
6.4. Microbial Leaching of Alumina from Fly Ash
6.5. Properties and Applications of Alumina Nanoparticles
7. Synthesis of SiNPs from Fly Ash
7.1. Silica Extraction from Fly Ash by Chemical Method
7.2. Microbial Leaching of Silicon and Silica Syntheses from Fly Ash
7.3. Properties and Applications of SiNPs
8. Role of Nanotechnology: Nano adsorbents for Heavy Metal Removal
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choudhary, N.; Yadav, V.K.; Malik, P.; Khan, S.H.; Inwati, G.K.; Suriyaprabha, R.; Singh, B.; Yadav, A.K.; Ravi, R.K. Recovery of Natural Nanostructured Minerals: Ferrospheres, Plerospheres, Cenospheres, and Carbonaceous Particles From Fly Ash. In Handbook of Research on Emerging Developments and Environmental Impacts of Ecological Chemistry; Gheorghe, D., Ashok, V., Eds.; IGI Global: Hershey, PA, USA, 2020; pp. 450–470. [Google Scholar] [CrossRef]
- Ohenoja, K.; Pesonen, J.; Yliniemi, J.; Illikainen, M. Utilization of Fly Ashes from Fluidized Bed Combustion: A Review. Sustainability 2020, 12, 2988. [Google Scholar] [CrossRef] [Green Version]
- Fuller, A.; Maier, J.; Karampinis, E.; Kalivodova, J.; Grammelis, P.; Kakaras, E.; Scheffknecht, G. Fly Ash Formation and Characteristics from (co-)Combustion of an Herbaceous Biomass and a Greek Lignite (Low-Rank Coal) in a Pulverized Fuel Pilot-Scale Test Facility. Energies 2018, 11, 1581. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Song, W. Mineralogical and Chemical Characteristics of Coal Ashes from Two High-Sulfur Coal-Fired Power Plants in Wuhai, Inner Mongolia, China. Minerals 2020, 10, 323. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, P.; Silvestre, J.D.; Flores-Colen, I.; Viegas, C.A.; Ahmed, H.H.; Kurda, R.; de Brito, J. Evaluation of the Ecotoxicological Potential of Fly Ash and Recycled Concrete Aggregates Use in Concrete. Appl. Sci. 2020, 10, 351. [Google Scholar] [CrossRef] [Green Version]
- Alam, J.; Akhtar, M. Fly ash utilization in different sectors in Indian scenario. Int. J. Emerg. Trends Eng. Dev. 2011, 1, 1–14. [Google Scholar]
- Yadav, V.K.; Fulekar, M.H. The current scenario of thermal power plants and fly ash: Production and utilization with a focus in India. Int. J. Adv. Eng. Res. Dev. 2018, 5, 768–777. [Google Scholar]
- Nisham, K.; Sridhar, M.B.; Kumar, V. Experimental study on class F fly ash cement bricks using partial replacement of fly ash by metakaolin. Int. J. Chem. Sci. 2016, 14, 227–234. [Google Scholar]
- Yadav, V.K.; Pandita, P.R. Fly Ash Properties and Their Applications as a Soil Ameliorant. In Amelioration Technology for Soil Sustainability; Rathoure, A.K., Ed.; IGI Global: Hershey, PA, USA, 2019; pp. 59–89. [Google Scholar] [CrossRef] [Green Version]
- Yadav, V.K.; Choudhary, N. An Introduction to Fly Ash: Natural Nanostructured Materials; Educreation: New Delhi, India, 2019; Volume 1, p. 162. [Google Scholar]
- Zhao, Y.; Soltani, A.; Taheri, A.; Karakus, M.; Deng, A. Application of Slag—Cement and Fly Ash for Strength Development in Cemented Paste Backfills. Minerals 2018, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Valentim, B.; Białecka, B.; Gonçalves, A.P.; Guedes, A.; Guimarães, R.; Cruceru, M.; Całus-Moszko, J.; Popescu, G.L.; Predeanu, G.; Santos, C.A. Undifferentiated Inorganics in Coal Fly Ash and Bottom Ash: Calcispheres, Magnesiacalcispheres, and Magnesiaspheres. Minerals 2018, 8, 140. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Qin, S.; Kang, L.; Liu, J.; Wang, J.; Li, Y. An Efficient Approach for Lithium and Aluminum Recovery from Coal Fly Ash by Pre-Desilication and Intensified Acid Leaching Processes. Metals 2017, 7, 272. [Google Scholar] [CrossRef] [Green Version]
- Habte, L.; Shiferaw, N.; Mulatu, D.; Thenepalli, T.; Chilakala, R.; Ahn, J. Synthesis of Nano-Calcium Oxide from Waste Eggshell by Sol-Gel Method. Sustainability 2019, 11, 3196. [Google Scholar] [CrossRef] [Green Version]
- Peng, X. Dynamic hydrothermal synthesis of xonotlite fibers by alkali silica extraction of fly ash. J. Eng. Fibers Fabr. 2019, 14, 155892501989034. [Google Scholar] [CrossRef]
- Purnomo, C.; Wirawan, S.; Hinode, H. The utilization of bagasse fly ash for mesoporous silica synthesis. IOP Conf. Ser. Mater. Sci. Eng. 2019, 543, 012040. [Google Scholar] [CrossRef]
- Guo, C.; Zou, J.; Ma, S.; Yang, J.; Wang, K. Alumina Extraction from Coal Fly Ash via Low-Temperature Potassium Bisulfate Calcination. Minerals 2019, 9, 585. [Google Scholar] [CrossRef]
- Gong, Y.; Sun, J.; Sun, S.-Y.; Lu, G.; Zhang, T.-A. Enhanced Desilication of High Alumina Fly Ash by Combining Physical and Chemical Activation. Metals 2019, 9, 411. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.; El-Naas, M.; Benamor, A.; Al-Sobhi, S.; Zhang, Z. Carbon Mineralization by Reaction with Steel-Making Waste: A Review. Processes 2019, 7, 115. [Google Scholar] [CrossRef] [Green Version]
- Valeev, D.; Mikhailova, A.; Atmadzhidi, A. Kinetics of Iron Extraction from Coal Fly Ash by Hydrochloric Acid Leaching. Metals 2018, 8, 533. [Google Scholar] [CrossRef] [Green Version]
- Yadav, V.K.; Fulekar, M.H. Biogenic synthesis of maghemite nanoparticles (γ-Fe2O3) using Tridax leaf extract and its application for removal of fly ash heavy metals (Pb, Cd). Mater. Today Proc. 2018, 5, 20704–20710. [Google Scholar] [CrossRef]
- Yao, Z.; Xia, M.S.; Sarker, P.; Chen, T. A review of the alumina recovery from coal fly ash, with a focus in China. Fuel 2014, 120, 74–85. [Google Scholar] [CrossRef] [Green Version]
- Miricioiu, M.G.; Niculescu, V.C. Fly Ash, from Recycling to Potential Raw Material for Mesoporous Silica Synthesis. Nanomaterials (Basel) 2020, 10, 474. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, P.K.; Kim, K.; Powell, M.A.; Equeenuddin, S.M. Recovery of metals and other beneficial products from coal fly ash: A sustainable approach for fly ash management. Int. J. Coal Sci. Technol. 2016, 3, 267–283. [Google Scholar] [CrossRef] [Green Version]
- Langmann, B. Volcanic Ash versus Mineral Dust: Atmospheric Processing and Environmental and Climate Impacts. ISRN Atmos. Sci. 2013, 2013, 17. [Google Scholar] [CrossRef] [Green Version]
- Sunjidmaa, D.; Batdemberel, G.; Takibai, S. A Study of Ferrospheres in the Coal Fly Ash. Open J. Appl. Sci. 2019, 9, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Murugesan, S.; Ramaswamy, J.; Parshwanath, R.; Sundararaj, J.; Jose, R. Evaluation of Suitability of Alumino-Silicate Precursor for Geopolymerization through Advance Analytical Techniques. Asian J. Chem. 2018, 30, 1771–1776. [Google Scholar] [CrossRef]
- Valentim, B.; Flores, D.; Guedes, A.; Shreya, N.; Paul, B.; Ward, C.R. Notes on the occurrence of char plerospheres in fly ashes derived from Bokaro and Jharia coals (Jharkhand, India) and the influence of the combustion conditions on their genesis. Int. J. Coal Geol. 2016, 158, 29–43. [Google Scholar] [CrossRef]
- Salah, N.; Al-Ghamdi, A.; Memic, A.; Habib, S.; Khan, Z. Formation of Carbon Nanotubes from Carbon Rich Fly Ash: Growth Parameters and Mechanism. Mater. Manuf. Process. 2015, 31, 150811005209005. [Google Scholar] [CrossRef]
- Silva, L.; Martinello, K.; Mardon, S.; Hower, J.; Serra-Rodríguez, C. Fullerenes and Metallofullerenes in Coal-Fired Stoker Fly Ash. Coal Combust. Gasif. Prod. 2010, 2. [Google Scholar] [CrossRef]
- Fu, B.; Hower, J.C.; Dai, S.; Mardon, S.M.; Liu, G. Determination of Chemical Speciation of Arsenic and Selenium in High-As Coal Combustion Ash by X-ray Photoelectron Spectroscopy: Examples from a Kentucky Stoker Ash. ACS Omega 2018, 3, 17637–17645. [Google Scholar] [CrossRef]
- Yoriya, S.; Intana, T.; Tepsri, P. Separation of Cenospheres from Lignite Fly Ash Using Acetone-Water Mixture. Appl. Sci. 2019, 9, 3792. [Google Scholar] [CrossRef] [Green Version]
