Neurovascular Coupling in Seizures
Abstract
:1. Introduction
2. Cerebral Blood Flow
3. Cerebrovascular Autoregulation
4. Physiological Neurovascular Coupling
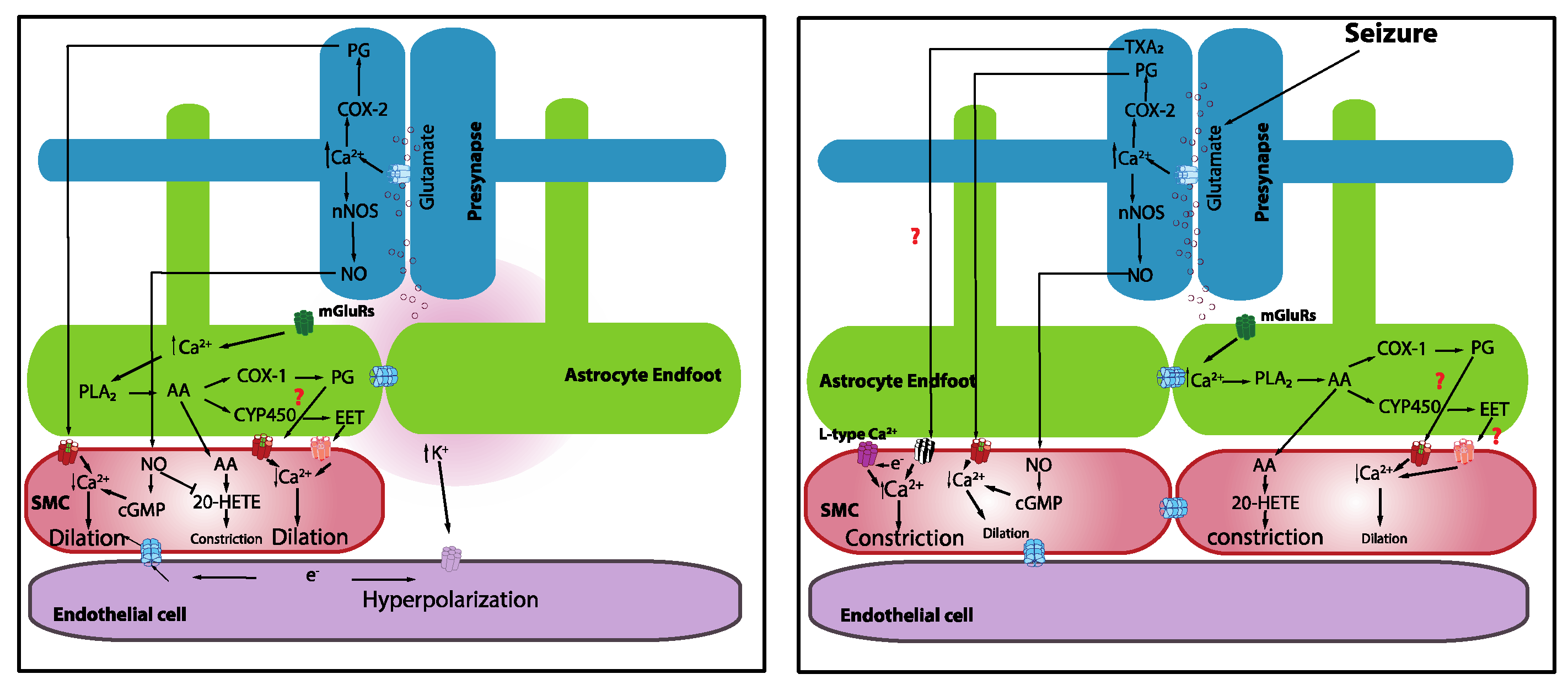
5. Neurovascular Coupling in Seizures
6. Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004, 5, 347–360. [Google Scholar] [CrossRef]
- Tarantini, S.; Tran, C.H.T.; Gordon, G.R.; Ungvari, Z.; Csiszar, A. Impaired neurovascular coupling in aging and Alzheimer’s disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp. Gerontol. 2017, 94, 52–58. [Google Scholar] [CrossRef]
- Kisler, K.; Nelson, A.R.; Montagne, A.; Zlokovic, B.V. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2017, 18, 419–434. [Google Scholar] [CrossRef] [Green Version]
- McCarron, J.G.; Osol, G.; Halpern, W. Myogenic Responses Are Independent of the Endothelium in Rat Pressurized Posterior Cerebral Arteries. J. Vasc. Res. 1989, 26, 315–319. [Google Scholar] [CrossRef]
- Attwell, D.; Buchan, A.; Charpak, S.; Lauritzen, M.; MacVicar, B.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef] [Green Version]
- Nippert, A.R.; Biesecker, K.R.; Newman, E.A. Mechanisms Mediating Functional Hyperemia in the Brain. Neuroscientist 2017, 24, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Longden, T.; Hill-Eubanks, D.C.; Nelson, M.T. Ion channel networks in the control of cerebral blood flow. Br. J. Pharmacol. 2015, 36, 492–512. [Google Scholar] [CrossRef] [Green Version]
- Stackhouse, T.L.; Mishra, A. Neurovascular Coupling in Development and Disease: Focus on Astrocytes. Front. Cell Dev. Biol. 2021, 9, 702832. [Google Scholar] [CrossRef] [PubMed]
- Baruah, J.; Vasudevan, A.; Köhling, R. Vascular Integrity and Signaling Determining Brain Development, Network Excitability, and Epileptogenesis. Front. Physiol. 2020, 10, 1583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tabatabaei, M.; Bélanger, S.; Girouard, H.; Moeini, M.; Lu, X.; Lesage, F. Astrocytic endfoot Ca2+ correlates with parenchymal vessel responses during 4-AP induced epilepsy: An in vivo two-photon lifetime microscopy study. Br. J. Pharmacol. 2017, 39, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, D.E.; Engel, J.; Phelps, M.E.; Selin, C. Epileptic patterns of local cerebral metabolism and perfusion in humans determined by emission computed tomography of 18FDG and 13NH3. Ann. Neurol. 1980, 8, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, T.; Teaw, S.; Bordey, A. Hypervascularization in mTOR-dependent focal and global cortical malformations displays differential rapamycin sensitivity. Epilepsia 2019, 60, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.Y.; Rühlmann, C.; Blinder, P.; Devor, A.; Drew, P.J.; Friedman, B.; Knutsen, P.M.; Lyden, P.D.; Mateo, C.; Mellander, L.; et al. Robust and fragile aspects of cortical blood flow in relation to the underlying angioarchitecture. Microcirculation 2015, 22, 204–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, S.; Lee, T.M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 1990, 87, 9868–9872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangold, R.; Sokoloff, L.; Conner, E.; Kleinerman, J.; Therman, P.-O.G.; Kety, S.S. The effects of sleep and lack of sleep on the cerebral circulation and metabolism of normal young men 1. J. Clin. Investig. 1955, 34, 1092–1100. [Google Scholar] [CrossRef] [Green Version]
