Streambank Legacy Sediments in Surface Waters: Phosphorus Sources or Sinks?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description, Streambank Sampling for Legacy Sediments, and Sediment Analysis
2.2. Phosphorus Sorption Index (PSI)
2.3. Equilibrium Phosphorus Concentration (EPC0)
2.4. Sorption and Desorption Under Anoxic and Oxic Conditions
3. Results and Discussion
3.1. Concentrations of P in Streambank Legacy Sediments and Comparisons Against Upland Soils, Stream Bed Sediments, and Water Quality Thresholds
3.2. Phosphorus Sorption Index (PSI) for Legacy Sediments
3.3. Equilibrium Phosphorus Concentration (EPC0)
3.4. Legacy Sediment Sorption Under Anoxic and Oxic Conditions:
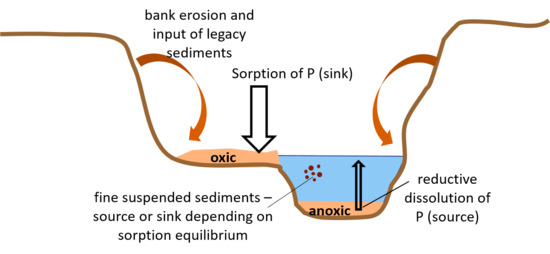
3.5. Conceptual Model for Source/Sink Behavior of Legacy Sediment P and Broader Implications for Water Quality and Watershed Management
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Correll, D.L. The Role of Phosphorus in the Eutrophication of Receiving Waters: A Review. J. Environ. Qual. 1998, 27, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Kleinman, P.J.A.; Fanelli, R.M.; Hirsch, R.M.; Buda, A.R.; Easton, Z.M.; Wainger, L.A.; Brosch, C.; Lowenfish, M.; Collick, A.S.; Shirmohammadi, A.; et al. Phosphorus and the Chesapeake Bay: Lingering Issues and Emerging Concerns for Agriculture. J. Environ. Qual. 2019, 48, 1191–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smil, V. Phosphorus in the environment: Natural Flows and Human Interferences. Annu. Rev. Energy Environ. 2000, 25, 53–88. [Google Scholar] [CrossRef] [Green Version]
- Henley, W.F.; Patterson, M.A.; Neves, R.J.; Lemly, A.D. Effects of Sedimentation and Turbidity on Lotic Food Webs: A Concise Review for Natural Resource Managers. Rev. Fish. Sci. 2000, 8, 125–139. [Google Scholar] [CrossRef] [Green Version]
- Smith, V.H. Eutrophication of freshwater and coastal marine ecosystems a global problem. Environ. Sci. Pollut. Res. 2003, 10, 126–139. [Google Scholar] [CrossRef]
- Goldes, S.A.; Ferguson, H.W.; Moccia, R.D.; Daoust, P. Histological effects of the inert suspended clay kaolin on the gills of juvenile rainbow trout, Salmo gairdneri Richardson. J. Fish Dis. 1988, 11, 23–33. [Google Scholar] [CrossRef]
- Waters, T.F. Sediment in Streams. Sources, Biological Effects, and Control; American Fisheries Society: Bethesda, MD, USA, 1995; Monograph 7; p. 251. [Google Scholar]
- Chesapeake Bay TMDL Document. Available online: https://www.epa.gov/chesapeake-bay-tmdl/chesapeake-bay-tmdl-document (accessed on 10 May 2020).