- Choo, T.F.; Salleh, M.A.M.; Kok, K.Y.; Matori, K.A.; Rashid, S.A. Effect of Temperature on Morphology, Phase Transformations and Thermal Expansions of Coal Fly Ash Cenospheres. Crystals 2020, 10, 481. [Google Scholar] [CrossRef]
- Hower, J.; Groppo, J.; Graham, U.; Ward, C.; Kostova, I.; Maroto-Valer, M.; Dai, S. Coal-derived unburned carbons in fly ash: A review. Int. J. Coal Geol. 2017, 179, 11–27. [Google Scholar] [CrossRef]
- Bayukov, O.A.; Anshits, N.N.; Balaev, A.D.; Sharonova, O.M.; Rabchevskii, E.V.; Petrov, M.I.; Anshits, A.G. Mössbauer study of magnetic microspheres isolated from power plant fly ash. Inorg. Mater. 2005, 41, 50–59. [Google Scholar] [CrossRef]
- Wang, P.; Massoudi, M. Slag Behavior in Gasifiers. Part I: Influence of Coal Properties and Gasification Conditions. Energies 2013, 6, 784–806. [Google Scholar] [CrossRef] [Green Version]
- Krishnamoorthy, V.; Pisupati, S. A Critical Review of Mineral Matter Related Issues during Gasification of Coal in Fixed, Fluidized, and Entrained Flow Gasifiers. Energies 2015, 8, 10430–10463. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Alrefaei, Y.; Dai, J.-G. Silico-Aluminophosphate and Alkali-Aluminosilicate Geopolymers: A Comparative Review. Front. Mater. 2019, 6, 106. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Sun, Q.; Wang, B.; Wang, P.; Zou, J. Morphology and Composition of Microspheres in Fly Ash from the Luohuang Power Plant, Chongqing, Southwestern China. Minerals 2016, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Sharonova, O.; Anshits, N.; Fedorchak, M.; Zhizhaev, A.; Anshits, A. Characterization of Ferrospheres Recovered from High-Calcium Fly Ash. Energy Fuels 2015, 29, 5404–5414. [Google Scholar] [CrossRef]
- Veranth, J.M.; Fletcher, T.H.; Pershing, D.W.; Sarofim, A.F. Measurement of soot and char in pulverized coal fly ash. Fuel 2000, 79, 1067–1075. [Google Scholar] [CrossRef]
- Vassilev, S.; Menendez, R.; Borrego, A.; Díaz-Somoano, M.; Martínez-Tarazona, M. Phase-mineral and chemical composition of coal fly ashes as a basis for their multicomponent utilization. 3. Characterization of magnetic and char concentrates. Fuel 2004, 83, 1563–1583. [Google Scholar] [CrossRef]
- Gupta, D.K.; Rai, U.N.; Tripathi, R.D.; Inouhe, M. Impacts of fly-ash on soil and plant responses. J. Plant Res. 2002, 115, 401–409. [Google Scholar] [CrossRef]
- Singh, G.B.; Subramaniam, K.V. Characterization of Indian fly ashes using different Experimental Techniques. Indian Concr. J. 2018, 92, 10–23. [Google Scholar]
- Šešlija, M.; Rosić, A.; Radović, N.; Vasić, M.; Đogo, M.; Jotić, M. Physiproperties of fly ash and slag from the power plants. Geol. Croat. 2016, 69, 317–324. [Google Scholar] [CrossRef]
- Eisele, T.C.; Kawatra, S.K.; Nofal, A. Comparison of class C and class F fly-ashes as foundry sand binders and the effectiveness of accelerators in reducing curing time. Miner. Process. Extr. Metall. Rev. 2004, 25, 269–278. [Google Scholar] [CrossRef]
- Vassilev, S.; Menendez, R.; Alvarez, D.; Díaz-Somoano, M.; Martínez-Tarazona, M. Phase-Mineral and Chemical Composition of Coal Fly Ashes as a Basis for Their Multicomponent Utilization. 1. Characterization of Feed Coals and Fly Ashes. Fuel 2003, 82, 1793–1811. [Google Scholar] [CrossRef]
- Basu, M.; Pande, M.; Bhadoria, P.B.S.; Mahapatra, S.C. Potential fly-ash utilization in agriculture: A global review. Prog. Nat. Sci. 2009, 19, 1173–1186. [Google Scholar] [CrossRef]
- Fulekar, M.H.; Dave, J.M. Heavy metals release from ash pond to soil water environment: A simulated technique. Environ. Int. 1992, 18, 283–295. [Google Scholar] [CrossRef]
- Fulekar, M.H.; Yadav, V.K. Method for Separation of Ferrous, Alumina and Silica from Fly Ash. 201721035720, 7 October 2017. [Google Scholar]
- Papatzani, S.; Paine, K. A Step by Step Methodology for Building Sustainable Cementitious Matrices. Appl. Sci. 2020, 10, 2955. [Google Scholar] [CrossRef]
- Davison, R.L.; Natusch, D.F.; Wallace, J.R.; Evans, C.A., Jr. Trace elements in fly ash. Dependence of concentration on particle size. Environ. Sci. Technol. 1974, 8, 1107–1113. [Google Scholar] [CrossRef]
- Boboc, V.; Rotaru, A.; Boboc, A. A material for substructure and road works: Mechanical characteristics of pozzolana fly ash from thermal power plant of iasi, Romania. WSEAS Trans. Environ. Dev. 2010, 42, 437–446. [Google Scholar]
- Yunusa, I.; Loganathan, P.; Nissanka, S.; Veeragathipillai, M.; Burchett, M.; Skilbeck, C.; Eamus, D. Application of Coal Fly Ash in Agriculture: A Strategic Perspective. Crit. Rev. Environ. Sci. Technol. 2012, 42, 559–600. [Google Scholar] [CrossRef]
- Kleinhans, U.; Wieland, C.; Frandsen, F.J.; Spliethoff, H. Ash formation and deposition in coal and biomass fired combustion systems: Progress and challenges in the field of ash particle sticking and rebound behavior. Prog. Energy Combust. Sci. 2018, 68, 65–168. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 20. [Google Scholar] [CrossRef] [Green Version]
- Fulekar, M.H.; Naik, D.S.; Dave, J.M. Heavy metals in Indian coals and corresponding fly-ash and their relationship with particulate size. Int. J. Environ. Stud. 1983, 21, 179–182. [Google Scholar] [CrossRef]
- Chou, M.-I.M. Fly Ash. In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2012; pp. 3820–3843. [Google Scholar] [CrossRef]
- Ngu, L.-N.; Wu, H.; Zhang, D.-k. Characterization of Ash Cenospheres in Fly Ash from Australian Power Stations. Energy Fuels 2007, 21, 3437–3445. [Google Scholar] [CrossRef]
- Żyrkowski, M.; Neto, R.C.; Santos, L.; Witkowski, K. Characterization of fly-ash cenospheres from coal-fired power plant unit. Fuel 2016, 174, 49–53. [Google Scholar] [CrossRef]
- Fisher, G.L.; Chang, D.P.Y.; Brummer, M. Fly Ash Collected from Electrostatic Precipitators: Microcrystalline Structures and the Mystery of the Spheres. Science 1976, 192, 553–555. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Sun, J.; Bai, X.; Zheng, C. Mineralogy, Chemical Composition, and Microstructure of Ferrospheres in Fly Ashes from Coal Combustion. Energy Fuels 2006, 20, 1490–1497. [Google Scholar] [CrossRef]
- Ibeto, C.N.; Obiefuna, C.J.; Ugwu, K.E. Environmental effects of concretes produced from partial replacement of cement and sand with coal ash. Int. J. Environ. Sci. Technol. 2020, 17, 2967–2976. [Google Scholar] [CrossRef]
- Meer, I.; Nazir, R. Removal techniques for heavy metals from fly ash. J. Mater. Cycles Waste Manag. 2017, 20, 703–722. [Google Scholar] [CrossRef]
- Zhang, W.; Noble, A.; Yang, X.; Honaker, R. A Comprehensive Review of Rare Earth Elements Recovery from Coal-Related Materials. Minerals 2020, 10, 451. [Google Scholar] [CrossRef]
- Kunecki, P.; Panek, R.; Wdowin, M.; Bień, T.; Franus, W. Influence of the fly ash fraction after grinding process on the hydrothermal synthesis efficiency of Na-A, Na-P1, Na-X and sodalite zeolite types. Int. J. Coal Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Kishor, P.; Ghosh, A.; Kumar, D. Use of Flyash in Agriculture: A Way to Improve Soil Fertility and its Productivity. Asian J. Agric. Res. 2010, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Gupta, A.K.; Ibrahim, M.H.; Mittal, A.K. Coal fly ash utilization in agriculture: Its potential benefits and risks. Rev. Environ. Sci. Bio/Technol. 2010, 9, 345–358. [Google Scholar] [CrossRef]
- Behera, A.; Mohapatra, S.S. Challenges in Recovery of Valuable and Hazardous Elements from Bulk Fly Ash and Options for Increasing Fly Ash Utilization. In Coal Fly Ash Beneficiation—Treatment of Acid Mine Drainage with Coal Fly Ash; Akinyemi, S., Gitari, M.W., Eds.; IntechOpen: London, UK, 2018; pp. 19–39. [Google Scholar] [CrossRef] [Green Version]
