Dense MgB2 Ceramics by Ultrahigh Pressure Field-Assisted Sintering
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
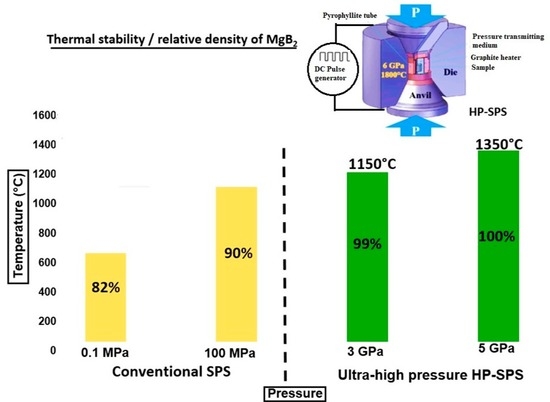
3.1. X-ray Diffraction and Density Analyses
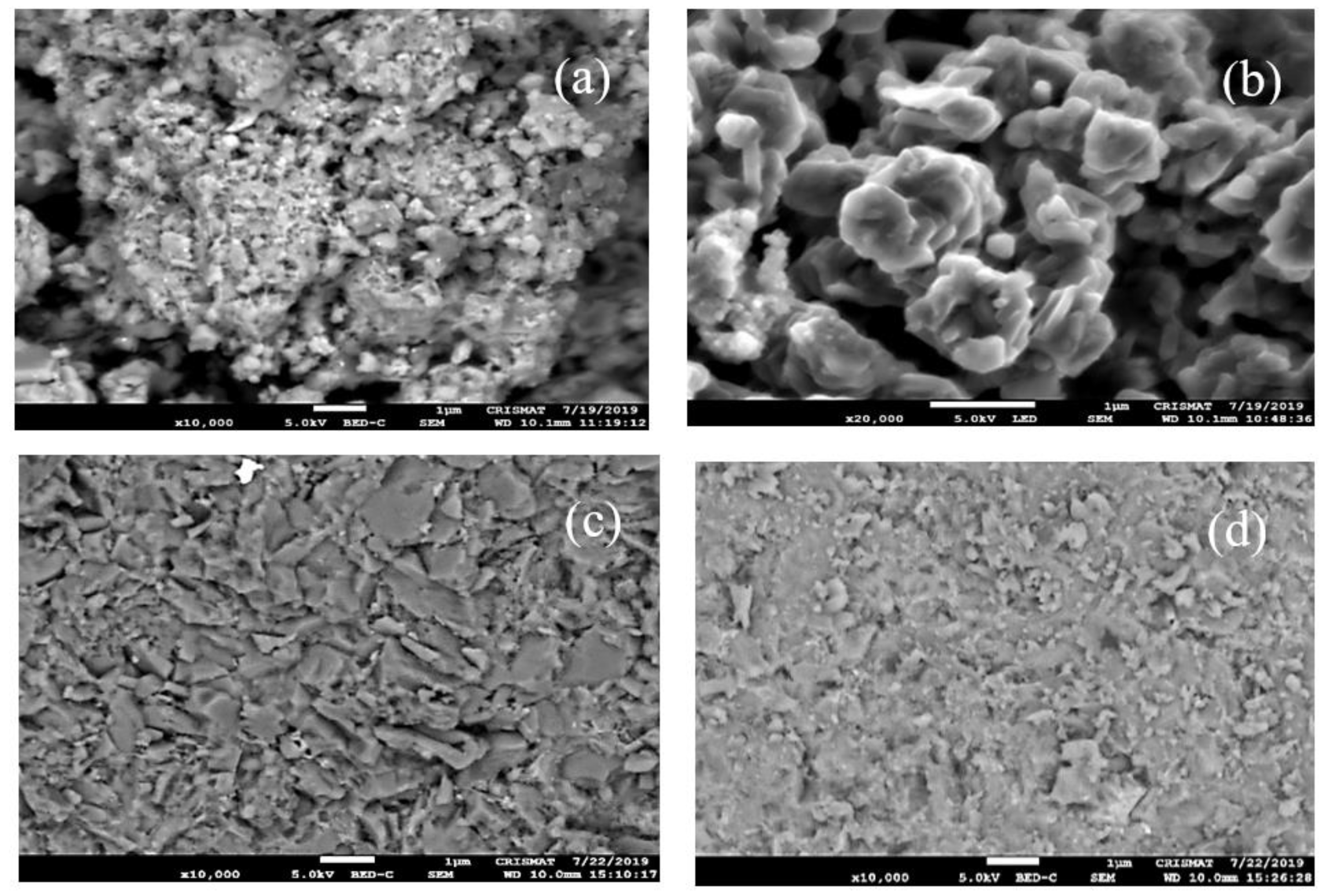
3.2. Microstructural Analyses of MgB2 Sintered Compacts
3.3. Vickers Microhardness Analyses
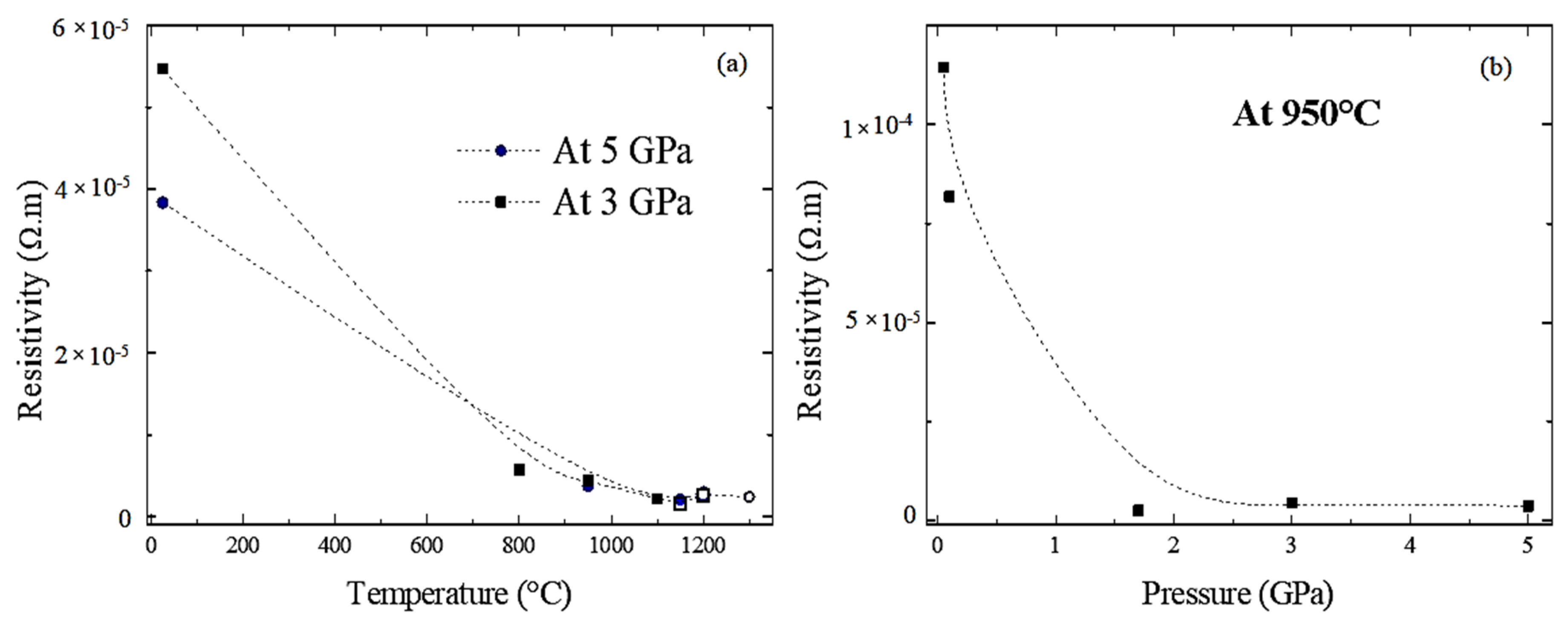
3.4. Resistance Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nagamatsu, J.; Nakagawa, N. Superconductivity at 39 K in magnesium diboride. Nature 2001, 410, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Yamashita, T. Review of superconducting properties of MgB2. Supercond. Sci. Technol. 2001, 14, R115–R146. [Google Scholar] [CrossRef] [Green Version]
- Dancer, C.E.J.; Prabhakaran, D.; Başoğlu, M.; Yanmaz, E.; Yan, H.; Reece, M.; Todd, R.I.; Grovenor, C.R.M. Fabrication and properties of dense ex situ magnesium diboride bulk material synthesized using spark plasma sintering. Supercond. Sci. Technol. 2009, 22, 095003. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yoo, S.I.; Kim, Y.W.; Hwang, N.M.; Kim, D.Y. Preparation of dense MgB2 bulk superconductors by spark plasma sintering. J. Am. Ceram. Soc. 2003, 86, 1800–1802. [Google Scholar] [CrossRef]
- Shim, S.H.; Shim, K.B.; Yoon, J.W. Superconducting characteristics of polycrystalline magnesium diboride ceramics fabricated by a spark plasma sintering technique. J. Am. Ceram. Soc. 2005, 88, 858–861. [Google Scholar] [CrossRef]
- Yamamoto, A.; Tanaka, H.; Shimoyama, J.; Ogino, H.; Kishio, K.; Matsushita, T. Towards the Realization of Higher Connectivity in MgB2 Conductors: In-situ or Sintered Ex-situ? Jpn. J. Appl. Phys. 2012, 51, 010105. [Google Scholar] [CrossRef] [Green Version]