- Kaufman, Z.; Abler, D.; Shortle, J.; Harper, J.; Hamlett, J.; Feather, P. Agricultural Costs of the Chesapeake Bay Total Maximum Daily Load. Environ. Sci. Technol. 2014, 48, 14131–14138. [Google Scholar] [CrossRef]
- Fanelli, R.M.; Blomquist, J.D.; Hirsch, R.M. Point sources and agricultural practices control spatial-temporal patterns of orthophosphate in tributaries to Chesapeake Bay. Sci. Total Environ. 2019, 652, 422–433. [Google Scholar] [CrossRef]
- Fox, G.A.; Purvis, R.A.; Penn, C.J. Streambanks: A net source of sediment and phosphorus to streams and rivers. J. Environ. Manag. 2016, 181, 602–614. [Google Scholar] [CrossRef] [Green Version]
- Zaimes, G.N.; Schultz, R.C.; Isenhart, T.M. Streambank Soil and Phosphorus Losses under Different Riparian Land-Uses in Iowa1. J. Am. Water Resour. Assoc. 2008, 44, 935–947. [Google Scholar] [CrossRef]
- James, L.A. Legacy sediment: Definitions and processes of episodically produced anthropogenic sediment. Anthropocene 2013, 2, 16–26. [Google Scholar] [CrossRef]
- Walter, R.C.; Merritts, D.J. Natural Streams and the Legacy of Water-Powered Mills. Science 2008, 319, 299–304. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.; Baker, M.; Boomer, K.; Merritts, D.; Prestegaard, K.; Smith, S. Legacy Sediment, Riparian Corridors, and Total Maximum Daily Loads; STAC Publication Number 19-001: Edgewater, MD, USA, 2019; p. 64. [Google Scholar]
- Johnson, K.M.; Snyder, N.P.; Castle, S.; Hopkins, A.J.; Waltner, M.; Merritts, D.J.; Walter, R.C. Legacy sediment storage in New England river valleys: Anthropogenic processes in a postglacial landscape. Geomorphology 2019, 327, 417–437. [Google Scholar] [CrossRef]
- Merritts, D.; Walter, R.; Rahnis, M.; Hartranft, J.; Cox, S.; Gellis, A.; Poter, N.; Hilgartner, W.; Langland, M.; Manion, L.; et al. Anthropocene streams and base-level controls from historic dams in the unglaciated mid-Atlantic region, USA. Philos. Trans. Math. Phys. Eng. Sci. 2011, 369, 976–1009. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, K.; Lewis, R.; Hunt, M. Historic mill ponds and piedmont stream water quality: Making the connection near Raleigh, North Carolina. In From the Blue Ridge to the Coastal Plain: Field Excursions in the Southeastern United States: Geological Society of America Field Guide; Geological Society of America: Boulder, CO, USA, 2012. [Google Scholar]
- Cashman, M.J.; Gellis, A.; Sanisaca, L.G.; Noe, G.B.; Cogliandro, V.; Baker, A. Bank-derived material dominates fluvial sediment in a suburban Chesapeake Bay watershed. River Res. Appl. 2018, 34, 1032–1044. [Google Scholar] [CrossRef]
- Gellis, A.; Noe, G. Sediment source analysis in the Linganore Creek watershed, Maryland, USA, using the sediment fingerprinting approach: 2008 to 2010. J. Soils Sediments 2013, 13, 1735–1753. [Google Scholar] [CrossRef]
- Jiang, G.; Lutgen, A.; Sienkiewicz, N.; Mattern, K.; Kan, J.; Inamdar, S. Streambank legacy sediment contributions to sediment-bound nutrient yields from a Mid-Atlantic, Piedmont Watershed. J. Am. Water Resour. Assoc. 2020, in press. [Google Scholar]
- Lutgen, A.; Jiang, G.; Siekiewicz, N.; Mattern, K.; Kan, J.; Inamdar, S. Nutrients and Heavy Metals in Legacy Sediments: Concentrations, Comparisons with Upland Soils, and Implications for Water Quality. J. Am. Water Resour. Assoc. 2020, in press. [Google Scholar] [CrossRef]
- Walter, R.; Merritts, D.; Rahnis, M. Estimating volume, nutrient content, and rates of stream bank erosion of legacy sediment in the Piedmont and Valley and Ridge Physiographic provinces of southeastern and central PA. In Report to the Pennsylvania Department of Environmental Protection, Lancaster, Pennsylvania; Franklin and Marshall: Lancaster, PA, USA, 2007. [Google Scholar]
- Niemitz, J.; Haynes, C.; Lasher, G. Legacy sediments and historic land use: Chemostratigraphic evidence for excess nutrient and heavy metal sources and remobilization. Geology 2013, 41, 47–50. [Google Scholar] [CrossRef]
- Lammers, R.W.; Bledsoe, B.P. What role does stream restoration play in nutrient management? Crit. Rev. Environ. Sci. Technol. 2017, 47, 335–371. [Google Scholar] [CrossRef]
- Fleming, P.M.; Merritts, D.J.; Walter, R.C. Legacy sediment erosion hot spots: A cost-effective approach for targeting water quality improvements. J. Soil Water Conserv. 2019, 74, 67A–73A. [Google Scholar] [CrossRef] [Green Version]
- Sims, J.T.; Maguire, R.O.; Leytem, A.B.; Gartley, K.L.; Pautler, M.C. Evaluation of Mehlich 3 as an Agri-Environmental Soil Phosphorus Test for the Mid-Atlantic United States of America. Soil Sci. Soc. Am. J. 2002, 66, 2016–2032. [Google Scholar] [CrossRef]
- Sienkiewicz, N. Microbial Characteristics and Fate of Legacy Sediments in Mid-Atlantic Streams. Master’s Thesis, University of Delaware, Newark, DE, USA, 2019. [Google Scholar]
- Nair, V.D.; Reddy, K.R. Phosphorus Sorption and Desorption in Wetland Soils. In Methods in Biogeochemistry of Wetlands; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 2013; pp. 667–681. [Google Scholar]
- Haggard, B.; Sharpley, A. Phosphorus Transport in Streams: Processes and Modeling Considerations. In Modeling Phosphorus in the Environment; Radcliffe, D., Carbrera, M., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 105–130. [Google Scholar]
- McDaniel, M.D.; David, M.B.; Royer, T.V. Relationships between Benthic Sediments and Water Column Phosphorus in Illinois Streams. J. Environ. Qual. 2009, 38, 607–617. [Google Scholar] [CrossRef]
- Young, E.O.; Ross, D.S. Phosphate Release from Seasonally Flooded Soils: A Laboratory Microcosm Study. J. Environ. Qual. 2001, 30, 91–101. [Google Scholar] [CrossRef]
- Sienkiewicz, N.; Bier, R.L.; Wang, J.; Zgleszewski, L.; Lutgen, A.; Jiang, G.; Mattern, K.; Inamdar, S.; Kan, J. Bacterial communities and nitrogen transformation genes in streambank legacy sediments and implications for biogeochemical processing. Biogeochemistry 2020, in press. [Google Scholar] [CrossRef]
- Kovar, J.; Pierzynski, G. Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Waters; Southern Cooperative Series Bulletin; Virginia Tech University: Blacksburg, VA, USA, 2009. [Google Scholar]
- Bache, B.W.; Williams, E.G. A phosphate sorption index for soils. J. Soil Sci. 1971, 22, 289–301. [Google Scholar] [CrossRef]
- Hongthanat, N.; Kovar, J.L.; Thompson, M.L.; Russell, J.R.; Isenhart, T.M. Phosphorus source-sink relationships of stream sediments in the Rathbun Lake watershed in southern Iowa, USA. Environ. Monit. Assess. 2016, 188, 453. [Google Scholar] [CrossRef] [Green Version]
- House, W.A.; Denison, F.H. Factors influencing the measurement of equilibrium phosphate concentrations in river sediments. Water Res. 2000, 34, 1187–1200. [Google Scholar] [CrossRef]
- Jiang, G. Stream Bank Legacy Sediment Contributions to Suspended Sediment and Nutrient Exports from a Mid-Atlantic Piedmont Watershed. Master’s Thesis, University of Delaware, Newark, DE, USA, 2019. [Google Scholar]
- McDowell, R.W.; Sharpley, A.N. A Comparison of Fluvial Sediment Phosphorus (P) Chemistry in Relation to Location and Potential to Influence Stream P Concentrations. Aquat. Geochem. 2001, 7, 255. [Google Scholar] [CrossRef]
- Rahutomo, S.; Kovar, J.; Kovar, J.; Thompson, M.; Thompson, M. Phosphorus transformations in stream bank sediments in Iowa, USA, at varying redox potentials. J. Soils Sediments 2019, 19, 1029–1039. [Google Scholar] [CrossRef] [Green Version]
- Perillo, V.L.; Ross, D.S.; Wemple, B.C.; Balling, C.; Lemieux, L.E. Stream Corridor Soil Phosphorus Availability in a Forested-Agricultural Mixed Land Use Watershed. J. Environ. Qual. 2019, 48, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Ishee, E.R.; Ross, D.S.; Garvey, K.M.; Bourgault, R.R.; Ford, C.R. Phosphorus Characterization and Contribution from Eroding Streambank Soils of Vermont’s Lake Champlain Basin. J. Environ. Qual. 2015, 44, 1745–1753. [Google Scholar] [CrossRef] [Green Version]
- McDowell, R.; Simpson, Z.; Simpson, Z.; Stenger, R.; Stenger, R.; Depree, C.; Depree, C. The influence of a flood event on the potential sediment control of baseflow phosphorus concentrations in an intensive agricultural catchment. J. Soils Sediments 2019, 19, 429–438. [Google Scholar] [CrossRef]
- Reddy, K.R.; Kadlec, R.H.; Flaig, E.; Gale, P.M. Phosphorus Retention in Streams and Wetlands: A Review. Crit. Rev. Environ. Sci. Technol. 1999, 29, 83–146. [Google Scholar] [CrossRef]
- Young, E.O.; Ross, D.S. Total and Labile Phosphorus Concentrations as Influenced by Riparian Buffer Soil Properties. J. Environ. Qual. 2016, 45, 294–304. [Google Scholar] [CrossRef]
- Mozaffari, M.; Sims, J.T. Phosphorus availability and sorption in an Atlantic coastal plain watershed dominated by animal-based agriculture. Soil Sci. 1994, 157, 97–107. [Google Scholar] [CrossRef]
- Sobotka, M. Legacy Sediments in Streams—Effects on Nutrient Partitioning during Simulated Re-Suspension Events. Master’s Thesis, Virginia Commonwealth University, Richmond, VA, USA, 2011. [Google Scholar]
- Grundtner, A.; Gupta, S.; Bloom, P. River bank materials as a source and as carriers of phosphorus to lake pepin. J. Environ. Qual. 2014, 43, 1991–2001. [Google Scholar] [CrossRef]
- Roberts, E.J.; Cooper, R.J. Riverbed sediments buffer phosphorus concentrations downstream of sewage treatment works across the River Wensum catchment, UK. J. Soils Sediments 2018, 18, 2107–2116. [Google Scholar] [CrossRef] [Green Version]
- Rahutomo, S.; Kovar, J.L.; Thompson, M.L. Varying redox potential affects P release from stream bank sediments. PLoS ONE 2018, 13, e0209208. [Google Scholar] [CrossRef]
- Ekka, S.A.; Haggard, B.E.; Matlock, M.D.; Chaubey, I. Dissolved phosphorus concentrations and sediment interactions in effluent–dominated Ozark streams. Ecol. Eng. 2006, 26, 375–391. [Google Scholar] [CrossRef]
- Jarvie, H.P.; Jürgens, M.D.; Williams, R.J.; Neal, C.; Davies, J.J.L.; Barrett, C.; White, J. Role of river bed sediments as sources and sinks of phosphorus across two major eutrophic UK river basins: The Hampshire Avon and Herefordshire Wye. J. Hydrol. 2005, 304, 51–74. [Google Scholar] [CrossRef]
- Young, E.O.; Ross, D.S. Phosphorus Mobilization in Flooded Riparian Soils from the Lake Champlain Basin, VT, USA. Front. Environ. Sci. 2018. [Google Scholar] [CrossRef]
- Rodens, E.; Edmonds, J. Phosphate Mobilization in Iron-Rich Anaerobic Sediments: Microbial Fe(III) Oxide Reduction Versus Iron-Sulfide Formation. Arch. Hydrobiol. 1997, 139, 347–378. [Google Scholar]
- Chambers, R.M.; Odum, W.E. Porewater oxidation, dissolved phosphate and the iron curtain. Biogeochemistry 1990, 10, 37–52. [Google Scholar] [CrossRef]
- Kumaragamage, D.; Concepcion, A.; Gregory, C.; Goltz, D.; Indraratne, S.; Amarawansha, G. Temperature and freezing effects on phosphorus release from soils to overlying floodwater under flooded-anaerobic conditions. J. Environ. Qual. 2020. [Google Scholar] [CrossRef]
- Vidon, P.; Allan, C.; Burns, D.; Duval, T.; Gurwick, N.; Inamdar, S.; Lowrance, R.; Okay, J.; Scott, D.; Sebestyen, S. Hot Spots and Hot Moments in Riparian Zones: Potential for Improved Water Quality Management. J. Am. Water Resour. Assoc. 2010, 46, 278–298. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X. Effect of Temperature and Salinity on Phosphate Sorption on Marine Sediments. Environ. Sci. Technol. 2011, 45, 6831–6837. [Google Scholar] [CrossRef]
- Upreti, K.; Joshi, S.R.; McGrath, J.; Jaisi, D.P. Factors Controlling Phosphorus Mobilization in a Coastal Plain Tributary to the Chesapeake Bay. Soil Sci. Soc. Am. J. 2015, 79, 826–837. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, D.S.; Mitchell, A.M. The effects of drying and re-flooding on the sediment and soil nutrient dynamics of lowland river–floodplain systems: A synthesis. Regul. Rivers Res. Manag. 2000, 16, 457–467. [Google Scholar] [CrossRef]
- Records, R.M.; Wohl, E.; Arabi, M. Phosphorus in the river corridor. Earth Sci. Rev. 2016, 158, 65–88. [Google Scholar] [CrossRef] [Green Version]
- Sharpley, A.; Jarvie, H.P.; Buda, A.; May, L.; Spears, B.; Kleinman, P. Phosphorus Legacy: Overcoming the Effects of Past Management Practices to Mitigate Future Water Quality Impairment. J. Environ. Qual. 2013, 42, 1308–1326. [Google Scholar] [CrossRef] [Green Version]
- Noe, G.B.; Boomer, K.; Gillespie, J.L.; Hupp, C.R.; Martin-Alciati, M.; Floro, K.; Schenk, E.R.; Jacobs, A.; Strano, S. The effects of restored hydrologic connectivity on floodplain trapping vs. release of phosphorus, nitrogen, and sediment along the Pocomoke River, Maryland USA. Ecol. Eng. 2019, 138, 334–352. [Google Scholar] [CrossRef]
- Garnache, C. Solving the phosphorus pollution puzzle. Am. J. Agric. Econ. 2016, 98, 1334–1359. [Google Scholar] [CrossRef] [Green Version]
- Merritts, D.; Walter, R.; Rahnis, M.; Cox, S.; Hartranft, J.; Scheid, C.; Potter, N.; Jenschke, M.; Reed, A.; Matuszewski, D.; et al. The rise and fall of Mid-Atlantic streams: Millpond sedimentation, milldam breaching, channel incision, and stream bank erosion. Geol. Soc. Am. Rev. Eng. Geol. 2013, XXI, 183–203. [Google Scholar]
- Foley, M.M.; Bellmore, J.R.; O’Connor, J.E.; Duda, J.J.; East, A.E.; Grant, G.E.; Anderson, C.W.; Bountry, J.A.; Collins, M.J.; Connolly, P.J.; et al. Dam removal: Listening in. Water Resour. Res. 2017, 53, 5229–5246. [Google Scholar] [CrossRef]
- Ryan Bellmore, J.; Duda, J.J.; Craig, L.S.; Greene, S.L.; Torgersen, C.E.; Collins, M.J.; Vittum, K. Status and trends of dam removal research in the United States. Wiley Interdiscip. Rev. Water 2017, 4, e1164. [Google Scholar] [CrossRef]
- Tonitto, C.; Riha, S. Planning and implementing small dam removals: Lessons learned from dam removals across the eastern United States. Sustain. Water Resour. Manag. 2016, 2, 489–507. [Google Scholar] [CrossRef] [Green Version]