- Attarde, S.; Marathe, S.; Sil, A. Utilization of fly ash in construction industries for environment management. Int. J. Environ. Sci. 2014, 3, 117–121. [Google Scholar]
- Luo, Y.; Zheng, S.; Ma, S.; Liu, C.; Wang, X. Ceramic tiles derived from coal fly ash: Preparation and mechanical characterization. Ceram. Int. 2017, 43, 11953–11966. [Google Scholar] [CrossRef]
- FİGen, A.; ÖZÇAy, Ü.; Pişkin, S. Manufacturing and Characterization of Roof Tiles a Mixture of Tile Waste and Coal Fly Ash. Süleyman Demirel Üniversitesi Fen Bilimleri Enstitüsü Derg. 2017, 21, 10. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Nordin, N.; Abdullah, M.M.A.B.; Tahir, M.F.M.; Sandu, A.V.; Hussin, K. Utilization of fly ash waste as construction material. Int. J. Conserv. Sci. 2016, 7, 161–166. [Google Scholar]
- Tyson, S.; Blackstock, T. Coal combustion fly ash—Overview of applications and opportunities in the USA. Fuel Energy Abstr. 1996, 37, 422. [Google Scholar]
- Martin, J.; Collins, R.; Browning, J.; Bichl, J. Properties and Use of Fly Ashes for Embankments. J. Energy Eng. 1990, 116, 71–86. [Google Scholar] [CrossRef]
- Baykal, G.; Edinçliler, A.; Saygılı, A. Highway embankment construction using fly ash in cold regions. Resour. Conserv. Recycl. 2004, 42, 209–222. [Google Scholar] [CrossRef]
- Dasgupta, M.; Kar, S.; Gupta, S.D.; Mukhopadhyay, R.; Bandyopadhyay, A. Effect of Fly Ash as Filler in Rubber—A Comprehensive Study of the Vulcanisate Properties of Styrene-Butadiene Rubber Compounds. Prog. Rubber Plast. Recycl. Technol. 2013, 29, 151–168. [Google Scholar] [CrossRef]
- Gaikwad, A.; Patel, B.K.; Verma, V.; Rai, A. Development of fly ash based new Bio-Composites Material as Wood Substitute. Int. J. Mech. Prod. Eng. Res. Dev. 2017, 7, 1–6. [Google Scholar]
- Nadesan, M.S.; Dinakar, P. Mix design and properties of fly ash waste lightweight aggregates in structural lightweight concrete. Case Stud. Constr. Mater. 2017, 7, 336–347. [Google Scholar] [CrossRef]
- Rudić, O.; Ducman, V.; Malešev, M.; Radonjanin, V.; Draganić, S.; Šupić, S.; Radeka, M. Aggregates Obtained by Alkali Activation of Fly Ash: The Effect of Granulation, Pelletization Methods and Curing Regimes. Materials 2019, 12, 776. [Google Scholar] [CrossRef] [Green Version]
- Valeev, D.; Kunilova, I.; Alpatov, A.; Varnavskaya, A.; Ju, D. Magnetite and Carbon Extraction from Coal Fly Ash Using Magnetic Separation and Flotation Methods. Minerals 2019, 9, 320. [Google Scholar] [CrossRef] [Green Version]
- Brännvall, E.; Kumpiene, J. Fly ash in landfill top covers—A review. Environ. Sci. Process. Impacts 2016, 18, 11–21. [Google Scholar] [CrossRef]
- Ahmed, S.; Saurikhia, A.; Haleem, A.; Gangopadhyay, S. Geographical spread of fly ash generation and residual potential for its utilization in India. Int. J. Innov. Res. Rev. 2016, 4, 8–19. [Google Scholar]
- Namkane, K.; Naksata, W.; Thiansem, S.; Sooksamiti, P.; Arqueropanyo, O.-A. Utilization of coal bottom ash as raw material for production of ceramic floor tiles. Environ. Earth Sci. 2016, 75, 386. [Google Scholar] [CrossRef]
- Dwivedi, A.; Jain, M. Fly ash—Waste management and overview: A Review. Recent Res. Sci. Technol. 2014, 6, 30–35. [Google Scholar]
- Ghosal, S.; Self, S.A. Particle size-density relation and cenosphere content of coal fly ash. Fuel 1995, 74, 522–529. [Google Scholar] [CrossRef]
- Amin, N.U. A multi-directional utilization of different ashes. RSC Adv. 2014, 4, 62769–62788. [Google Scholar] [CrossRef]
- Suriyanarayanan, N.; Nithin, K.K.; Bernardo, E. Mullite glass ceramics production from coal fly ash and alumina by high temperture plasma. J. Non-Oxide Glasses 2009, 1, 247–260. [Google Scholar]
- Zhao, Y.; Zhang, J.; Tian, C.; Li, H.; Shao, X.; Zheng, C. Mineralogy and Chemical Composition of High-Calcium Fly Ashes and Density Fractions from a Coal-Fired Power Plant in China. Energy Fuels 2010, 24, 834–843. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Kim, A.G. Fly ash characterization by SEM–EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Ashrafi, F. Synthesis of alumina nano powder using sol-gel method and chelate precursor. Asian Acad. Res. J. Multidiscip. 2015, 2, 2319–2801. [Google Scholar]
- Song, X.L.; Qu, P.; Yang, H.P.; He, X.; Qiu, G.Z. Synthesis of γ-Al2O3 nanoparticles by chemical precipitation method. J. Cent. South Univ. Technol. 2005, 12, 536–541. [Google Scholar] [CrossRef]
- Narayanan, M.V.; Rakesh, S.G. Synthesis of colloidal alumina nanoparticles using green method. IOP Conf. Ser. Mater. Sci. Eng. 2018, 402, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Itskos, G.; Koutsianos, A.; Koukouzas, N.; Vasilatos, C. Zeolite development from fly ash and utilization in lignite mine-water treatment. Int. J. Miner. Process. 2015, 139, 43–50. [Google Scholar] [CrossRef]
- Park, H.C.; Park, Y.J.; Stevens, R. Synthesis of alumina from high purity alum derived from coal fly ash. Mater. Sci. Eng. A 2004, 367, 166–170. [Google Scholar] [CrossRef]
- Liang, G.; Li, Y.; Yang, C.; Zi, C.; Zhang, Y.; Hu, X.; Zhao, W. Production of biosilica nanoparticles from biomass power plant fly ash. Waste Manag. 2020, 105, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Kgabi, N.A.; Waanders, F.B.; Taole, S.H. Iron-Sulphur Compounds of South African Coal. Eur. J. Sci. Res. 2009, 34, 190–195. [Google Scholar]
- Xie, P.; Song, H.; Wei, J.; Li, Q. Mineralogical Characteristics of Late Permian Coals from the Yueliangtian Coal Mine, Guizhou, Southwestern China. Minerals 2016, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Waanders, F.; Vinken, E.; Mans, A.; Mulaba-Bafubiandi, A. Iron Minerals in Coal, Weathered Coal and Coal Ash—SEM and Mössbauer Results. Hyperfine Interact. 2003, 148–149, 21–29. [Google Scholar] [CrossRef]
- Biggs, D.L.; Lindsay, C.G. High-Temperature Interactions among Minerals Occurring in Coal. In Mineral Matter and Ash in Coal; American Chemical Society: Washington, DC, USA, 1986; Volume 301, pp. 128–137. [Google Scholar]
- Waanders, F.B.; Mohamed, W.; Wagner, N.J. Changes of pyrite and pyrrhotite in coal upon microwave treatment. J. Phys. Conf. Ser. 2010, 217, 012051. [Google Scholar] [CrossRef]
- Cohn, C.A.; Laffers, R.; Simon, S.R.; O’Riordan, T.; Schoonen, M.A.A. Role of pyrite in formation of hydroxyl radicals in coal: Possible implications for human health. Part. Fibre Toxicol. 2006, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- de Aldecoa, A.L.; Roldan, F.V.; Menor-Salvan, C. Natural pyrrhotite as a catalyst in prebiotic chemical evolution. Life 2013, 3, 502–517. [Google Scholar] [CrossRef] [Green Version]
- Querol, X.; Alastuey, A.; Chinchon, J.S.; Turiel, J.F.; Soler, A.L. Determination of pyritic sulphur and organic matter contents in Spanish subbituminous coals by X-ray power diffraction. Int. J. Coal Geol. 1993, 22, 279–293. [Google Scholar] [CrossRef]
- Laita, E.; Bauluz, B.; Yuste, A. High-Temperature Mineral Phases Generated in Natural Clinkers by Spontaneous Combustion of Coal. Minerals 2019, 9, 213. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Tang, S.; Zhang, S. Geochemical and Mineralogical Characteristics of the Middle Jurassic Coals from the Tongjialiang Mine in the Northern Datong Coalfield, Shanxi Province, China. Minerals 2019, 9, 184. [Google Scholar] [CrossRef] [Green Version]
- Spears, D.A.; Caswell, S.A. Mineral matter in coals: Cleat minerals and their origin in some coals from the english midlands. Int. J. Coal Geol. 1986, 6, 107–125. [Google Scholar] [CrossRef]
- Lin, Z.; Li, P.; Hou, D.; Kuang, Y.; Wang, G. Aggregation Mechanism of Particles: Effect of Ca2+ and Polyacrylamide on Coagulation and Flocculation of Coal Slime Water Containing Illite. Minerals 2017, 7, 30. [Google Scholar] [CrossRef]