- Viznichenko, R.V.; Kordyuk, A.A.; Fuchs, G.; Nenkov, K.; Müller, K.-H.; Prikhna, T.A.; Gawalek, W. Temperature dependence of trapped magnetic field in MgB2 bulk superconductor. Appl. Phys. Lett. 2003, 83, 4360–4362. [Google Scholar] [CrossRef] [Green Version]
- Aldica, G.; Burdusel, M.; Popa, S.; Enculescu, M.; Pasuk, I.; Badica, P. The influence of heating rate on superconducting characteristics of MgB2 obtained by spark plasma sintering technique. Phys. C 2015, 519, 184–193. [Google Scholar] [CrossRef]
- Aldica, G.; Popa, S.; Enculescu, M.; Pasuk, I.; Ionescu, A.M.; Badica, P. Dwell time influence on spark plasmasintered MgB2. J. Supercond. Novel Magn. 2018, 31, 317–342. [Google Scholar] [CrossRef]
- Takano, Y.; Takeya, H.; Fujii, H.; Kumakura, H.; Hatano, T.; Togano, K.; Kito, H.; Ihara, H. Superconducting properties of MgB2 bulk materials prepared by high-pressure sintering. Appl. Phys. Lett. 2001, 78, 2914–2920. [Google Scholar] [CrossRef] [Green Version]
- Häßler, W.; Scheiter, J.; Hädrich, P.; Kauffmann-Wei, S.; Holzapfel, B.; Oomen, M.; Nielsch, K. Properties of ex-situ MgB2 bulk samples prepared by uniaxial hot pressing and spark plasma sintering. Phys. C 2018, 551, 48–54. [Google Scholar] [CrossRef]
- Krinitsina, T.P.; Kuznetsova, E.I.; Blinova, Y.V.; Degtyarev, M.V. Microstructure and critical current of bulk MgB2 superconductor. J. Phys. Conf. Ser. 2019, 1389, 012068. [Google Scholar] [CrossRef]
- Prakasam, M.; Balima, F.; Cygan, S.; Klimczyk, P.; Jaworska, L.; Largeteau, A. Chapter 9-Ultrahigh pressure SPS (HP-SPS) as new syntheses and exploration tool in materials science. In Spark Plasma Sintering; Giacomo, C., Claude, E., Javier, G., Roberto, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 201–218. [Google Scholar] [CrossRef]