- Gusnard, D.A.; Raichle, M.E. Searching for a baseline: Functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001, 2, 685–694. [Google Scholar] [CrossRef]
- Hyder, F.; Rothman, D.L.; Shulman, R.G. Total neuroenergetics support localized brain activity: Implications for the interpretation of fMRI. Proc. Natl. Acad. Sci. USA 2002, 99, 10771–10776. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.J.; Blumenfeld, H.; Behar, K.; Rothman, D.L.; Shulman, R.G.; Hyder, F. Cerebral energetics and spiking frequency: The neurophysiological basis of fMRI. Proc. Natl. Acad. Sci. USA 2002, 99, 10765–10770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attwell, D.; Laughlin, S. An Energy Budget for Signaling in the Grey Matter of the Brain. Br. J. Pharmacol. 2001, 21, 1133–1145. [Google Scholar] [CrossRef]
- Howarth, C.; Gleeson, P.; Attwell, D. Updated Energy Budgets for Neural Computation in the Neocortex and Cerebellum. Br. J. Pharmacol. 2012, 32, 1222–1232. [Google Scholar] [CrossRef]
- Faraci, F.; Heistad, D. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ. Res. 1990, 66, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Cipolla, M.J.; Smith, J.; Kohlmeyer, M.M.; Godfrey, J.A. SKCa and IKCa Channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: Effect of ischemia and reperfusion. Stroke 2009, 40, 1451–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, P.; Raichle, M.E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl. Acad. Sci. USA 1986, 83, 1140–1144. [Google Scholar] [CrossRef] [Green Version]
- Lecoq, J.; Tiret, P.; Najac, M.; Shepherd, G.M.; Greer, C.A.; Charpak, S. Odor-evoked oxygen consumption by action potential and synaptic transmission in the olfactory bulb. J. Neurosci. 2009, 29, 1424–1433. [Google Scholar] [CrossRef]
- Parpaleix, A.; Houssen, Y.G.; Charpak, S. Imaging local neuronal activity by monitoring PO2 transients in capillaries. Nat. Med. 2013, 19, 241–246. [Google Scholar] [CrossRef]
- Devor, A.; Sakadžić, S.; Saisan, P.A.; Yaseen, M.A.; Roussakis, E.; Srinivasan, V.J.; Vinogradov, S.A.; Rosen, B.R.; Buxton, R.B.; Dale, A.M.; et al. ‘Overshoot’ of O2 is required to maintain baseline tissue oxygenation at locations distal to blood vessels. J. Neurosci. 2011, 31, 13676–13681. [Google Scholar] [CrossRef] [Green Version]
- Paulson, O.B.; Hasselbalch, S.G.; Rostrup, E.; Knudsen, G.M.; Pelligrino, D. Cerebral Blood Flow Response to Functional Activation. Br. J. Pharmacol. 2009, 30, 2–14. [Google Scholar] [CrossRef]
- Logothetis, N.K.; Pauls, J.; Augath, M.; Trinath, T.; Oeltermann, A. Neurophysiological investigation of the basis of the fMRI signal. Nature 2001, 412, 150–157. [Google Scholar] [CrossRef]
- Devor, A.; Ulbert, I.; Dunn, A.K.; Narayanan, S.N.; Jones, S.; Andermann, M.L.; Boas, D.A.; Dale, A.M. Coupling of the cortical hemodynamic response to cortical and thalamic neuronal activity. Proc. Natl. Acad. Sci. USA 2005, 102, 3822–3827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shmuel, A.; Yacoub, E.; Pfeuffer, J.; Van de Moortele, P.-F.; Adriany, G.; Hu, X.; Ugurbil, K. Sustained Negative BOLD, Blood Flow and Oxygen Consumption Response and Its Coupling to the Positive Response in the Human Brain. Neuron 2002, 36, 1195–1210. [Google Scholar] [CrossRef] [Green Version]