- Giménez-García, R.; Vigil de la Villa Mencía, R.; Rubio, V.; Frías, M. The Transformation of Coal-Mining Waste Minerals in the Pozzolanic Reactions of Cements. Minerals 2016, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Kholodov, V.N.; Butuzova, G.Y. Siderite formation and evolution of sedimentary iron ore deposition in the Earth’s history. Geol. Ore Depos. 2008, 50, 299. [Google Scholar] [CrossRef]
- O’Brien, G.; Firth, B.; Adair, B. The Application of the Coal Grain Analysis Method to Coal Liberation Studies. Int. J. Coal Prep. Util. 2011, 31, 96–111. [Google Scholar] [CrossRef]
- Sokol, E.; Kalugin, V.; Nigmatulina, E.N.; Volkova, N.; Frenkel, A.E.; Maksimova, N.V. Ferrospheres from fly ashes of Chelyabinsk coals: Chemical composition, morphology and formation conditions. Fuel 2002, 81, 867–876. [Google Scholar] [CrossRef]
- Yamaura, M.; Fungaro, D.A. Synthesis and characterization of magnetic adsorbent prepared by magnetite nanoparticles and zeolite from coal fly ash. J. Mater. Sci. 2013, 48, 5093–5101. [Google Scholar] [CrossRef]
- Lu, S.G.; Chen, Y.Y.; Shan, H.D.; Bai, S.Q. Mineralogy and heavy metal leachability of magnetic fractions separated from some Chinese coal fly ashes. J. Hazard. Mater. 2009, 169, 246–255. [Google Scholar] [CrossRef]
- Joseph, A.; Snellings, R.; Van den Heede, P.; Matthys, S.; De Belie, N. The Use of Municipal Solid Waste Incineration Ash in Various Building Materials: A Belgian Point of View. Materials 2018, 11, 141. [Google Scholar] [CrossRef] [Green Version]
- Bayukov, O.A.; Anshits, N.N.; Balaev, A.D.; Sharonova, O.M.; Petrov, M.I.; Rabchevskii, E.V.; Anshits, A.G. Mossbauer and magnetic study of microspheres extracted from fly ashes of power stations. Phys. Met. Metallogr. 2006, 102, S53–S56. [Google Scholar] [CrossRef]
- Shoumkova, A.S. Magnetic separation of coal fly ash from Bulgarian power plants. Waste Manag. Res. 2010, 29, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Shoumkova, A.S. Physico-chemical characterization and magnetic separation of coal fly ashes from “varna”, “bobov Dol” and “maritza- Istok” power plants, Bulgaria, i—Phisico-chemical characteristics. J. Univ. Chem. Technol. Metall. 2006, 41, 175–180. [Google Scholar]
- Xue, Q.-F.; Lu, S.-G. Microstructure of ferrospheres in fly ashes: SEM, EDX and ESEM analysis. J. Zhejiang Univ. Sci. A Appl. Phys. Eng. 2008, 9, 1595–1600. [Google Scholar] [CrossRef]
- Oliveira, M.; Marostega, F.; Taffarel, S.; Saikia, B.K.; Waanders, F.; Martinello, K.; Baruah, B.P.; Silva, L. Nano-mineralogical investigation of coal and fly ashes from coal-based captive power plant (India): An introduction of occupational health hazards. Sci. Total Environ. 2013, 468–469, 1128–1137. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Anshits, N.N.; Kushnerova, O.A.; Akimochkina, G.V.; Kukhtetskiy, S.V.; Anshits, A.G. Separation of Nonmagnetic Fine Narrow Fractions of PM10 from Coal Fly Ash and Their Characteristics and Mineral Precursors. Energy Fuels 2019, 33, 3584–3593. [Google Scholar] [CrossRef]
- Devasahayam, S.; Raman, R.K.; Chennakesavulu, K.; Bhattacharya, S. Plastics—Villain or Hero? Polymers and Recycled Polymers in Mineral and Metallurgical Processing—A Review. Materials 2019, 12, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vereshchagin, S.; Kondratenko, E.; Rabchevskii, E.; Anshits, N.; Solov’ev, L.A.; Anshits, A.G. New approach to the preparation of catalysts for the oxidative coupling of methane. Kinet. Catal. 2012, 53, 449–455. [Google Scholar] [CrossRef]
- Saraswat, M.; Musante, L.; Ravidá, A.; Shortt, B.; Byrne, B.; Holthofer, H. Preparative Purification of Recombinant Proteins: Current Status and Future Trends. BioMed Res. Int. 2013, 2013, 18. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Uphade, B.S.; Mulla, S.A.R. Oxidative Coupling of Methane over a Sr-Promoted La2O3 Catalyst Supported on a Low Surface Area Porous Catalyst Carrier. Ind. Eng. Chem. Res. 1997, 36, 3594–3601. [Google Scholar] [CrossRef]
- Anshits, N.N.; Fedorchak, M.A.; Fomenko, E.V.; Mazurova, E.V.; Anshits, A.G. Composition, Structure, and Formation Routes of Blocklike Ferrospheres Separated from Coal and Lignite Fly Ashes. Energy Fuels 2020, 34, 3743–3754. [Google Scholar] [CrossRef]
- Anshits, A.G.; Bayukov, O.A.; Kondratenko, E.V.; Anshits, N.N.; Pletnev, O.N.; Rabchevskii, E.V.; Solovyov, L.A. Catalytic properties and nature of active centers of ferrospheres in oxidative coupling of methane. Appl. Catal. A Gen. 2016, 524, 192–199. [Google Scholar] [CrossRef]
- Gomes, S.; François, M.; Abdelmoula, M.; Refait, P.; Pellissier, C.; Evrard, O. Characterization of magnetite in silico-aluminous fly ash by SEM, TEM, XRD, magnetic susceptibility, and Mössbauer spectroscopy. Cem. Concr. Res. 1999, 29, 1705–1711. [Google Scholar] [CrossRef]
- Yadav, V.K.; Fulekar, M.H. Isolation and Charcterization of Iron Nanoparticles From Coal Fly Ash From Gandhinagar (Gujarat)Thermal Power Plant (A Mechanical Method of Isolation). Int. J. Eng. Res. Technol. 2014, 3, 471–479. [Google Scholar]
- Orwat, K.; Bernard, P.; Migdał-Mikuli, A. Obtaining and Investigating Amphoteric Properties of Aluminum Oxide in a Hands-On Laboratory Experiment for High School Students. J. Chem. Educ. 2016, 93, 906–909. [Google Scholar] [CrossRef]
- Kelmers, A.D.; Canon, R.M.; Egan, B.Z.; Felker, L.K.; Gilliam, T.M.; Jones, G.; Owen, G.D.; Seeley, F.G.; Watson, J.S. Chemistry of the direct acid leach, calsinter, and pressure digestion-acid leach methods for the recovery of alumina from fly ash. Resour. Conserv. 1982, 9, 271–279. [Google Scholar] [CrossRef]
- Jung, J.; Park, H.; Stevens, R. Mullite ceramics derived from coal fly ash. J. Mater. Sci. Lett. 2001, 20, 1089–1091. [Google Scholar] [CrossRef]
- Dos Santos, R.P.; Martins, J.; Gadelha, C.; Cavada, B.; Albertini, A.V.; Arruda, F.; Vasconcelos, M.; Teixeira, E.; Alves, F.; Filho, J.L.; et al. Coal fly ash ceramics: Preparation, characterization, and use in the hydrolysis of sucrose. Sci. World J. 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Peng, J.; Zhang, L.; Wang, S.; Huang, S.; He, S. Extraction of Aluminum from Coal Fly Ash by Alkali Activation with Microwave Heating. J. Residuals Sci. Technol. 2016, 13, S181–S187. [Google Scholar] [CrossRef]
- Su, S.Q.; Yang, J.; Ma, H.W.; Jiang, F.; Liu, Y.Q.; Li, G. Preparation of Ultrafine Aluminum Hydroxide from Coal Fly Ash by Alkali Dissolution Process. Integr. Ferroelectr. 2011, 128, 155–162. [Google Scholar] [CrossRef]
- Li, H.; Hui, J.; Wang, C.; Bao, W.; Sun, Z. Extraction of alumina from coal fly ash by mixed-alkaline hydrothermal method. Hydrometallurgy 2014, 147–148, 183–187. [Google Scholar] [CrossRef]
- Matjie, R.H.; Bunt, J.R.; van Heerden, J.H.P. Extraction of alumina from coal fly ash generated from a selected low rank bituminous South African coal. Miner. Eng. 2005, 18, 299–310. [Google Scholar] [CrossRef]
- Qin, J.; Gu, S. Process for Recovery of Silica Followed by Alumina from Coal Fly Ash. U.S. Patent 7,871,583, 18 January 2011. [Google Scholar]
- Rampou, M.; Ndlovu, S.; Shemi, A. Purification of Coal Fly Ash Leach Liquor for Alumina Recovery Using an Integrated Precipitation and Solvent Extraction Process. J. Sustain. Metall. 2017, 3, 782–792. [Google Scholar] [CrossRef]
- Boudreault, R.; Fournier, J.; Dumont, H.; Samuel, J.F.; Bouffard, J.; Lepage, S.; Huard, A.C.; Gravel-Rouleau, C.; Labrecque-Gilbert, M.M. Methods for Purifying Aluminium Ions. U.S. Patent 9,534,274, 3 January 2017. [Google Scholar]