- Balima, F.; Bellin, F.; Michau, D.; Viraphong, O.; Poulon-Quintin, A.; Chung, U.-C.; Dourfaye, A.; Largeteau, A. High pressure pulsed electric current activated equipment (HP-SPS) for material processing. Mater. Des. 2018, 139, 541–548. [Google Scholar] [CrossRef]
- Balima, F.; Largeteau, A. Phase transformation of alumina induced by high pressure spark plasma sintering (HP-SPS). Scr. Mater. 2019, 158, 20–23. [Google Scholar] [CrossRef]
- Largeteau, A.; Prakasam, M. Trends in high pressure developments for new perspectives. Solid State Sci. 2018, 80, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Cannon, J.F. Behavior of elements at high pressure. J. Phys. Chem. Data 1974, 3, 781–825. [Google Scholar] [CrossRef] [Green Version]
- Collings, E.W.; Sumption, M.D.; Bhatia, M.; Susner, M.A.; Bohnenstiehl, S.D. Prospects for improving the intrinsic and extrinsic properties of magnesium diboride superconducting strands. Supercond. Sci. Technol. 2008, 21, 103001–103057. [Google Scholar] [CrossRef]
- Ma, H.A.; Jia, X.P.; Chen, L.X.; Zhu, P.W.; Ren, G.Z.; Guo, W.L.; Fu, X.Q.; Zou, G.T.; Ren, Z.A.; Che, G.C.; et al. Superhard MgB2 bulk material prepared by high-pressure sintering. J. Phys. Condens. Matter 2002, 14, 11181–11184. [Google Scholar] [CrossRef]
- Matthews, G.A.B.; Liu, J.; Grovenor, C.R.M.; Grant, P.S.; Speller, S. Design and characterisation of ex situ bulk MgB2 superconductors containing a nanoscale dispersion of artificial pinning centres. Supercond. Sci. Technol. 2020, 33, 034006. [Google Scholar] [CrossRef]
- Matthews, G.A.B.; Santra, S.; Ma, R.; Grovenor, C.R.M.; Grant, P.S.; Speller, S.C. Effect of the sintering temperature on the microstructure and superconducting properties of MgB2 bulks manufactured by the field assisted sintering technique. Supercond. Sci. Technol. 2020, 33, 054003–054014. [Google Scholar] [CrossRef]