- Shmuel, A.; Augath, M.; Oeltermann, A.; Logothetis, N.K. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat. Neurosci. 2006, 9, 569–577. [Google Scholar] [CrossRef]
- Klingner, C.M.; Ebenau, K.; Hasler, C.; Brodoehl, S.; Görlich, Y.; Witte, O.W. Influences of negative BOLD responses on positive BOLD responses. NeuroImage 2011, 55, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Harel, N.; Lee, S.-P.; Nagaoka, T.; Kim, D.-S.; Kim, S.-G. Origin of negative blood oxygenation lev-el-dependent fMRI signals. J. Cereb. Blood Flow Metab. 2002, 22, 908–917. [Google Scholar] [CrossRef]
- Blinder, P.; Tsai, P.S.; Kaufhold, J.; Knutsen, P.M.; Suhl, H.; Kleinfeld, D. The cortical angiome: An interconnected vascular network with noncolumnar patterns of blood flow. Nat. Neurosci. 2013, 16, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, D.A.; Berthiaume, A.-A.; Grant, R.I.; Harrill, S.A.; Koski, T.; Tieu, T.; McDowell, K.P.; Faino, A.V.; Kelly, A.L.; Shih, A.Y. Brain capillary pericytes exert a substantial but slow influence on blood flow. Nat. Neurosci. 2021, 24, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.J.; Hill, M. Signaling Mechanisms Underlying the Vascular Myogenic Response. Physiol. Rev. 1999, 79, 387–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayliss, W.M.; Hill, L.; Gulland, G.L. On Intra-Cranial Pressure and the Cerebral Circulation: Part I. Physiological; Part II. Histological. J. Physiol. 1895, 18, 334–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassen, N.A. Cerebral Blood Flow and Oxygen Consumption in Man. Physiol. Rev. 1959, 39, 183–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.O.; Hamner, J.W.; Taylor, J.A. The role of myogenic mechanisms in human cerebrovascular regulation. J. Physiol. 2013, 591, 5095–5105. [Google Scholar] [CrossRef]
- Brayden, J.E.; Earley, S.; Nelson, M.T.; Reading, S. Transient receptor potential (trp) channels, vascular tone and autoregulation of cerebral blood flow. Clin. Exp. Pharmacol. Physiol. 2008, 35, 1116–1120. [Google Scholar] [CrossRef] [Green Version]
- Meininger, G.A.; Davis, M.J. Cellular mechanisms involved in the vascular myogenic response. Am. J. Physiol. Circ. Physiol. 1992, 263, H647–H659. [Google Scholar] [CrossRef]
- VanBavel, E.; Wesselman, J.P.M.; Spaan, J.A.E. Myogenic Activation and Calcium Sensitivity of Cannulated Rat Mesenteric Small Arteries. Circ. Res. 1998, 82, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Brayden, J.; Nelson, M. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 1992, 256, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Harder, D.R.; Lange, A.R.; Gebremedhin, D.; Birks, E.K.; Roman, R.J. Cytochrome P450 metabolites of arachidonic acid as intracellular signaling molecules in vascular tissue. J. Vasc. Res. 1997, 34, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.H.; Gordon, G.R. Acute two-photon imaging of the neurovascular unit in the cortex of active mice. Front. Cell. Neurosci. 2015, 9, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busija, D.W.; Bari, F.; Domoki, F.; Louis, T. Mechanisms involved in the cerebrovascular dilator effects of N-methyl-d-aspartate in cerebral cortex. Brain Res. Rev. 2007, 56, 89–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domoki, F.; Perciaccante, J.V.; Shimizu, K.; Puskar, M.; Busija, D.W.; Bari, F. N-methyl-d-aspartate-induced vasodilation is mediated by endothelium-independent nitric oxide release in piglets. Am. J. Physiol. Circ. Physiol. 2002, 282, H1404–H1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Meng, W.; Ayata, C.; Huang, P.L.; Fishman, M.C.; Moskowitz, M.A. L-NNA-sensitive regional cerebral blood flow augmentation during hypercapnia in type III NOS mutant mice. Am. J. Physiol. Circ. Physiol. 1996, 271, H1717–H1719. [Google Scholar] [CrossRef]
- Tran, C.H.; Peringod, G.; Gordon, G.R. Astrocytes Integrate Behavioral State and Vascular Signals during Functional Hyperemia. Neuron 2018, 100, 1133–1148.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindauer, U.; Megow, D.; Matsuda, H.; Dirnagl, U. Nitric oxide: A modulator, but not a mediator, of neuro-vascular coupling in rat somatosensory cortex. Am. J. Physiol. 1999, 277, H799–H811. [Google Scholar] [CrossRef]
- Uhlirova, H.; Kılıç, K.; Tian, P.; Thunemann, M.; Desjardins, M.; Saisan, P.A.; Sakadžić, S.; Ness, T.V.; Mateo, C.; Cheng, Q.; et al. Cell type specificity of neurovascular coupling in cerebral cortex. eLife 2016, 5, e14315. [Google Scholar] [CrossRef] [PubMed]
- Zonta, M.; Angulo, M.C.; Gobbo, S.; Rosengarten, B.; Hossmann, K.-A.; Pozzan, T.; Carmignoto, P. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat. Neurosci. 2002, 6, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, S.J.; MacVicar, B. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 2004, 431, 195–199. [Google Scholar] [CrossRef]
- Girouard, H.; Bonev, A.D.; Hannah, R.M.; Meredith, A.; Aldrich, R.W.; Nelson, M.T. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc. Natl. Acad. Sci. USA 2010, 107, 3811–3816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, G.R.J.; Choi, H.B.; Rungta, R.; Ellis-Davies, G.C.R.; MacVicar, B.A. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 2008, 456, 745–749. [Google Scholar] [CrossRef] [Green Version]
- Nett, W.J.; Oloff, S.H.; McCarthy, K.D. Hippocampal Astrocytes In Situ Exhibit Calcium Oscillations That Occur Independent of Neuronal Activity. J. Neurophysiol. 2002, 87, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Lind, B.L.; Brazhe, A.R.; Jessen, S.B.; Tan, F.C.C.; Lauritzen, M. Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, E4678–E4687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind, B.L.; Jessen, S.B.; Lønstrup, M.; Joséphine, C.; Bonvento, G.; Lauritzen, M. Fast Ca2+ responses in astrocyte end-feet and neurovascular coupling in mice. Glia 2017, 66, 348–358. [Google Scholar] [CrossRef] [Green Version]
- Winship, I.R.; Plaa, N.; Murphy, T.H. Rapid Astrocyte Calcium Signals Correlate with Neuronal Activity and Onset of the Hemodynamic Response In Vivo. J. Neurosci. 2007, 27, 6268–6272. [Google Scholar] [CrossRef] [Green Version]
- Nizar, K.; Uhlirova, H.; Tian, P.; Saisan, P.A.; Cheng, Q.; Reznichenko, L.; Weldy, K.L.; Steed, T.; Sridhar, V.B.; Macdonald, C.L.; et al. In vivo Stimulus-Induced Vasodilation Occurs without IP3 Receptor Activation and May Precede Astrocytic Calcium Increase. J. Neurosci. 2013, 33, 8411–8422. [Google Scholar] [CrossRef]
- Bonder, D.E.; McCarthy, K.D. Astrocytic Gq-GPCR-Linked IP3R-Dependent Ca2+ Signaling Does Not Mediate Neurovascular Coupling in Mouse Visual Cortex In Vivo. J. Neurosci. 2014, 34, 13139–13150. [Google Scholar] [CrossRef]
- Stobart, J.; Ferrari, K.D.; Barrett, M.J.; Glück, C.; Stobart, M.J.; Zuend, M.; Weber, B. Cortical Circuit Activity Evokes Rapid Astrocyte Calcium Signals on a Similar Timescale to Neurons. Neuron 2018, 98, 726–735.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iadecola, C.; Nedergaard, M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 2007, 10, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Gordon, G.R.J.; Tran, C.H.T. Heterogeneity of Sensory-Induced Astrocytic Ca2+ Dynamics During Functional Hyperemia. Front. Physiol. 2020, 11, 587-10. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, T.H. Neurovascular Coupling and Epilepsy: Hemodynamic Markers for Localizing and Predicting Seizure Onset. Epilepsy Curr. 2007, 7, 91–94. [Google Scholar] [CrossRef]
- Farrell, J.S.; Wolff, M.D.; Teskey, G.C. Neurodegeneration and Pathology in Epilepsy: Clinical and Basic Perspectives. Neurodegener. Dis. 2017, 15, 317–334. [Google Scholar] [CrossRef]
- Leonhardt, G.; De Greiff, A.; Weber, J.; Ludwig, T.; Wiedemayer, H.; Forsting, M.; Hufnagel, A. Brain Perfusion Following Single Seizures. Epilepsia 2005, 46, 1943–1949. [Google Scholar] [CrossRef]
- Fong, G.C.Y.; Fong, K.Y.; Mak, W.; Tsang, K.L.; Chan, K.H.; Cheung, R.T.F.; Ho, S.L. Postictal psychosis related regional cerebral hyperfusion. J. Neurol. Neurosurg. Psychiatr. 2000, 68, 100–101. [Google Scholar] [CrossRef] [Green Version]
- Tatlidil, R. Persistent postictal hyperperfusion demonstrated with PET. Epilepsy Res. 2000, 42, 83–88. [Google Scholar] [CrossRef]
- Gotman, J.; Kobayashi, E.; Bagshaw, A.; Bénar, C.-G.; Dubeau, F. Combining EEG and fMRI: A multimodal tool for epilepsy research. J. Magn. Reson. Imaging 2006, 23, 906–920. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, H.; Suh, M.; Schwartz, T.H. Spatiotemporal Dynamics of Perfusion and Oximetry during Ictal Discharges in the Rat Neocortex. J. Neurosci. 2009, 29, 2814–2823. [Google Scholar] [CrossRef] [Green Version]
- Penfield, W.; Santha von, K.; Cipriani, A. Cerebral Blood Flow During Induced Epileptiform Seizures in Animals and Man. J. Neurophysiol. 1939, 2, 257–267. [Google Scholar] [CrossRef]
- Penfield, W. Epilepsy and the cerebral lesions of birth and infancy. Can. Med. Assoc. J. 1939, 41, 527–534. [Google Scholar]
- Bahar, S.; Suh, M.; Zhao, M.; Schwartz, T.H. Intrinsic optical signal imaging of neocortical seizures: The ‘epileptic dip’. NeuroReport 2006, 17, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.; Ma, H.; Zhao, M.; Sharif, S.; Schwartz, T.H. Neurovascular Coupling and Oximetry During Epileptic Events. Mol. Neurobiol. 2006, 33, 181–198. [Google Scholar] [CrossRef]
- Tran, C.H.T.; George, A.G.; Teskey, G.C.; Gordon, G.R. Seizures elevate gliovascular unit Ca2+ and cause sustained vasoconstriction. JCI Insight 2020, 5, e136469. [Google Scholar] [CrossRef]
- Gómez-Gonzalo, M.; Losi, G.; Chiavegato, A.; Zonta, M.; Cammarota, M.; Brondi, M.; Vetri, F.; Uva, L.; Pozzan, T.; de Curtis, M.; et al. An excitatory loop with astrocytes contributes to drive neurons to seizure thresh-old. PLoS Biol. 2010, 8, e1000352. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Gonzalo, M.; Losi, G.; Brondi, M.; Uva, L.; Sato, S.S.; de Curtis, M.; Ratto, G.M.; Carmignoto, G. Ictal but Not Interictal Epileptic Discharges Activate Astrocyte Endfeet and Elicit Cerebral Arteriole Responses. Front. Cell. Neurosci. 2011, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Nguyen, J.; Ma, H.; Nishimura, N.; Schaffer, C.B.; Schwartz, T.H. Preictal and Ictal Neurovascular and Metabolic Coupling Surrounding a Seizure Focus. J. Neurosci. 2011, 31, 13292–13300. [Google Scholar] [CrossRef] [Green Version]
- Devor, A.; Hillman, E.M.C.; Tian, P.; Waeber, C.; Teng, I.C.; Ruvinskaya, L.; Shalinsky, M.H.; Zhu, H.; Haslinger, R.H.; Narayanan, S.N.; et al. Stimulus-Induced Changes in Blood Flow and 2-Deoxyglucose Uptake Dissociate in Ipsilateral Somatosensory Cortex. J. Neurosci. 2008, 28, 14347–14357. [Google Scholar] [CrossRef] [Green Version]
- Rowe, C.C.; Berkovic, S.; Austin, M.C.; Saling, M.; Kalnins, R.M.; McKay, W.J.; Bladin, P.F. Visual and quantitative analysis of interictal SPECT with technetium-99m-HMPAO in temporal lobe epilepsy. J. Nucl. Med. 1991, 32, 1688–1694. [Google Scholar] [PubMed]
- Newton, M.R.; Berkovic, S.F.; Austin, M.C.; Rowe, C.C.; McKay, W.J.; Bladin, P.F. Postictal switch in blood flow distribution and temporal lobe seizures. J. Neurol. Neurosurg. Psychiatry 1992, 55, 891–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrell, J.S.; Gaxiola-Valdez, I.; Wolff, M.D.; David, L.S.; Dika, H.I.; Geeraert, B.L.; Wang, X.R.; Singh, S.; Spanswick, S.C.; Dunn, J.F.; et al. Postictal behavioural impairments are due to a severe prolonged hypoperfusion/hypoxia event that is COX-2 dependent. Elife 2016, 5, e19352. [Google Scholar] [CrossRef] [PubMed]
- Gokina, N.I.; Osol, G. Temperature and protein kinase C modulate myofilament Ca2+ sensitivity in pressurized rat cerebral arteries. Am. J. Physiol. 1998, 274, H1920–H1927. [Google Scholar] [CrossRef]
- Farrell, J.S.; Colangeli, R.; Dong, A.; George, A.G.; Addo-Osafo, K.; Kingsley, P.J.; Morena, M.; Wolff, M.D.; Dudok, B.; He, K.; et al. In vivo endocannabinoid dynamics at the timescale of physiological and pathological neural activity. Neuron 2021, 109, 2398–2403.e4. [Google Scholar] [CrossRef]
- Devor, A.; Tian, P.; Nishimura, N.; Teng, I.C.; Hillman, E.M.; Narayanan, S.N.; Ulbert, I.; Boas, D.A.; Kleinfeld, D.; Dale, A.M. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J. Neurosci. 2007, 27, 4452–4459. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teskey, G.C.; Tran, C.H.T. Neurovascular Coupling in Seizures. Neuroglia 2021, 2, 36-47. https://0-doi-org.brum.beds.ac.uk/10.3390/neuroglia2010005
Teskey GC, Tran CHT. Neurovascular Coupling in Seizures. Neuroglia. 2021; 2(1):36-47. https://0-doi-org.brum.beds.ac.uk/10.3390/neuroglia2010005
Chicago/Turabian StyleTeskey, G. Campbell, and Cam Ha T. Tran. 2021. "Neurovascular Coupling in Seizures" Neuroglia 2, no. 1: 36-47. https://0-doi-org.brum.beds.ac.uk/10.3390/neuroglia2010005