- Sibanda, V.; Ndlovu, S.; Dombo, G.; Shemi, A.; Rampou, M. Towards the Utilization of Fly Ash as a Feedstock for Smelter Grade Alumina Production: A Review of the Developments. J. Sustain. Metall. 2016, 2, 167–184. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liang, Z.; Sun, F. Recovery of Alumina from Coal Fly Ash by CaCl2 Calcination Followed by H2SO4 Leaching. J. Environ. Anal. Toxicol. 2017, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.-C.; Zhai, Y.-C.; Ning, Z.-Q. Thermodynamics and kinetics of alumina extraction from fly ash using an ammonium hydrogen sulfate roasting method. Int. J. Miner. Metall. Mater. 2014, 21, 144–149. [Google Scholar] [CrossRef]
- Park, J.; Son, Y.; Noh, S.; Bong, T. An Evaluation of the Environmental Safety and Geochemical Characteristics of Coal Combustion Products. KSCE J. Civ. Eng. 2017, 22, 1–9. [Google Scholar] [CrossRef]
- Wang, R.-C.; Zhai, Y.-C.; Wu, X.-W.; Ning, Z.-Q.; Pei, H. Extraction of alumina from fly ash by ammonium hydrogen sulfate roasting technology. Trans. Nonferr. Met. Soc. China 2014, 24, 1596–1603. [Google Scholar] [CrossRef]
- Nayak, N.; Panda, C. Aluminium extraction and leaching characteristics of Talcher Thermal Power Station fly ash with sulphuric acid. Fuel 2010, 89, 53–58. [Google Scholar] [CrossRef]
- Li, L.S.; Wu, Y.S.; Liu, Y.Y.; Zhai, Y.C. Extraction of alumina from coal fly ash with sulfuric acid leaching method. Chin. J. Process Eng. 2011, 11, 255–258. [Google Scholar]
- Shi, Z.; Bonneville, S.C.; Krom, M.; Carslaw, K.; Jickells, T.; Baker, A.; Benning, L. Iron dissolution kinetics of mineral dust at low pH during simulated atmospheric processing. Atmos. Chem. Phys. 2011, 11, 995–1007. [Google Scholar] [CrossRef] [Green Version]
- Bai, G.; Teng, W.; Wang, X.; Zhang, H.; Xu, P. Processing and kinetics studies on the alumina enrichment of coal fly ash by fractionating silicon dioxide as nano particles. Fuel Process. Technol. 2010, 91, 175–184. [Google Scholar] [CrossRef]
- Wu, C.; Yu, H.-F.; Zhang, H.-F. Extraction of aluminum by pressure acid-leaching method from coal fly ash. Trans. Nonferr. Met. Soc. China 2012, 22, 2282–2288. [Google Scholar] [CrossRef]
- Shemi, A.; Ndlovu, S.; Sibanda, V.; van Dyk, L. Extraction of alumina from coal fly ash using an acid leach-sinter-acid leach technique. Hydrometallurgy 2015, 157, 348–355. [Google Scholar] [CrossRef]
- Valeev, D.; Shoppert, A.; Mikhailova, A.; Kondratiev, A. Acid and Acid-Alkali Treatment Methods of Al-Chloride Solution Obtained by the Leaching of Coal Fly Ash to Produce Sandy Grade Alumina. Metals 2020, 10, 585. [Google Scholar] [CrossRef]
- Das, A.; Mishra, A.K. Role of Thiobacillus ferrooxidans and sulphur (sulphide)-dependent ferric-ion-reducing activity in the oxidation of sulphide minerals. Appl. Microbiol. Biotechnol. 1996, 45, 377–382. [Google Scholar] [CrossRef]
- Wang, R.; Lin, J.-Q.; Liu, X.-M.; Pang, X.; Zhang, C.-J.; Yang, C.-L.; Gao, X.-Y.; Lin, C.-M.; Li, Y.-Q.; Li, Y.; et al. Sulfur Oxidation in the Acidophilic Autotrophic Acidithiobacillus spp. Front. Microbiol. 2019, 9, 3290. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.; Servulo, E.; da Costa, A.C.; Ferreira, D.; Godoy, M.; Oliveira, F. Bioleaching of metals from a spent diesel hydrodesulfurization catalyst employing Acidithiobacillus thiooxidans FG-01. Braz. J. Chem. Eng. 2017, 34, 119–129. [Google Scholar] [CrossRef]
- Koschorreck, M. Microbial sulphate reduction at a low pH. FEMS Microbiol. Ecol. 2008, 64, 329–342. [Google Scholar] [CrossRef]
- Kimura, S.; Johnson, D. Sulfidogenesis in Low pH (3.8–4.2) Media by a Mixed Population of Acidophilic Bacteria. Biodegradation 2006, 17, 159–167. [Google Scholar] [CrossRef]
- Monrroy, M.; Rueda, L.; Aparicio, A.; García, J. Fermentation of Musa paradisiaca Peels to Produce Citric Acid. J. Chem. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ferreira-Guedes, S.; Mendes, B.; Leitão, A.L. Degradation of 2,4-dichlorophenoxyacetic acid by a halotolerant strain of Penicillium chrysogenum: Antibiotic production. Environ. Technol. 2012, 33, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Bazgha, A.; Haq, N.B.; Sadia, I. Bio-extraction of metal ions from laterite ore by Penicillium chrysogenum. Afr. J. Biotechnol. 2011, 10, 11196–11205. [Google Scholar] [CrossRef]
- Bosshard, P.P.; Bachofen, R.; Brandl, H. Metal Leaching of Fly Ash from Municipal Waste Incineration by Aspergillus niger. Environ. Sci. Technol. 1996, 30, 3066–3070. [Google Scholar] [CrossRef]
- Khan, A.H.; Karuppayil, S.M. Fungal pollution of indoor environments and its management. Saudi J. Biol. Sci. 2012, 19, 405–426. [Google Scholar] [CrossRef] [Green Version]
- Vachon, P.; Tyagi, R.D.; Auclair, J.C.; Wilkinson, K.J. Chemical and biological leaching of aluminum from red mud. Environ. Sci. Technol. 1994, 28, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.-J.; Ting, Y.-P. Optimisation on bioleaching of incinerator fly ash by Aspergillus niger—Use of central composite design. Enzym. Microb. Technol. 2004, 35, 444–454. [Google Scholar] [CrossRef]
- Xu, T.-J.; Ramanathan, T.; Ting, Y.-P. Bioleaching of incineration fly ash by Aspergillus niger—Precipitation of metallic salt crystals and morphological alteration of the fungus. Biotechnol. Rep. 2014, 3, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.-J.; Ting, Y.-P. Fungal bioleaching of incineration fly ash: Metal extraction and modeling growth kinetics. Enzym. Microb. Technol. Enzym. Microb. Technol. 2009, 44, 323–328. [Google Scholar] [CrossRef]
- Krishna, P.; Babu, A.G.; Reddy, M.S. Bacterial diversity of extremely alkaline bauxite residue site of alumina industrial plant using culturable bacteria and residue 16S rRNA gene clones. Extremophiles 2014, 18, 665–676. [Google Scholar] [CrossRef]
- Argyle, M.; Bartholomew, C. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef] [Green Version]
- Hassanpour, P.; Panahi, Y.; Ebrahimi-Kalan, A.; Akbarzadeh, A.; Davaran, S.; Nasibova, A.N.; Khalilov, R.; Kavetskyy, T. Biomedical applications of aluminium oxide nanoparticles. Micro Nano Lett. 2018, 13, 1227–1231. [Google Scholar] [CrossRef]
- Hakuta, Y.; Nagai, N.; Suzuki, Y.H.; Kodaira, T.; Bando, K.K.; Takashima, H.; Mizukami, F. Preparation of α-alumina nanoparticles with various shapes via hydrothermal phase transformation under supercritical water conditions. IOP Conf. Ser. Mater. Sci. Eng. 2013, 47, 012045. [Google Scholar] [CrossRef]
- Daimatsu, K.; Sugimoto, H.; Kato, Y.; Nakanishi, E.; Inomata, K.; Amekawa, Y.; Kazuki, T. Preparation and physical properties of flame retardant acrylic resin containing nano-sized aluminum hydroxide. Polym. Degrad. Stab. 2007, 92, 1433–1438. [Google Scholar] [CrossRef]
- Suchanek, W.L. Hydrothermal Synthesis of Alpha Alumina (α-Al2O3) Powders: Study of the Processing Variables and Growth Mechanisms. J. Am. Ceram. Soc. 2010, 93, 399–412. [Google Scholar] [CrossRef]
- Matori, K.A.; Wah, L.C.; Hashim, M.; Ismail, I.; Zaid, M.H.M. Phase Transformations of α-Alumina Made from Waste Aluminum via a Precipitation Technique. Int. J. Mol. Sci. 2012, 13, 16812–16821. [Google Scholar] [CrossRef] [Green Version]
- Ilyas, S.; Pendyala, R.; Marneni, N. Settling Characteristics of Alumina Nanoparticles in Ethanol-Water Mixtures. Appl. Mech. Mater. 2013, 372, 143–148. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, F.L. Extracting alumina from fly ash by soda lime sintering method. Min. Metall. Eng. 2008, 28, 73–75. [Google Scholar]
- Zhu, P.-W.; Dai, H.; Han, L.; Xu, X.-L.; Cheng, L.-M.; Wang, Q.-H.; Shi, Z.-L. Aluminum extraction from coal ash by a two-step acid leaching method. J. Zhejiang Univ. Sci. A 2015, 16, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Dong, J.; Guo, F.; Shao, Z.; Wu, J. Zeolite Synthesized from Coal Fly Ash Produced by a Gasification Process for Ni2+ Removal from Water. Minerals 2018, 8, 116. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, S.; Banerjee, D.K. Speciation of some heavy metals in coal fly ash. Chem. Speciat. Bioavailab. 2015, 19, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Falayi, T.; Okonta, F.N.; Ntuli, F. Desilication of fly ash and development of lightweight construction blocks from alkaline activated desilicated fly ash. Int. J. Environ. Waste Manag. 2017, 20, 233–253. [Google Scholar] [CrossRef]
- Piekos, R.; Paslawska, S. Leaching characteristics of flouride from coal fly ash. Digit. Arch. Fluoride J. 1988, 31, 188–192. [Google Scholar]
- Yadav, V.K.; Fulekar, M.H. Green synthesis and characterization of amorphous silica nanoparticles from fly ash. Mater. Today Proc. 2019, 18, 4351–4359. [Google Scholar] [CrossRef]
- Adnan, M.; Shah, Z.; Fahad, S.; Arif, M.; Alam, M.; Khan, I.A.; Mian, I.A.; Basir, A.; Ullah, H.; Arshad, M.; et al. Phosphate-Solubilizing Bacteria Nullify the Antagonistic Effect of Soil Calcification on Bioavailability of Phosphorus in Alkaline Soils. Sci. Rep. 2017, 7, 16131. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Waqas, M.; Shahzad, R.; You, Y.-H.; Asaf, S.; Khan, M.A.; Lee, K.-E.; Joo, G.-J.; Kim, S.-J.; Lee, I.-J. Isolation and characterization of a novel silicate-solubilizing bacterial strain Burkholderia eburnea CS4-2 that promotes growth of japonica rice (Oryza sativa L. cv. Dongjin). Soil Sci. Plant Nutr. 2017, 63, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Xu, G.; Ma, P.; Lin, Y.; Yang, X.; Cao, C. Isolation and Characterization of a Phosphorus-Solubilizing Bacterium from Rhizosphere Soils and Its Colonization of Chinese Cabbage (Brassica campestris ssp. chinensis). Front. Microbiol. 2017, 8, 1270. [Google Scholar] [CrossRef]
- Janczura, E.; Perkins, H.R.; Rogers, H.J. Teichuronic acid: A mucopolysaccharide present in wall preparations from vegetative cells of Bacillus subtilis. Biochem. J. 1961, 80, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Zhan, S.; Liu, J.; Chen, Y.; Sun, D. Single and Coorperative Bauxite Bioleaching by Silicate Bacteria. Ieri Procedia 2013, 5, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.A.; Uddin, I.; Moeez, S.; Ahmad, A. Fungus-Mediated Preferential Bioleaching of Waste Material Such as Fly—Ash as a Means of Producing Extracellular, Protein Capped, Fluorescent and Water Soluble Silica Nanoparticles. PLoS ONE 2014, 9, e0107597. [Google Scholar] [CrossRef]
- Nakamura, L.K.; Swezey, J. Taxonomy of Bacillus circulans Jordan 1890: Base Composition and Reassociation of Deoxyribonucleic Acid. Int. J. Syst. Evol. Microbiol. 1983, 33, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2009, 156, 609–643. [Google Scholar] [CrossRef] [PubMed]
- Bahira, S.; Raut, S.; Sharma, D. Bioleaching of Alumina from low grade Indian bauxite (41% Al2O3) by indigenous bacteria with reference to pH, Time and Carbon source. Int. Res. J. Environ. Sci. 2018, 7, 20–27. [Google Scholar]
- Vrvic, M.; Matić, V.; Vučetić, J.; Vitorović, D. Demineralization of an oil shale by Bacillus circulans (“siliceous bacteria”). Org. Geochem. 1990, 16, 1203–1209. [Google Scholar] [CrossRef]
- Savostin, P. Microbial transformation of silicates. Z. Für Pflanz. Und Bodenkd. 1972, 132, 37–45. [Google Scholar] [CrossRef]
- Groudev, S. Biobeneficiation of mineral raw material. In Mineral Biotechnology: Microbial Aspects of Mineral Beneficiation, Metal Extraction, and Environmental Control; Kawatra, S.K., Natarajan, K.A., Eds.; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2001; Volume 16, pp. 37–54. [Google Scholar]
- Li, C.; Wang, X.; Jiao, Z.; Zhang, Y.; Yin, X.; Cui, X.; Wei, Y. Functionalized Porous Silica-Based Nano/Micro Particles for Environmental Remediation of Hazard Ions. Nanomaterials 2019, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Salata, O.V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Asefa, T.; Tao, Z. Biocompatibility of Mesoporous Silica Nanoparticles. Chem. Res. Toxicol. 2012, 25, 2265–2284. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, W.; Tan, W. Bioconjugated Silica Nanoparticles: Development and Applications. Nano Res. 2008, 1, 99–115. [Google Scholar] [CrossRef] [Green Version]
- Bae, S.W.; Tan, W.; Hong, J.-I. Fluorescent dye-doped silica nanoparticles: New tools for bioapplications. Chem. Commun. 2012, 48, 2270–2282. [Google Scholar] [CrossRef]
- Luigi, P.; Antonella, L.; Diego, S.; Sebastiano, A.; Catia, M. Mesoporous Silica Nanoparticles in Cancer Therapy: Relevance of the Targeting Function. Mini-Rev. Med. Chem. 2016, 16, 743–753. [Google Scholar] [CrossRef]
- Rosenholm, J.; Mamaeva, V.; Sahlgren, C.; Lindén, M. Nanoparticles in targeted cancer therapy: Mesoporous silica nanoparticles entering preclinical development stage. Nanomed. (Lond. Engl.) 2012, 7, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Enrichi, F.; Ricco, R.; Meneghello, A.; Pierobon, R.; Canton, G.; Cretaio, E. Enhancing the Sensitivity of DNA Microarray Using Dye-Doped Silica Nanoparticles: Detection of Human Papilloma Virus. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2010; Volume 1275, pp. 154–157. [Google Scholar]
- Wang, L.; Tan, W. Multicolor FRET silica nanoparticles by single wavelength excitation. Nano Lett. 2006, 6, 84–88. [Google Scholar] [CrossRef] [Green Version]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Farrell, N.; Houlton, A.; Horrocks, B.R. Silicon nanoparticles: Applications in cell biology and medicine. Int. J. Nanomed. 2006, 1, 451–472. [Google Scholar] [CrossRef]
- Ahmad, I.Z.; Ahmad, A.; Tabassum, H.; Kuddus, M. Applications of Nanoparticles in the Treatment of Wastewater. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 275–299. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, L.; Tan, W. Fluorescent Nanoparticle for Bacteria and DNA Detection. In Bio-Applications of Nanoparticles; Chan, W.C.W., Ed.; Springer: New York, NY, USA, 2007; pp. 129–135. [Google Scholar] [CrossRef]
- Chitra, K.; Annadurai, G. Fluorescent Silica Nanoparticles in the Detection and Control of the Growth of Pathogen. J. Nanotechnol. 2013, 2013, 7. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Lu, H.; Wang, J.; Stoller, M.; Wang, T.; Bao, Y.; Hao, H. An Overview of Nanomaterials for Water and Wastewater Treatment. Adv. Mater. Sci. Eng. 2016, 2016, 10. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Keswani, C.; Abhilash, P.C.; Fraceto, L.F.; Singh, H.B. Integrated Approach of Agri-nanotechnology: Challenges and Future Trends. Front. Plant Sci. 2017, 8, 471. [Google Scholar] [CrossRef] [Green Version]
- Kaushal, A.; Singh, S. Removal of heavy metals by nanoadsorbents: A review. J. Environ. Biotechnol. Res. 2017, 6, 96–104. [Google Scholar]
- Agarwal, P.; Gupta, R.; Agarwal, N. Advances in Synthesis and Applications of Microalgal Nanoparticles for Wastewater Treatment. J. Nanotechnol. 2019, 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.; Liang, K.; Qin, Y.; Ma, H.; Zhao, X.; Zhang, L.; Zhu, S.; Yu, Y.; Bian, D.; Yang, J. Hydrothermal Conversion of Red Mud into Magnetic Adsorbent for Effective Adsorption of Zn(II) in Water. Appl. Sci. 2019, 9, 1519. [Google Scholar] [CrossRef] [Green Version]
- Ge, J.; Yoon, S.; Choi, N. Application of Fly Ash as an Adsorbent for Removal of Air and Water Pollutants. Appl. Sci. 2018, 8, 1116. [Google Scholar] [CrossRef] [Green Version]
- Ahmaruzzaman, M.; Gupta, V.K. Rice Husk and Its Ash as Low-Cost Adsorbents in Water and Wastewater Treatment. Ind. Eng. Chem. Res. 2011, 50, 13589–13613. [Google Scholar] [CrossRef]