- Demazeau, G. High Pressure and Chemical Bonding in Materials Chemistry. Z. Nat. 2006, 61, 799–808. [Google Scholar]



| Pressure | Temperature | Crystallite Size (nm) |
|---|---|---|
| 50 MPa | 950 °C | 70 |
| 100 MPa | 950 °C | 73 |
| 100 MPa | 1050 °C | 91 |
| 100 MPa | 1100 °C | 104 |
| 1.7 GPa | 950 °C | 59 |
| 3 GPa | 800 °C | 67 |
| 3 GPa | 950 °C | 46 |
| 3 GPa | 1100 °C | 55 |
| 3 GPa | 1150 °C | 116 |
| 3 GPa | 1200 °C | 126 |
| 3 GPa | 1300 °C | 141 |
| 5 GPa | 950 °C | 44 |
| 5 GPa | 1150 °C | 75 |
| 5 GPa | 1200 °C | 68 |
| 5 GPa | 1300 °C | 131 |
| Pressure (GPa) | Temperature (°C) | Density (g/cm3) | Hardness (HV) |
|---|---|---|---|
| 0.05 (SPS) | 950 | 2.14 ± 0.01 | Not measurable |
| 0.1 (SPS) | 950 | 2.37 ± 0.01 | Not measurable |
| 1.7 (HP-SPS) | 950 | 2.59 ± 0.01 | 1347 ± 85 |
| 3 (HP-SPS) | 950 | 2.57 ± 0.01 | 1404 ± 37 |
| 3 (HP-SPS) | 1100 | 2.60 ± 0.01 | 1058 ± 88 |
| 3 (HP-SPS) | 1150 | 2.61 ± 0.01 | 1039 ± 62 |
| 5 (HP-SPS) | 950 | 2.62 ± 0.01 | 1465 ± 38 |
| 5 (HP-SPS) | 1150 | 2.62 ± 0.01 | 1418 ± 51 |
| 5 (HP-SPS) | 1200 | 2.62 ± 0.01 | 1460 ± 80 |
| 5 (HP-SPS) | 1300 | 2.59 ± 0.01 | 1072 ± 80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakasam, M.; Balima, F.; Noudem, J.; Largeteau, A. Dense MgB2 Ceramics by Ultrahigh Pressure Field-Assisted Sintering. Ceramics 2020, 3, 521-532. https://0-doi-org.brum.beds.ac.uk/10.3390/ceramics3040042
Prakasam M, Balima F, Noudem J, Largeteau A. Dense MgB2 Ceramics by Ultrahigh Pressure Field-Assisted Sintering. Ceramics. 2020; 3(4):521-532. https://0-doi-org.brum.beds.ac.uk/10.3390/ceramics3040042
Chicago/Turabian StylePrakasam, Mythili, Felix Balima, Jacques Noudem, and Alain Largeteau. 2020. "Dense MgB2 Ceramics by Ultrahigh Pressure Field-Assisted Sintering" Ceramics 3, no. 4: 521-532. https://0-doi-org.brum.beds.ac.uk/10.3390/ceramics3040042