- Tullos, D.D.; Collins, M.J.; Bellmore, J.R.; Bountry, J.A.; Connolly, P.J.; Shafroth, P.B.; Wilcox, A.C. Synthesis of Common Management Concerns Associated with Dam Removal. J. Am. Water Resour. Assoc. 2016, 52, 1179–1206. [Google Scholar] [CrossRef]
- Palmer, M.A.; Hondula, K.L.; Koch, B.J. Ecological Restoration of Streams and Rivers: Shifting Strategies and Shifting Goals. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 247–269. [Google Scholar] [CrossRef] [Green Version]
- Newcomer Johnson, T.; Kaushal, S.; Mayer, P.; Smith, R.; Sivirichi, G. Nutrient Retention in Restored Streams and Rivers: A Global Review and Synthesis. Water 2016, 8, 116. [Google Scholar] [CrossRef] [Green Version]
- Filoso, S.; Palmer, M.A. Assessing stream restoration effectiveness at reducing nitrogen export to downstream waters. Ecol. Appl. 2011, 21, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Hartranft, J.; Merritts, D.; Walter, R.; Rahnis, M. The Big Spring Run Restoration Experiment: Policy, Geomorphology, and Aquatic Ecosystems in the Big Spring Run Watershed, Lancaster County, PA. Sustain 2011, 24, 24–30. [Google Scholar]
- Jarvie, H. Murky waters: Phosphorus mitigation to control river eutrophication. J. Environ. Qual. 2013, 42, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, S.; Johnson, E.; Rowland, R.; Warner, D.; Walter, R.; Merritts, D. Freeze–thaw processes and intense rainfall: The one-two punch for high sediment and nutrient loads from mid-Atlantic watersheds. Biogeochemistry 2018, 141, 333–349. [Google Scholar] [CrossRef] [Green Version]






| Reference | Sample Description | Grain Size (μm) | P (mg/kg) | M3-P (mg/kg) | % DPS |
|---|---|---|---|---|---|
| [22] | streambank legacy sediment | <63 | 80–1293 | 0.25–52.8 | 4.6–16 |
| [67] | (551) | (11.8) | (6.8) | ||
| >63 | 25–668 | 0.51–48.8 | 4.7–19.7 | ||
| [67] | (255) | (10.3) | (7.7) | ||
| [23] | streambank legacy sediment | Bulk | 340–958 (556) | - | - |
| [36] | streambanks bed sediments | Bulk | 209–306 | 26–68 | 3–8 |
| (269) | (39) | (5) | |||
| 177–454 | 17–37 | 5–8 | |||
| (315) | (29) | (6) | |||
| [39] | streambanks bed sediment | Bulk | 417 ± 28.7 | 14 ± 2.4 | - |
| bulk | 281 ± 37 | 22 ± 2.7 | - | ||
| [40] | streambanks bed sediment | Bulk | 370–847 | 5–55 | - |
| Bulk | 558–1134 | 13–85 | - | ||
| [41] | streambanks | bulk | 710 ± 203 | - | 15–21 |
| [42] | streambanks | bulk | 138–1140 (621) | - | 15.7–17.3 |
| [21] | forest | <63 | 368–1229 | 6.4–26.8 | 5.6–7.9 |
| [7] | (850) | (15.4) | (6.7) | ||
| >63 | 136–620 | 5.6–36.3 | 6.2–10 | ||
| [7] | (372) | (17.4) | (8) | ||
| cropland | <63 | 924–1780 | 30–237 | 10.3–60 | |
| [7] | (1260) | (149.6) | (40.4) | ||
| >63 | 280–1142 | 23–223 | 10–92 | ||
| [7] | (781) | (126) | (49) | ||
| developed | <63 | 231–2594 | 17.5–871 | 7.1–275.3 | |
| [6] | (548) | (27.7) | (12.9) | ||
| >63 | 66–911 | 6.4–380 | 8.4–293 | ||
| [6] | (192) | (14.5) | (14.2) | ||
| streambank legacy sediment | <63 | 79–719 | 0.25–28 | 4.6–8.9 | |
| [23] | (549) | (9.8) | (6.1) | ||
| >63 | 34–526 | 0.5–16.9 | 4.7–10.4 | ||
| [23] | (248) | (8.8) | (7) | ||
| bed sediment | <63 | 252–921 | 14–36 | 0–12 | |
| [32] | (668) | (25) | (9.1) | ||
| >63 | 99–611 | 7.6–23 | 10–16 | ||
| [23] | (199) | (15) | (13) |
| Location | Soil Depth | Soil Type | PSI (mg/kg) | Reference |
|---|---|---|---|---|
| Mid-Atlantic watersheds | Stream bank legacy sediments (Coarse) | 293 | This study | |
| Stream bank legacy sediments (Fine) | 652 | |||
| Delaware Inland Bays Watershed | 0–20 cm | Evesboro loamy-sand * | 149 | [46] |
| 20–40 cm | Evesboro loamy-sand * | 136 | ||
| 40–60 cm | Evesboro loamy-sand * | 217 | ||
| 60–80 cm | Evesboro loamy-sand * | 263 | ||
| 0–20 cm | Matawan sandy loam * | 588 | ||
| 20–40 cm | Matawan sandy loam * | 2083 | ||
| 40–60 cm | Matawan sandy loam * | 2564 | ||
| 60–80 cm | Matawan sandy loam * | 1886 | ||
| 0–20 cm | Matawan sandy loam ** | 434 | ||
| 20–40 cm | Matawan sandy loam ** | 1562 | ||
| 40–60 cm | Matawan sandy loam ** | 2000 | ||
| 60–80 cm | Matawan sandy loam ** | 1923 | ||
| 0–20 cm | Pocomoke sandy clay loam * | 95 | ||
| 20–40 cm | Pocomoke sandy clay loam * | 714 | ||
| 40–60 cm | Pocomoke sandy clay loam * | 212 | ||
| 60–80 cm | Pocomoke sandy clay loam * | 303 | ||
| Mahantango Creek Catchment (Central PA) | Agricultural catchment exposed stream bank | 259 | [39] | |
| Agricultural catchment submerged bank sediment | 214 |
| Site Name | Depth (cm) | EPC0 (mg/L) | Stream PO43− Concentration (mg/L) | Land Use | Sink or Source |
|---|---|---|---|---|---|
| Gramies Run (GMT) | 107 | 0.028 | 0 | Forest | Source |
| Middle Run (MR) | 91 | 0.044 | 0.036 | Forest | Source |
| Cider Mill (CDM) | 183 | 0.024 | 0.01 | Suburban | Source |
| Casho Mill (CM) | 102 | 0.020 | 0.005 | Suburban | Source |
| Cottage Mill (RH) | 76 | 0.000 | 0.001 | Suburban | Sink |
| Byrnes Mill (BYR) | 163 | 0.035 | 0.023 | Urban | Source |
| Brandywine Zoo (BZ) | 76 | 0.240 | 0.064 | Urban | Source |
| Cooch’s Bridge (COB) | 38 | 0.136 | 0.004 | Urban | Source |
| Woolen Mill (WM) | 61 | 0.001 | 0.205 | Urban | Sink |
| Big Elk Bridge (BEB) | 122 | 0.027 | 0.004 | Agriculture | Source |
| Camp Bonsul Road (CB) | 137 | 0.010 | 0.066 | Agriculture | Sink |
| Nature Center Beach (NCB) | 114 | 0.027 | 0.032 | Agriculture | Sink |
| Scotts Mill 2 (SM2) | 122 | 0.011 | 0.002 | Agriculture | Source |
| Scotts Mill 3 (SM3) | 183 | 0.033 | 0.002 | Agriculture | Source |
| Tweeds Mill (TM) | 81 | 0.026 | 0.039 | Agriculture | Sink |
| 10 sources and 5 sinks | |||||
| Location | EPC0 (mg/L) | Reference |
|---|---|---|
| Delaware, Maryland, & Pennsylvania Legacy Sediment (mean) | 0.044 | This study |
| Lake Pepin stream bank till sediment | <0.1 | [48] |
| River Wensum Catchment (UK) | 0.085 | [49] |
| Mahantango Creek Catchment (Central PA) | [39] | |
| Bed sediment | 0.043 | |
| Bank sediment | 0.02 | |
| Courthouse Creek Sediment VA | 0.090 | [47] |
| Kimages Creek Sediment VA (Legacy sediment) | 0.010 | [47] |
| Rathburn Lake Watershed (Iowa): | [36] | |
| Bed sediments (mean) | 0.09 | |
| Bank sediments (mean) | 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inamdar, S.; Sienkiewicz, N.; Lutgen, A.; Jiang, G.; Kan, J. Streambank Legacy Sediments in Surface Waters: Phosphorus Sources or Sinks? Soil Syst. 2020, 4, 30. https://0-doi-org.brum.beds.ac.uk/10.3390/soilsystems4020030
Inamdar S, Sienkiewicz N, Lutgen A, Jiang G, Kan J. Streambank Legacy Sediments in Surface Waters: Phosphorus Sources or Sinks? Soil Systems. 2020; 4(2):30. https://0-doi-org.brum.beds.ac.uk/10.3390/soilsystems4020030
Chicago/Turabian StyleInamdar, Shreeram, Nathan Sienkiewicz, Alyssa Lutgen, Grant Jiang, and Jinjun Kan. 2020. "Streambank Legacy Sediments in Surface Waters: Phosphorus Sources or Sinks?" Soil Systems 4, no. 2: 30. https://0-doi-org.brum.beds.ac.uk/10.3390/soilsystems4020030