- Pham, T.; Bui, T.; Nguyen, V.; Bui, T.; Tran, T.; Phan, Q.; Pham, T.; Hoang, T. Adsorption of Polyelectrolyte onto Nanosilica Synthesized from Rice Husk: Characteristics, Mechanisms, and Application for Antibiotic Removal. Polymers 2018, 10, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plohl, O.; Finšgar, M.; Gyergyek, S.; Ajdnik, U.; Ban, I.; Zemljič, L.F. Efficient Copper Removal from an Aqueous Anvironment using a Novel and Hybrid Nanoadsorbent Based on Derived-Polyethyleneimine Linked to Silica Magnetic Nanocomposites. Nanomaterials 2019, 9, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wu, A.; Ciacchi, L.C.; Wei, G. Recent Advances in Nanoporous Membranes for Water Purification. Nanomaterials 2018, 8, 65. [Google Scholar] [CrossRef] [Green Version]
- Guerra, F.; Attia, M.; Whitehead, D.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef] [Green Version]
- Ray, P.C.; Yu, H.; Fu, P.P. Toxicity and Environmental Risks of Nanomaterials: Challenges and Future Needs. J. Environ. Sci. Health Part C 2009, 27, 1–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khin, M.M.; Nair, A.S.; Babu, V.J.; Murugan, R.; Ramakrishna, S. A review on nanomaterials for environmental remediation. Energy Environ. Sci. 2012, 5, 8075–8109. [Google Scholar] [CrossRef]
- Singh, N.; Sallem, F.; Mirjolet, C.; Nury, T.; Sahoo, S.K.; Millot, N.; Kumar, R. Polydopamine Modified Superparamagnetic Iron Oxide Nanoparticles as Multifunctional Nanocarrier for Targeted Prostate Cancer Treatment. Nanomaterials 2019, 9, 138. [Google Scholar] [CrossRef] [Green Version]
- Pham, B.; Colvin, E.; Pham, N.; Kim, B.; Fuller, E.; Moon, E.; Barbey, R.; Yuen, S.; Rickman, B.; Bryce, N.; et al. Biodistribution and Clearance of Stable Superparamagnetic Maghemite Iron Oxide Nanoparticles in Mice Following Intraperitoneal Administration. Int. J. Mol. Sci. 2018, 19, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, L.; Pessan, J.; Vieira, A.; Lima, T.; Delbem, A.; Monteiro, D. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Barahona, I.; Muñoz-Hernando, M.; Herranz, F. Microwave-Driven Synthesis of Iron-Oxide Nanoparticles for Molecular Imaging. Molecules 2019, 24, 1224. [Google Scholar] [CrossRef] [Green Version]
- Maity, D.; Agrawal, D. Synthesis of Iron Oxide Nanoparticles under Oxidizing Environment and Their Stabilization in Aqueous and Non-Aqueous Media. J. Magn. Magn. Mater. 2007, 308, 46–55. [Google Scholar] [CrossRef]
- Teja, A.S.; Koh, P.-Y. Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Asim, T.; Chen, Y.; Adil, S. Polymeric Nanocomposites of Iron–Oxide Nanoparticles (IONPs) Synthesized Using Terminalia chebula Leaf Extract for Enhanced Adsorption of Arsenic (V) from Water. Colloids Interfaces 2019, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-H.; Zhuang, Y.-N.; Wang, H.-T.; Wei, M.-F.; Ko, W.-C.; Chang, W.-J.; Way, T.-F.; Rwei, S.-P. Fabrication of Self-Healable Magnetic Nanocomposites via Diels−Alder Click Chemistry. Appl. Sci. 2019, 9, 506. [Google Scholar] [CrossRef] [Green Version]
- Dar, M.I.; Shivashankar, S.A. Single crystalline magnetite, maghemite, and hematite nanoparticles with rich coercivity. RSC Adv. 2014, 4, 4105–4113. [Google Scholar] [CrossRef]
- Nguyen, K.; Nguyen, B.; Nguyen, H.; Nguyen, H. Adsorption of Arsenic and Heavy Metals from Solutions by Unmodified Iron-Ore Sludge. Appl. Sci. 2019, 9, 619. [Google Scholar] [CrossRef] [Green Version]
- Charerntanyarak, L. Heavy metals removal by chemical coagulation and precipitation. Water Sci. Technol. 1999, 39, 135–138. [Google Scholar] [CrossRef]
- Inam, M.; Khan, R.; Park, D.; Lee, Y.-W.; Yeom, I. Removal of Sb(III) and Sb(V) by Ferric Chloride Coagulation: Implications of Fe Solubility. Water 2018, 10, 418. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Wang, Z.; Zhou, X.; Li, S. Efficient Removal of Heavy Metal Ions in Wastewater by Using a Novel Alginate-EDTA Hybrid Aerogel. Appl. Sci. 2019, 9, 547. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Hou, B.; Wang, J.; Tian, B.; Bi, J.; Wang, N.; Li, X.; Huang, X. Nanomaterials for the Removal of Heavy Metals from Wastewater. Nanomaterials 2019, 9, 424. [Google Scholar] [CrossRef] [Green Version]
- Da̧browski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Wang, Z.; Yang, Q.; Huang, R. Efficient Removal of Lead, Copper and Cadmium Ions from Water by a Porous Calcium Alginate/Graphene Oxide Composite Aerogel. Nanomaterials 2018, 8, 957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yantasee, W.; Warner, C.L.; Sangvanich, T.; Addleman, R.S.; Carter, T.G.; Wiacek, R.J.; Fryxell, G.E.; Timchalk, C.; Warner, M.G. Removal of Heavy Metals from Aqueous Systems with Thiol Functionalized Superparamagnetic Nanoparticles. Environ. Sci. Technol. 2007, 41, 5114–5119. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kong, H.; Jang, J. Adsorption of Heavy Metal Ions from Aqueous Solution by Polyrhodanine-Encapsulated Magnetic Nanoparticles. J. Colloid Interface Sci. 2011, 359, 505–511. [Google Scholar] [CrossRef]
- Chou, C.-M.; Lien, H.-L. Dendrimer-conjugated magnetic nanoparticles for removal of zinc(II) from aqueous solutions. J. Nanopart. Res. 2010, 13, 2099–2107. [Google Scholar] [CrossRef]
- Predescu, A.; Nicolae, A. Adsorption of Zn, Cu and Cd from waste waters by means of maghemite nanoparticles. UPB Sci. Bull. Ser. B Chem. Mater. Sci. 2012, 74, 255–264. [Google Scholar]
- Sheet, I.; Kabbani, A.; Holail, H. Removal of Heavy Metals Using Nanostructured Graphite Oxide, Silica Nanoparticles and Silica/Graphite Oxide Composite. Energy Procedia 2014, 50, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Karnib, M.; Kabbani, A.; Holail, H.; Olama, Z. Heavy Metals Removal Using Activated Carbon, Silica and Silica Activated Carbon Composite. Energy Procedia 2014, 50, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Yang, B.; Xiong, H.; Zhou, Y.; Xu, B.-Q.; Wang, S.-X. Selective removal of heavy metal ions from aqueous solutions with surface functionalized silica nanoparticles by different functional groups. J. Cent. South Univ. 2014, 21, 3575–3579. [Google Scholar] [CrossRef]
- Yadav, V.K.; Ali, D.; Khan, S.H.; Gnanamoorthy, G.; Choudhary, N.; Yadav, K.K.; Thai, V.N.; Hussain, S.A.; Manhrdas, S. Synthesis and Characterization of Amorphous Iron Oxide Nanoparticles by the Sonochemical Method and Their Application for the Remediation of Heavy Metals from Wastewater. Nanomaterials 2020, 10, 1551. [Google Scholar] [CrossRef] [PubMed]
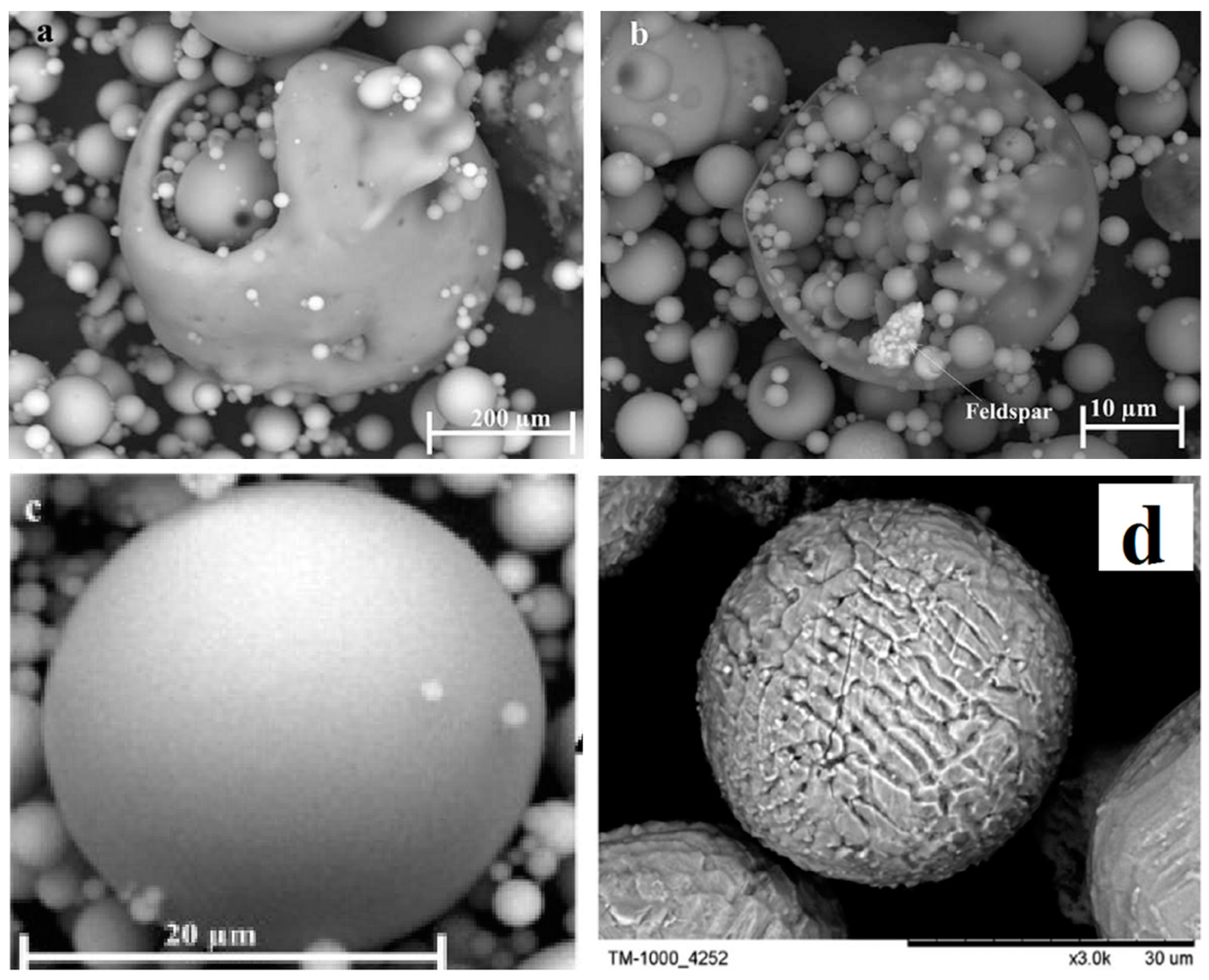
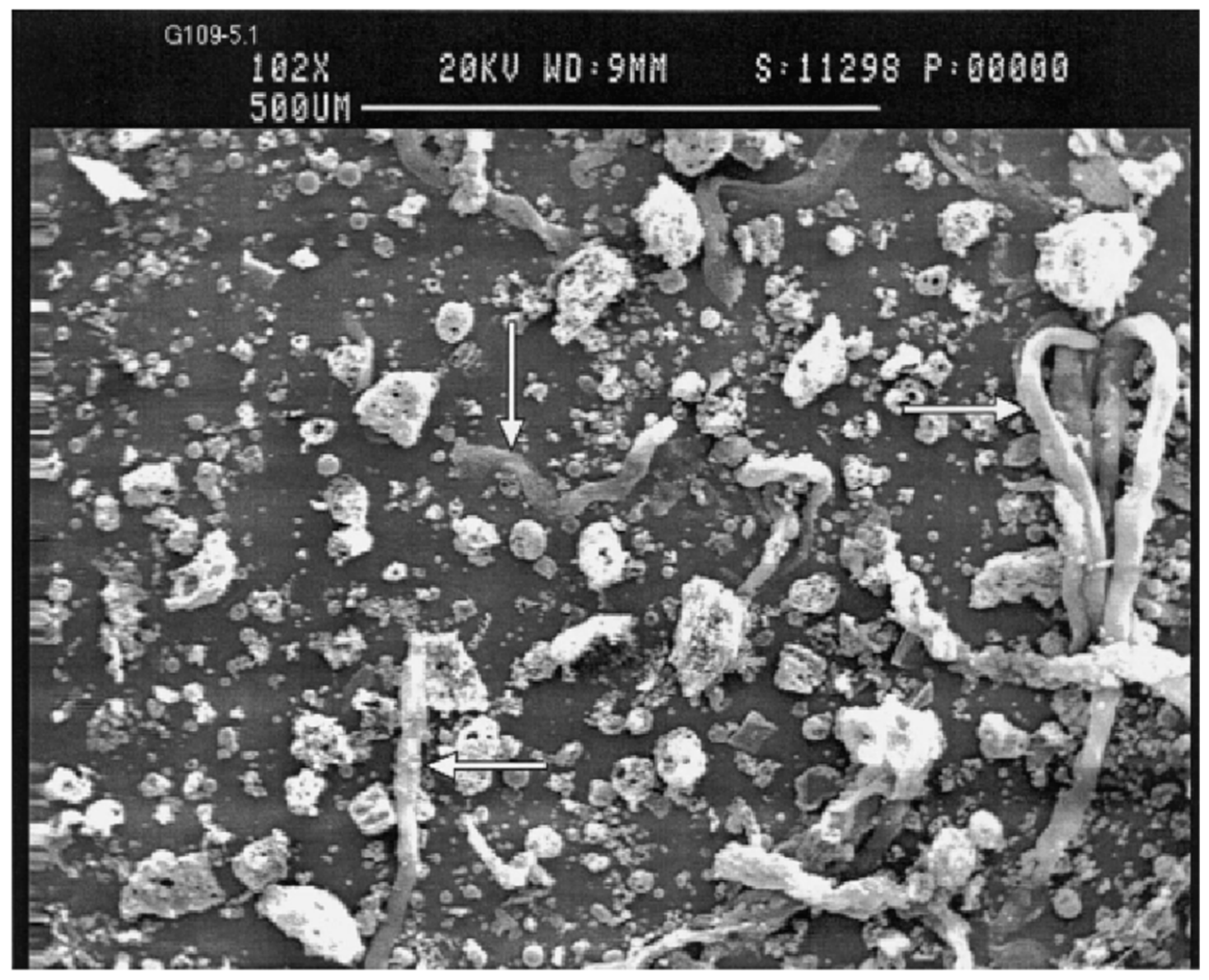





| Components | Bituminous | Subbituminous | Lignite |
|---|---|---|---|
| SiO2% | 20–60 | 40–60 | 15–45 |
| Al2O3% | 5–35 | 20–30 | 10–25 |
| Fe2O3% | 10–40 | 4–10 | 4–15 |
| CaO% | 1–12 | 5–30 | 15–40 |
| MgO% | 0–5 | 1–6 | 3–10 |
| SO3% | 0–4 | 0–2 | 0–10 |
| Na2O% | 0–4 | 0–2 | 0–6 |
| K2O% | 0–3 | 0–4 | 0–4 |
| Loss on ignition (LOI) % | 0–15 | 0–3 | 0–5 |
| Authors | Ferrous Particles | Instruments | Impurities and Findings |
|---|---|---|---|
| Gomes et al. [129] | Magnetite | Scanning Electron Microscope-Electron Diffraction Spectroscopy (SEM-EDS), X-ray Diffraction (XRD), Transmission Electron Microscope (TEM), Mossbauer spectroscopy and Vibrating sample magnetometer (VSM) | Mg has substituted Fe in the spinel structure |
| Bayukov et al. [35] | Mossbauer spectroscopy | Al, Mg and Ti were the major phases | |
| Shoumkova et al. [118] | Studied the comparative properties of magnetic and non-magnetic fractions | ||
| Olga et al. [40] | SEM-EDS | Ferrospheres of sizes 0.4 to 0.02 mm were recovered from high-calcium fly ash, SEM-EDS study | |
| Feng and Gao [120] | Magnetite and hematite | SEM-EDX and ESEM analysis | Studied the microstructures of ferrospheres in fly ashes with their detailed SEM, EDX and ESEM analysis. |
| Yadav and Fulekar [130] | Magnetite and hematite | TEM, Fourier transform infrared (FTIR) and Particle Size Analyzer (PSA) | Reported the nanosized, magnetic particles in class F fly ash from Gandhinagar, Gujarat, India. |
| Fulekar and Yadav [50] | Magnetite, hematite | TEM, XRD, SEM-EDS, Raman, FTIR, VSM, PSA | Studied the morphological, elemental and mineralogical properties of class F fly ash |
| Fulekar and Yadav [50] | Synthesized: Ferrous carbonate, Magnetite, Hematite, magnetite | TEM, XRD, SEM-EDS, Raman, FTIR, PSA | Synthesized ferrous carbonate, magnetite, maghemite and hematite by using extracted ferrous particles with high purity. |
| Authors/References | Operating Conditions | Leaching Agent | Product | Findings | Efficiency % |
|---|---|---|---|---|---|
| Park et al. [145] | CFA with ammonia in water at controlled pH followed by successive crystallization | NH4Al (SO4)2 | Alumina/alum | Alumina derived from the microwave assisted derived alum was finer powder with a high surface area | - |
| Su, S. et al.; Su, Yang [136] | Alkali- dissolution process | Ultrafine aluminum hydroxide | 2 steps:
| ~89% | |
| Huiquan Li et al. [137] | Mixed-alkaline hydrothermal method | Alumina leaching was done by mixed hydroxides of NaOH and Ca(OH)2 through the hydrothermal methods | Alumina | Al leaching was seen with increased temperature, calcium–silicon ratio and solid–liquid ratio. | 91.3% (optimized conditions) |
| Wang et al. [146] | NH4HSO4 Roasting technology | Aluminum hydroxide, Alumina | A two-step procedure in the first step Al and Fe was extracted while in the second step leached Al and Fe was precipitated with NH4HCO3 solution | - | |
| Wang et al. [146] | Ammonium hydrogen sulfate roasting technology | Alumina | Studied thermodynamics and kinetics of alumina extraction from fly ash. It was achieved when the CFA: ammonium hydrogen sulfate ratio was 1:8 mole at 673 K for 60 min | 90.5% (optimized conditions) |
| Authors/References | Operating Conditions | Leaching Agent | Product | Findings |
|---|---|---|---|---|
| Matjie et al. [138] | Mixing CFA with CaO and then calcinated at 1000–1200 °C | CaO, sulphuric acid | First calcium aluminate, Second alumina | Firstly, calcium aluminate was produced, further treated with sulphuric acid, and ~85% Al was extracted |
| Nayak and Panda 2010 [147] | Sulphuric acid based extraction of alumina and leaching behaviors from the fly ash collected | Sulphuric acid | Alumina | Reported: Not possible to get high recovery of alumina by direct acid leaching at low acid concentration and ambient temperature. Higher extraction of alumina is possible only at a higher solid: liquid ratio. Leaching of metals also depends on the nature of leaching medium, solid: liquid ratio, temperature and leaching time. |
| Shi et al. [149] | Sulphuric acid | Coarse alumina nanoparticles | Al extraction rate-87% | |
| Bai et al. [150] | Thermal decomposition—Fly ash + concentrated sulphuric acid and calcined at 300 °C, due to this, most of alumina is converted to aluminum sulfate | Sulphuric acid | Alumina Aluminum sulfate | Al extraction up to 85% |
| Wu et al. [151] | concentrated sulphuric acid + along with pressure | Alumina | Reported effect of coal size, reaction time and temperature on the Al leaching from fly ash. Pressure as well as smaller size have positive effects on the Al extraction. Al extraction efficiency was 82.4% under optimal conditions. | |
| Shemi et al. [152] | Al extraction by 6 M sulphuric acid by using acetylacetone in the gas phase. Temp: 250 °C for 6 h for optimum yields | Acetylacetone | Alumina | Application of acetylacetone in gas phase for alumina extraction. |
| Fulekar and Yadav [50] | Al extraction by 4–8 M using sulphuric acid. Temp: 125 °C for 90 min with stirring | Sulphuric acid | Alumina, Aluminum sulfate Aluminum hydroxides | Aluminum extraction was 40%. Obtained mixtures of alumina, aluminum sulfate and aluminum hydroxides with low Al content—i.e., below 15%. |
| Authors/References | Operating Conditions | Fungus Used | Product | Findings |
|---|---|---|---|---|
| Xu and Ting [165] | Citric acid, Gluconic acid | Aspergillus niger | Al in the medium | Reported that the optimal parameters for bioleaching of metals by varying CFA pulp density, spore concentration, sucrose concentration and time of addition of CFA. Leaching of Al and Fe was 12.3 ppm, which was far lower than the Zn, which was 77.6 ppm. The responsible acids for the leaching were citric acid and gluconic acid. |
| Xu and Ting [166] | Aspergillusniger | Al in the medium | Showed that the leaching concentration of metals was directly related to the citric acid productions. | |
| Fulekar and Yadav [50] | Citric acid | Aspergillus niger | Al, Al2(SO4)3 | Showed that the lesser yield of alumina present in mixtures of alumina, aluminum sulfate and Al(OH)3 |
| Authors/References | Operating Conditions | Leaching Agent | Product | Findings |
|---|---|---|---|---|
| Falayi et al. [180] | Optimum leaching parameters were time 6 h, Molarity of KOH = 3M, rpm 500, 25 S/L ratio, temperature: 100 °C | Leaching of silica by KOH | silica leachate | Found the optimal conditions of silica leaching for time, temperature, molarity of KOH |
| Wang et al. [144] | Concentrated NaOH | Studied the kinetics of silica and alumina leaching from the extracted slag of fly ash. Studied the effect of leaching temperature, stirring speed and mass ratio of NaOH to SiO2, on silica leaching rate. The silica leaching was 95.6% under the optimized conditions | ||
| Piekos and Paslawska [181] | Distilled water, sea water, synthetic sea water with the variable ratios of water and fly ash | Leaching of assimilable silicon species from fly ash | ||
| Fulekar and Yadav [50] | Temp: 90–95 °C Time: 90 min Stirring: 300–500 rpm | 4–16 M NaOH | Clustered silica nanoparticles | Amorphous, nanosilica, aggregated to form a cluster of size 20–80 nm with 90–97% purity |
| Authors | Operating Conditions | Leaching Agent | Product | Findings |
|---|---|---|---|---|
| Zhan et al. [187] | Muco-polysaccharides | Soluble silica in the medium | Studied the leaching of silica from the bauxite ores by individual bacteria as well as in co-operation. | |
| Mixed culture leached more silica from the solution in comparison to the individual. | ||||
| Khan et al. [188] | Synthesized silica nanoparticles from fly ash by using fungus F. oxysporum. | |||
| The purity of the biologically synthesized silica was up to 40% only with more than 50% as carbon | ||||
| Fulekar and Yadav [50] | Incubation at required temperatures | F. oxysporum: oxalic acid B. circulans—EPS, MPS | Synthesized amorphous 30–80 nm, aggregated, clustered silica nanoparticles using F. oxysporum supernatant and sodium silicate from fly ash | |
| Synthesized amorphous 60–120 nm porous nanosheets by using B. circulans supernatant and sodium silicate from fly ash |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, V.K.; Fulekar, M.H. Advances in Methods for Recovery of Ferrous, Alumina, and Silica Nanoparticles from Fly Ash Waste. Ceramics 2020, 3, 384-420. https://0-doi-org.brum.beds.ac.uk/10.3390/ceramics3030034
Yadav VK, Fulekar MH. Advances in Methods for Recovery of Ferrous, Alumina, and Silica Nanoparticles from Fly Ash Waste. Ceramics. 2020; 3(3):384-420. https://0-doi-org.brum.beds.ac.uk/10.3390/ceramics3030034
Chicago/Turabian StyleYadav, Virendra Kumar, and Madhusudan Hiraman Fulekar. 2020. "Advances in Methods for Recovery of Ferrous, Alumina, and Silica Nanoparticles from Fly Ash Waste" Ceramics 3, no. 3: 384-420. https://0-doi-org.brum.beds.ac.uk/10.3390/ceramics3030034






