Raman Microspectroscopic Imaging of Binder Remnants in Historical Mortars Reveals Processing Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Raman Microspectroscopy
2.3. Scanning Electron Microscopy
3. Results and Discussion
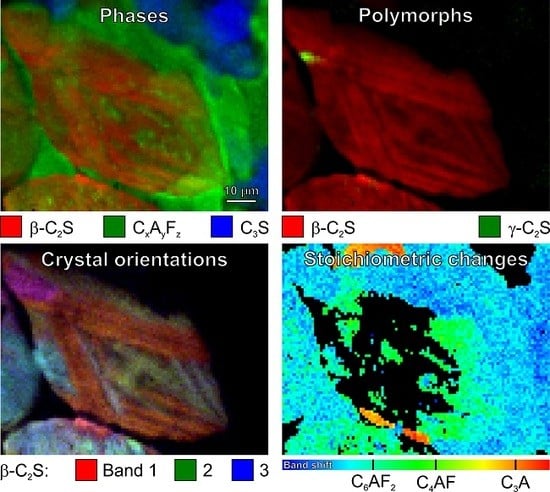
3.1. Intensities of Marker Bands Provide Access to Distributions of Phases in Clinker Remnants in Early Cement Mortars
3.2. Raman Spectra Enable the Discrimination of Polymorphs of Cement Clinker Phases
3.3. Changes in Relative Band Intensities Visualise Different Crystal Orientations
3.4. Band Shifts Elucidate Stoichiometric Changes and Members of Solid Solution Series
3.5. Band Widths are Measures for Crystallinities and Provide Access to the Thermal History of Medieval Gypsum Mortars
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaszowska, Z.; Malek, K.; Staniszewska-Slezak, E.; Niedzielska, K. Raman scattering or fluorescence emission? Raman spectroscopy study on lime-based building and conservation materials. Spectrochim. Acta A 2016, 169, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Schmid, T.; Dariz, P. Determination and imaging of binder remnants and aggregates in historic artificial stone by Raman microscopy. J. Raman Spectrosc. 2013, 44, 882–891. [Google Scholar] [CrossRef]
- Schmid, T.; Dariz, P. Chemical imaging of historical mortars by Raman microscopy. Constr. Build. Mater. 2016, 114, 506–516. [Google Scholar] [CrossRef]
- Jallad, K.; Santhanam, M.; Cohen, M.; Ben-Amotz, D. Chemical mapping of thaumasite in sulfate-attacked cement mortar using near-infrared Raman imaging microscopy. Cem. Concr. Res. 2001, 31, 953–958. [Google Scholar] [CrossRef]
- Sahu, S.; Exline, D.; Nelson, M. Identification of thaumasite in concrete by Raman chemical imaging. Cem. Concrete Comp. 2002, 24, 347–350. [Google Scholar] [CrossRef]
- García-Florentino, C.; Maguregui, M.; Morillas, H.; Balziskueta, U.; Azcarate, A.; Arana, G.; Madariaga, J. Portable and Raman imaging usefulness to detect decaying on mortars from Punta Begoña Galleries (Getxo, North of Spain). J. Raman Spectrosc. 2016, 47, 1458–1466. [Google Scholar] [CrossRef]
- Irazola, M.; Olivares, M.; Castro, K.; Maguregui, M.; Martínez-Arkarazo, I.; Madariaga, J. In situ Raman spectroscopy analysis combined with Raman and SEM-EDS imaging to assess the conservation state of 16th century wall paintings. J. Raman Spectrosc. 2012, 43, 1676–1684. [Google Scholar] [CrossRef]
- Maguregui, M.; Knuutinen, U.; Trebolazabala, J.; Morillas, H.; Castro, K.; Martínez-Arkarazo, I.; Madariaga, J. Use of in situ and confocal Raman spectroscopy to study the nature and distribution of carotenoids in brown patinas from a deteriorated wall painting in Marcus Lucretius House (Pompeii). Anal. Bioanal. Chem. 2012, 402, 1529–1539. [Google Scholar] [CrossRef]
- Veneranda, M.; Irazola, M.; Pitarch, A.; Olivares, M.; Iturregui, A.; Castro, K.; Madariaga, J. In-situ and laboratory Raman analysis in the field of cultural heritage: The case of a mural painting. J. Raman Spectrosc. 2014, 45, 228–237. [Google Scholar] [CrossRef]
- Raman, C.V.; Krishnan, K.S. A new type of secondary radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Opilik, L.; Schmid, T.; Zenobi, R. Modern Raman imaging: Vibrational spectroscopy on the micrometer and nanometer scales. Annu. Rev. Anal. Chem. 2013, 6, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Rayleigh, L. Investigations in Optics, with Special Reference to the Spectroscope. Philos. Mag. 1879, 8, 261–274. [Google Scholar] [CrossRef]
- Schmid, T.; Schäfer, N.; Levcenko, S.; Rissom, T.; Abou-Ras, D. Orientation-distribution mapping of polycrystalline materials by Raman microspectroscopy. Sci. Rep. 2015, 5, 18410. [Google Scholar] [CrossRef] [PubMed]
- Schmid, T.; Schäfer, N.; Abou-Ras, D. Raman microspectroscopy provides access to compositional and microstructural details of polycrystalline materials. Spectrosc. Eur. 2016, 28, 16–20. [Google Scholar]
- Campbell, D. Microscopical Examination and Interpretation of Portland Cement and Clinker, 2nd ed.; Portland Cement Association: Skokie, IL, USA, 1999. [Google Scholar]
- Gille, F.; Dreizler, I.; Grade, K.; Krämer, H.; Woermann, E. Mikroskopie des Zementklinkers: Bilderatlas; Beton-Verlag: Düsseldorf, Germany, 1965. [Google Scholar]
- Hofmänner, F. Portlandzement-Klinker. Kleine Gefügekunde; Holderbank: Heerbrugg, Switzerland, 1973. [Google Scholar]
- Stutzman, P. Microscopy of clinker and hydraulic cements. Rev. Mineral. Geochem. 2012, 74, 101–146. [Google Scholar] [CrossRef]
- Du Toit, P.; Caragacean, L. Benefits of microscopy for raw material preparation and clinker processing. In Proceedings of the 15th Euroseminar on Microscopy Applied to Building Materials, Delft, The Netherlands, 17–19 June 2015; Çopuroglu, O., Ed.; Delft University of Technology: Delft, The Netherlands, 2015; pp. 25–32. [Google Scholar]
- French, W. Concrete petrography: A review. Q. J. Eng. Geol. 1991, 24, 17–48. [Google Scholar] [CrossRef]
- Poole, A.; Sims, I. Concrete Petrography: A Handbook of Investigative Techniques, 2nd ed.; Taylor & Francis Group: London, UK, 2016. [Google Scholar]
- Blezard, R. Technical aspects of Victorian cement. Chem. Ind. 1981, 17, 630–636. [Google Scholar]
- Pintér, F.; Gosselin, C. Material characteristics of prefabricated concrete elements from a late 19th century church in lower Austria. In Proceedings of the 15th Euroseminar on Microscopy Applied to Building Materials, Delft, The Netherlands, 17–19 June 2015; Çopuroglu, O., Ed.; Delft University of Technology: Delft, The Netherlands, 2015; pp. 131–138. [Google Scholar]
- Pintér, F.; Gosselin, C. The origin, composition and early age hydration mechanisms of Austrian natural Portland cement. Cem. Concr. Res. 2018, 110, 1–12. [Google Scholar] [CrossRef]
- Ando, Y.; Hirono, S.; Sawaki, D.; Katayama, T. Microscopy to evaluate the properties of cement and alterations in historic mortar/concrete of old Nobiru Port project, Northeast Japan. In Proceedings of the 36th Conference on Cement Microscopy, Milan, Italy, 13–17 April 2014; International Cement Microscopy Association: Montgomery, AL, USA, 2014; pp. 212–233. [Google Scholar]
- Katayama, T.; Ando, Y.; Hirono, S.; Sawaki, D. Relicts of unhydrated cement clinker in a historic concrete from the 19th century—Microscopy with EDS analysis of old training dyke at Yokohama Port, Japan. In Proceedings of the 36th Conference on Cement Microscopy, Milan, Italy, 13–17 April 2014; International Cement Microscopy Association: Montgomery, AL, USA, 2014; pp. 432–458. [Google Scholar]
- Katayama, T.; Sakai, K. Petrography of 100-year-old concrete from Otaru Port, Japan. In Proceedings of the 2nd International Conference on Concrete under Severe Conditions, Tromsø, Norway, 21–24 June 1998; Gjørv, O., Sakai, K., Banthia, N., Eds.; E & FN Spon: London, UK, 1998; pp. 250–261. [Google Scholar]
- Francis, A. The Cement Industry 1796–1914: A History; David & Charles: Newton Abbot, UK, 1997. [Google Scholar]
- Black, L. Raman spectroscopy of cementitious materials. Spectrosc. Prop. Inorg. Organomet. Compd. 2009, 40, 72–127. [Google Scholar]
- Black, L.; Brooker, A. SEM-SCA: Combined SEM-Raman spectrometer for analysis of OPC clinker. Adv. Appl. Ceram. 2007, 6, 327–334. [Google Scholar] [CrossRef]
- Potgieter-Vermaak, S.; Potgieter, J.H.; Van Grieken, R. The application of Raman spectroscopy to investigate and characterize cement, