Correlations of Soil Fungi, Soil Structure and Tree Vigour on an Apple Orchard with Replant Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Site and Sampling Design
2.2. Measurement of Tree Vigour
2.3. Soil Sampling
2.4. Size Fractionation of Soil
2.5. Fractionation of Aggregate-Stability Classes
2.5.1. Calibration of the Ultrasonication Device
2.5.2. Dispersive Treatment
2.6. Quantification of Soil Fungal Densities
2.7. Statistical Analyses
3. Results
3.1. Soil Fungal Densities in No-Replant Soil and Replant Soil
3.2. Aggregation Level in No-Replant and Replant Soil
3.3. Aggregate-Stability of No-Replant and Replant Soil
3.4. Correlations between Soil Fungi and Structural Parameters
3.5. Correlation between Tree Vigour (CSA), Soil Fungi and Soil Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Winkelmann, T.; Smalla, K.; Amelung, W.; Baab, G.; Grunewaldt-Stöcker, G.; Kanfra, X.; Meyhöfer, R.; Reim, S.; Schmitz, M.; Vetterlein, D.; et al. Apple Replant Disease: Causes and Mitigation Strategies. Curr. Issues Mol. Biol. 2019, 30, 89–106. [Google Scholar] [CrossRef] [Green Version]
- Rani, G.D. Advances in Soil Borne Plant Diseases; New India Publishing: New Delhi, India, 2008. [Google Scholar]
- Verma, L.R.; Sharma, R.C. Diseases of Horticultural Crops: Fruits; Indus Publishing: New Delhi, India, 1999. [Google Scholar]
- Mazzola, M.; Manici, L.M. Apple Replant Disease: Role of Microbial Ecology in Cause and Control. Annu. Rev. Phytopathol. 2012, 50, 45–65. [Google Scholar] [CrossRef]
- Mai, W.F. Determining the Cause and Extent of Apple, Cherry, and Pear Replant Diseases Under Controlled Conditions. Phytopathology 1978, 68, 1540–1544. [Google Scholar] [CrossRef]
- Mazzola, M. Elucidation of the Microbial Complex Having a Causal Role in the Development of Apple Replant Disease in Washington. Phytopathology 1998, 88, 930–938. [Google Scholar] [CrossRef] [Green Version]
- Spath, M.; Insam, H.; Peintner, U.; Kelderer, M.; Kuhnert, R.; Franke-Whittle, I.H. Linking Soil Biotic and Abiotic Factors to Apple Replant Disease: A Greenhouse Approach. J. Phytopathol. 2015, 163, 287–299. [Google Scholar] [CrossRef]
- Yim, B.; Smalla, K.; Winkelmann, T. Evaluation of apple replant problems based on different soil disinfection treatments—Links to soil microbial community structure? Plant Soil 2013, 366, 617–631. [Google Scholar] [CrossRef]
- Franke-Whittle, I.H.; Manici, L.M.; Insam, H.; Stres, B. Rhizosphere bacteria and fungi associated with plant growth in soils of three replanted apple orchards. Plant Soil 2015, 395, 317–333. [Google Scholar] [CrossRef]
- Manici, L.; Kelderer, M.; Franke-Whittle, I.; Rühmer, T.; Baab, G.; Nicoletti, F.; Caputo, F.; Topp, A.; Insam, H.; Naef, A. Relationship between root-endophytic microbial communities and replant disease in specialized apple growing areas in Europe. Appl. Soil Ecol. 2013, 72, 207–214. [Google Scholar] [CrossRef]
- Tewoldemedhin, Y.T.; Mazzola, M.; Labuschagne, I.; McLeod, A. A multi-phasic approach reveals that apple replant disease is caused by multiple biological agents, with some agents acting synergistically. Soil Biol. Biochem. 2011, 43, 1917–1927. [Google Scholar] [CrossRef]
- Nicola, L.; Insam, H.; Pertot, I.; Stres, B. Reanalysis of microbiomes in soils affected by apple replant disease (ARD): Old foes and novel suspects lead to the proposal of extended model of disease development. Appl. Soil Ecol. 2018, 129, 24–33. [Google Scholar] [CrossRef]
- Fazio, G.; Kviklys, D.; Grusak, M.A.; Robinson, T.L. Soil pH, soil type and replant disease affect growth and nutrient absorption in apple rootstocks. N. Y. Fruit Q. 2012, 20, 22–28. [Google Scholar]
- Utkhede, R.S. Soil sickness, replant problem or replant disease and its integrated control. Allelopath. J. 2006, 18, 23–38. [Google Scholar]
- Mahnkopp, F.; Simon, M.; Lehndorff, E.; Pätzold, S.; Wrede, A.; Winkelmann, T. Induction and diagnosis of apple replant disease (ARD): A matter of heterogeneous soil properties? Sci. Hortic. 2018, 241, 167–177. [Google Scholar] [CrossRef]
- Sheng, Y.; Wang, H.; Wang, M.; Li, H.; Xiang, L.; Pan, F.; Chen, X.; Shen, X.; Yin, C.; Mao, Z.; et al. Effects of Soil Texture on the Growth of Young Apple Trees and Soil Microbial Community Structure under Replanted Conditions. Hortic. Plant J. 2020, 6, 123–131. [Google Scholar] [CrossRef]
- Simon, M.; Lehndorff, E.; Wrede, A.; Amelung, W. In-field heterogeneity of apple replant disease: Relations to abiotic soil properties. Sci. Hortic. 2020, 259, 108809. [Google Scholar] [CrossRef]
- Tilston, E.L.; Deakin, G.; Bennett, J.; Passey, T.; Harrison, N.; O’Brien, F.; Fernández-Fernández, F.; Chen, W.-H. Candidate Causal Organisms for Apple Replant Disease in the United Kingdom. Phytobiomes J. 2018, 2, 261–274. [Google Scholar] [CrossRef] [Green Version]
- Cavael, U.; Diehl, K.; Lentzsch, P. Assessment of growth suppression in apple production with replant soils. Ecol. Indic. 2020, 109, 105846. [Google Scholar] [CrossRef]
- Van Schoor, L.; Denman, S.; Cook, N. Characterisation of apple replant disease under South African conditions and potential biological management strategies. Sci. Hortic. 2009, 119, 153–162. [Google Scholar] [CrossRef]
- Bach, E.M.; Williams, R.J.; Hargreaves, S.K.; Yang, F.; Hofmockel, K.S. Greatest soil microbial diversity found in micro-habitats. Soil Biol. Biochem. 2018, 118, 217–226. [Google Scholar] [CrossRef]
- Fox, A.; Ikoyi, I.; Torres-Sallan, G.; Lanigan, G.; Schmalenberger, A.; Wakelin, S.; Creamer, R. The influence of aggregate size fraction and horizon position on microbial community composition. Appl. Soil Ecol. 2018, 127, 19–29. [Google Scholar] [CrossRef]
- Nunan, N. The microbial habitat in soil: Scale, heterogeneity and functional consequences. J. Plant Nutr. Soil Sci. 2017, 180, 425–429. [Google Scholar] [CrossRef] [Green Version]
- Ditterich, F. Microbial Community Structure and Function is Shaped by Microhabitat Characteristics in Soil. Ph.D. Thesis, Universität Hohenheim, Hohenheim, Germany, 2016. [Google Scholar]
- Torsvik, V.; Øvreås, L. Microbial diversity and function in soil: From genes to ecosystems. Curr. Opin. Microbiol. 2002, 5, 240–245. [Google Scholar] [CrossRef]
- Büks, F.; Rebensburg, P.; Lentzsch, P.; Kaupenjohann, M. Relation of aggregate stability and microbial diversity in an incubated sandy soil. SOIL Discuss. 2016. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A.; et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2017, 181, 104–136. [Google Scholar] [CrossRef] [Green Version]
- Kandeler, E.; Tscherko, D.; Bruce, K.D.; Stemmer, M.; Hobbs, P.J.; Bardgett, R.D.; Amelung, W. Structure and function of the soil microbial community in microhabitats of a heavy metal polluted soil. Biol. Fertil. Soils 2000, 32, 390–400. [Google Scholar] [CrossRef]
- Kravchenko, A.N.; Negassa, W.; Guber, A.K.; Hildebrandt, B.; Marsh, T.; Rivers, M.L. Intra-aggregate Pore Structure Influences Phylogenetic Composition of Bacterial Community in Macroaggregates. Soil Sci. Soc. Am. J. 2014, 78, 1924–1939. [Google Scholar] [CrossRef]
- Lǚ, L.-H.; Zou, Y.-N.; Wu, Q.-S. Mycorrhizas Mitigate Soil Replant Disease of Peach Through Regulating Root Exudates, Soil Microbial Population, and Soil Aggregate Stability. Commun. Soil Sci. Plant Anal. 2019, 50, 909–921. [Google Scholar] [CrossRef]
- Büks, F.; Kaupenjohann, M. Enzymatic biofilm detachment causes a loss of aggregate stability in a sandy soil. SOIL Discuss. 2016. [Google Scholar] [CrossRef]
- Chotte, J.L. Importance of microorganisms for soil aggregation. In Microorganisms in Soils: Roles in Genesis and Functions; Varma, A., Buscot, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 3, pp. 107–119. [Google Scholar]
- Lynch, J.M.; Bragg, E. Microorganisms and Soil Aggregate Stability. In Advances in Soil Science; Stewart, B.A., Ed.; Springer: New York, NY, USA, 1985; pp. 133–171. [Google Scholar]
- Helliwell, J.; Miller, A.; Whalley, W.; Mooney, S.; Sturrock, C. Quantifying the impact of microbes on soil structural development and behaviour in wet soils. Soil Biol. Biochem. 2014, 74, 138–147. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; DeGryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. Eur. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Aspiras, R.; Allen, O.; Harris, R.; Chesters, G. The role of microorganisms in the stabilization of soil aggregates. Soil Biol. Biochem. 1971, 3, 347–353. [Google Scholar] [CrossRef]
- Abiven, S.; Menasseri, S.; Chenu, C. The effects of organic inputs over time on soil aggregate stability—A literature analysis. Soil Biol. Biochem. 2009, 41, 1–12. [Google Scholar] [CrossRef]
- Bach, E.M.; Hofmockel, K.S. A time for every season: Soil aggregate turnover stimulates decomposition and reduces carbon loss in grasslands managed for bioenergy. GCB Bioenergy 2016, 8, 588–599. [Google Scholar] [CrossRef] [Green Version]
- Rumberger, A.; Merwin, I.A.; Thies, J.E. Microbial community development in the rhizosphere of apple trees at a replant disease site. Soil Biol. Biochem. 2007, 39, 1645–1654. [Google Scholar] [CrossRef]
- Shishido, M.; Sakamoto, K.; Yokoyama, H.; Momma, N.; Miyashita, S.-I. Changes in microbial communities in an apple orchard and its adjacent bush soil in response to season, land-use, and violet root rot infestation. Soil Biol. Biochem. 2008, 40, 1460–1473. [Google Scholar] [CrossRef]
- LBGR (Landesamt für Bergbau, Geologie und Rohstoffe Brandenburg). Bodengeologie Brandenburg—Grundkarten, Boden—Grundkarten. Available online: http://www.geo.brandenburg.de/boden/ (accessed on 1 July 2020).
- WetterKontor GmbH. Monats und Jahreswerte. Available online: https://www.wetterkontor.de/de/wetter/deutschland/monatswerte-station.asp (accessed on 1 July 2020).
- Roberson, E.B.; Firestone, M.K. Relationship between Desiccation and Exopolysaccharide Production in a Soil Pseudomonas sp. Appl. Environ. Microbiol. 1992, 58, 1284–1291. [Google Scholar] [CrossRef] [Green Version]
- Zornoza, R.; Guerrero, C.; Mataix-Solera, J.; Arcenegui, V.; García-Orenes, F.; Mataix-Beneyto, J. Assessing the effects of air-drying and rewetting pre-treatment on soil microbial biomass, basal respiration, metabolic quotient and soluble carbon under Mediterranean conditions. Eur. J. Soil Biol. 2007, 43, 120–129. [Google Scholar] [CrossRef]
- Blaud, A.; Menon, M.; Van der Zaan, B.; Lair, G.J.; Banwart, S.A. Effects of dry and wet sieving of soil on identification and interpretation of microbial community composition. In Advances in Agronomy; Banwart, A.S., Sparks, D.L., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 142, pp. 119–142. [Google Scholar] [CrossRef]
- Wentworth, C.K. A Scale of Grade and Class Terms for Clastic Sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Díaz-Zorita, M.; Villegas, E.I.G.; Grove, A.J.; Perfect, E. Aggregation, fragmentation, and structural stability measurement. In Encyclopedia of Soil Science; Lal, R., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 37–40. [Google Scholar]
- Van Bavel, C.H.M. Mean Weight-Diameter of Soil Aggregates as a Statistical Index of Aggregation. Soil Sci. Soc. Am. J. 1949, 14, 20–23. [Google Scholar] [CrossRef] [Green Version]
- North, P.F. Towards an absolute measurement of soil structural stability using ultrasound. Eur. J. Soil Sci. 1976, 27, 451–459. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Rumpel, C.; Kögel-Knabner, I. Evaluation of an ultrasonic dispersion procedure to isolate primary organomineral complexes from soils. Eur. J. Soil Sci. 1999, 50, 87–94. [Google Scholar] [CrossRef]
- Graf-Rosenfellner, M.; Kayser, G.; Guggenberger, G.; Kaiser, K.; Büks, F.; Kaiser, M.; Mueller, C.W.; Schrumpf, M.; Rennert, T.; Welp, G.; et al. Replicability of aggregate disruption by sonication-an inter-laboratory test using three different soils from Germany. J. Plant Nutr. Soil Sci. 2018, 181, 894–904. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.C. Aggregate stability and size distribution. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; American Society of Agronomy: Madison, Wisconsin, 1986; Volume 5, pp. 425–442. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols—A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Grube, S.; Schönling, J.; Prange, A. Evaluation of a triplex real-time PCR system to detect the plant-pathogenic molds Alternaria spp., Fusarium spp. and C. purpurea. J. Microbiol. Methods 2015, 119, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.P.; Gannibal, P.B.; Peever, T.; Pryor, B.M. The sections of Alternaria: Formalizing species-group concepts. Mycologia 2013, 105, 530–546. [Google Scholar] [CrossRef] [Green Version]
- Woudenberg, J.; Seidl, M.F.; Groenewald, J.; De Vries, M.; Stielow, J.; Thomma, B.P.H.J.; Crous, P. Alternaria section Alternaria: Species, formae speciales or pathotypes? Stud. Mycol. 2015, 82, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Müller, T.; Ruppel, S.; Behrendt, U.; Lentzsch, P.; Müller, M.E.H. Antagonistic Potential of Fluorescent Pseudomonads Colonizing Wheat Heads Against Mycotoxin Producing Alternaria and Fusaria. Front. Microbiol. 2018, 9, 2124. [Google Scholar] [CrossRef]
- Korn, U.; Müller, T.; Ulrich, A.; Müller, M.E.H. Impact of aggressiveness of Fusarium graminearum and F. culmorum isolates on yield parameters and mycotoxin production in wheat. Mycotoxin Res. 2011, 27, 195–206. [Google Scholar] [CrossRef]
- Hoestra, H. Replant Diseases of Apple in the Netherlands. Ph.D. Thesis, Agricultural University, Wageningen, The Netherlands, 1968. [Google Scholar]
- Zhang, Z.Z.; Srivastava, A.K.; Wu, Q.S.; Li, G.H. Growth performance and rhizospheric traits of peach (Prunus persica) in response to mycorrhization on replant versus non-replant soil. Indian J. Agric. Sci. 2015, 85, 125–130. [Google Scholar]
- Szczygiel, A.; Zepp, A. An occurrence and importance of apple replant disease in Polish orchards. In: IV International Symposium on Replant Problems. Acta Hortic. 1996, 477, 99–102. [Google Scholar] [CrossRef]
- Lehmann, A.; Zheng, W.; Rillig, M.C. Soil biota contributions to soil aggregation. Nat. Ecol. Evol. 2017, 1, 1828–1835. [Google Scholar] [CrossRef]
- Lehmann, A.; Zheng, W.; Ryo, M.; Soutschek, K.; Roy, J.; Rongstock, R.; Maaß, S.; Rillig, M.C. Fungal Traits Important for Soil Aggregation. Front. Microbiol. 2020, 10, 2904. [Google Scholar] [CrossRef] [PubMed]
- Jastrow, J. Soil aggregate formation and the accrual of particulate and mineral-associated organic matter. Soil Biol. Biochem. 1996, 28, 665–676. [Google Scholar] [CrossRef]
- van Marle, G.S. Bodemmoeheid in de fruitteelt; resultaten van een enquete in het rivier kleigebied. Tijdschr. PIZiekt. 1961, 67, 57–68. [Google Scholar]
- Eissenstat, D.; Bauerle, T.; Comas, L.; Lakso, A.; Neilsen, D.; Neilsen, G.; Smart, D. Seasonal patterns of root growth in relation to shoot phenology in grape and apple. Proc. Vth International Symposium on Mineral Nutrition of Fruit Plants. Acta Hortic. 2005, 721, 21–26. [Google Scholar] [CrossRef]
- Psarras, G.; Merwin, I.A.; Lakso, A.N.; Ray, J.A. Root Growth Phenology, Root Longevity, and Rhizosphere Respiration of Field Grown ‘Mutsu’ Apple Trees on ‘Malling 9’ Rootstock. J. Am. Soc. Hortic. Sci. 2000, 125, 596–602. [Google Scholar] [CrossRef] [Green Version]
- Kaspar, F.; Zimmermann, K.; Polte-Rudolf, C. An overview of the phenological observation network and the phenological database of Germany’s national meteorological service (Deutscher Wetterdienst). Adv. Sci. Res. 2014, 11, 93–99. [Google Scholar] [CrossRef] [Green Version]
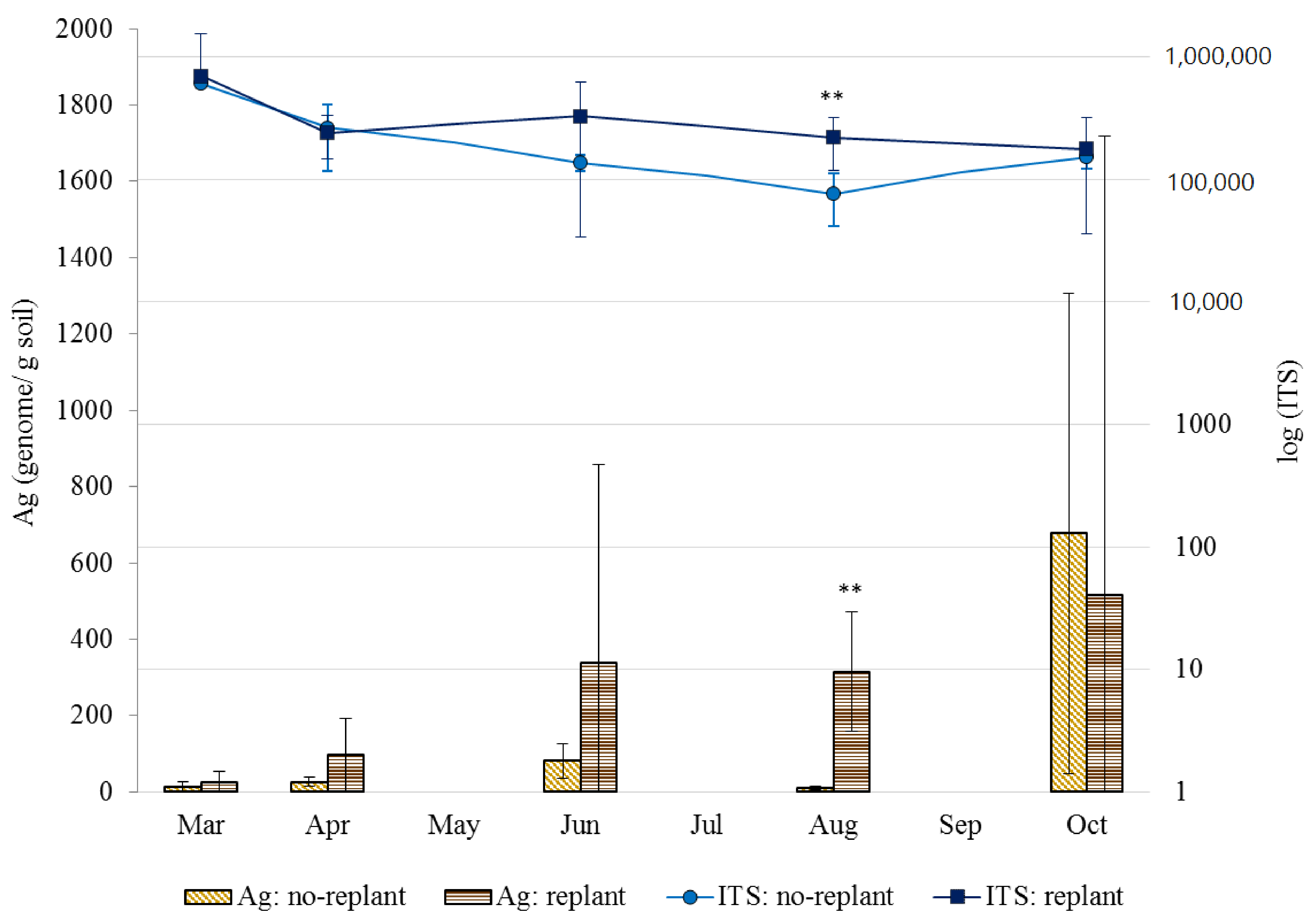

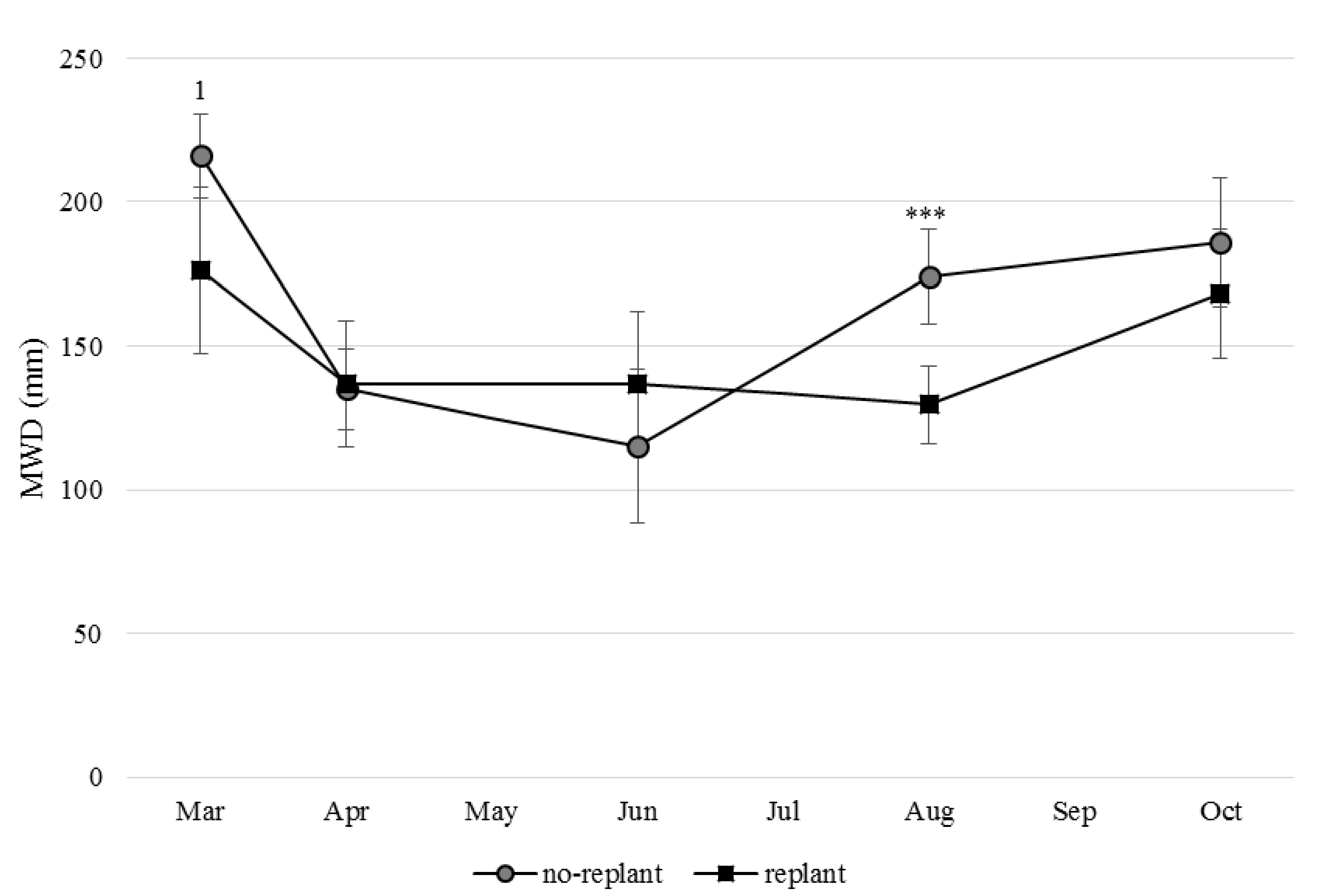
| Sieve-Fraction (µm) | Soil | March | April | June | August | October | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MW | SD | MW | SD | MW | SD | MW | SD | MW | SD | ||
| 2000–6300 | nr | 45.15 | 1.28 | 22.34 | 3.42 | 18.86 | 5.86 | 33.28 | 4.19 | 38.58 | 6.53 |
| r | 35.93 | 7.14 | 22.39 | 4.82 | 22.92 | 5.96 | 21.24 *** | 3.52 | 31.89 | 6.78 | |
| 1000–2000 | nr | 11.46 | 4.88 | 13.64 | 1.45 | 10.96 | 2.04 | 13.86 | 1.02 | 8.34 | 3.14 |
| r | 8.57 | 1.05 | 14.84 | 2.73 | 14.54 | 2.53 | 13.31 | 1.13 | 12.70 * | 2.88 | |
| 500–1000 | nr | 6.56 | 2.76 | 15.44 | 1.70 | 12.09 | 0.85 | 9.47 | 2.05 | 6.81 | 2.08 |
| r | 7.13 | 0.59 | 15.81 | 1.08 | 13.84 | 2.72 | 14.57 *** | 1.64 | 11.06 * | 2.99 | |
| 250–500 | nr | 7.98 | 7.24 | 15.85 | 0.46 | 14.97 | 0.88 | 10.57 | 1.93 | 6.81 | 0.71 |
| r | 11.38 | 1.51 | 14.85 | 2.34 | 13.61 | 3.48 | 15.16 *** | 1.34 | 10.90 | 2.18 | |
| 125–250 | nr | 13.71 | 7.71 | 18.16 | 2.34 | 23.24 | 4.42 | 16.24 | 1.25 | 18.21 | 0.67 |
| r | 17.88 | 3.35 | 17.54 | 3.23 | 18.97 | 3.20 | 20.89 ** | 2.02 | 17.68 | 1.24 | |
| ≤125 | nr | 15.14 | 7.83 | 14.56 | 1.98 | 19.88 | 3.83 | 16.59 | 2.78 | 18.83 | 2.90 |
| r | 19.11 | 3.26 | 14.58 | 2.54 | 16.12 | 1.85 | 14.83 | 1.20 | 15.77 * | 1.73 | |
| Aggregate-Stability Class | Soil | 125–250 µm | 250–500 µm | 500–1000 µm | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MW | SD | % | MW | SD | % | MW | SD | % | ||
| WL | nr | 42 | 17 | 1 | 280 | 84 | 12 | 711 | 200 | 24 |
| r | 58 | 14 | 2 | 279 | 60 | 9 | 693 | 140 | 23 | |
| WS ≤ 50 J mL−1 | nr | 405 | 100 | 14 | 892 | 139 | 31 | 1203 | 224 | 40 |
| r | 679 *** | 81 | 23 | 1235 *** | 75 | 41 | 1369 | 122 | 46 | |
| WS ≤ 100 J mL−1 | nr | 58 | 25 | 2 | 183 | 82 | 6 | 71 | 23 | 2 |
| r | 79 | 25 | 3 | 132 | 57 | 4 | 65 | 16 | 2 | |
| WS > 100 J mL−1 | nr | 2494 | 95 | 83 | 1645 | 248 | 51 | 1015 | 146 | 34 |
| r | 2184 *** | 90 | 73 | 1355 * | 82 | 45 | 873 * | 30 | 29 | |
| Soil Structure Parameter | Fungal Parameter | March | April | June | August | October | |
|---|---|---|---|---|---|---|---|
| MWD | Ag/ITS | −0.442 | −0.527 | −0.147 | −0.790 ** | (R2 = 0.674) | 0.238 |
| Ag | −0.624 | −0.482 | −0.154 | −0.895 *** | (R2 = 0.825) | 0.102 | |
| ITS | −0.055 | −0.082 | 0.154 | −0.671 * | (R2 = 0.570) | −0.140 | |
| 2000–6300 µm | Ag/ITS | −0.527 | −0.564 | −0.049 | −0.846 | 0.294 | |
| Ag | −0.685 * (R2 = 0.215) | −0.500 | −0.140 | −0.951 ** | (R2 = 0.827) | 0.137 | |
| ITS | −0.079 | −0.100 | 0.084 | −0.629 * | (R2 = 0.562) | −0.133 | |
| 1000–2000 µm | Ag/ITS | −0.336 | −0.136 | −0.294 | −0.280 | −0.483 | |
| Ag | −0.445 | −0.227 | −0.217 | −0.399 | −0.238 | ||
| ITS | 0.045 | −0.327 | 0.154 | −0.399 | 0.119 | ||
| 500–1000 µm | Ag/ITS | −0.527 | −0.564 | −0.049 | −0.846 ** | (R2 = 0.554) | 0.294 |
| Ag | 0.309 | −0.245 | 0.021 | 0.650 * | (R2 = 0.675) | −0.294 | |
| ITS | 0.027 | −0.464 | 0.259 | 0.545 | 0.056 | ||
| 250–500 µm | Ag/ITS | 0.482 | 0.182 | −0.133 | 0.664 * | (R2 = 0.598) | −0.385 |
| Ag | 0.700 * (R2 = 0.021) | 0.191 | 0.021 | 0.797 ** | (R2 = 0.809) | −0.371 | |
| ITS | 0.264 | 0.055 | −0.007 | 0.762 ** | (R2 = 0.688) | −0.189 | |
| 125–250 µm | Ag/ITS | 0.418 | 0.391 | 0.413 | 0.804 ** | (R2 = 0.635) | 0.154 |
| Ag | 0.564 | 0.373 | 0.308 | 0.867 ** | (R2 = 0.745) | 0.186 | |
| ITS | −0.036 | 0.227 | −0.203 | 0.605 * | (R2 = 0.466) | 0.168 | |
| ≤125 µm | Ag/ITS | 0.427 | 0.409 | 0.476 | −0.063 | 0.552 | |
| Ag | 0.664 * (R2 = 0.007) | 0.427 | 0.301 | −0.126 | 0.406 | ||
| ITS | 0.182 | 0.227 | −0.196 | −0.399 | 0.315 | ||
| Soil 1 | March | April | June | August | October | |
|---|---|---|---|---|---|---|
| Ag/ITS | nr + r | −0.109 | −0.392 | 0.332 | −0.818 ** (R2 = 0.77) | 0.574 |
| r | −0.059 | −0.228 | 0.197 | −0.679 | 0.418 | |
| Ag | nr + r | −0.273 | −0.469 | 0.056 | −0.782 ** (R2 = 0.70) | 0.420 |
| r | −0.301 | −0.287 | 0.192 | −0.429 | 0.117 | |
| ITS | nr + r | 0.118 | −0.255 | −0.242 | −0.370 | −0.018 |
| r | −0.059 | −0.431 | 0.084 | 0.679 | −0.059 | |
| MWD | nr + r | 0.483 | −0.027 | 0.021 | 0.595 | 0.266 |
| r | 0.151 | −0.156 | 0.360 | 0.084 | −0.033 | |
| 2000–6300 µm | nr + r | 0.452 | 0.000 | 0.109 | 0.687 *(R2 = 0.51) | 0.308 |
| r | 0.101 | −0.024 | 0.460 | 0.268 | −0.117 | |
| 1000–2000 µm | nr + r | 0.487 | −0.328 | −0.186 | 0.039 | −0.447 |
| r | 0.426 | −0.359 | 0.209 | −0.218 | 0.004 | |
| 500–1000 µm | nr + r | 0.494 | −0.169 | −0.613 | −0.716 ** (R2 = 0.55) | −0.550 |
| r | 0.445 | −0.539 | −0.747 | −0.387 | −0.226 | |
| 250–500 µm | nr + r | −0.158 | 0.200 | −0.137 | −0.712 ** (R2 = 0.62) | −0.343 |
| r | −0.244 | −0.156 | −0.510 | −0.328 | −0.076 | |
| 125–250 µm | nr + r | −0.095 | 0.173 | 0.161 | −0.487 | 0.028 |
| r | −0.177 | 0.252 | −0.134 | 0.109 | −0.259 | |
| ≤125 µm | nr + r | −0.126 | 0.183 | 0.483 | 0.106 | 0.441 |
| r | −0.226 | 0.383 | 0.351 | 0.029 | 0.276 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavael, U.; Tost, P.; Diehl, K.; Büks, F.; Lentzsch, P. Correlations of Soil Fungi, Soil Structure and Tree Vigour on an Apple Orchard with Replant Soil. Soil Syst. 2020, 4, 70. https://0-doi-org.brum.beds.ac.uk/10.3390/soilsystems4040070
Cavael U, Tost P, Diehl K, Büks F, Lentzsch P. Correlations of Soil Fungi, Soil Structure and Tree Vigour on an Apple Orchard with Replant Soil. Soil Systems. 2020; 4(4):70. https://0-doi-org.brum.beds.ac.uk/10.3390/soilsystems4040070
Chicago/Turabian StyleCavael, Ulrike, Philipp Tost, Katharina Diehl, Frederick Büks, and Peter Lentzsch. 2020. "Correlations of Soil Fungi, Soil Structure and Tree Vigour on an Apple Orchard with Replant Soil" Soil Systems 4, no. 4: 70. https://0-doi-org.brum.beds.ac.uk/10.3390/soilsystems4040070





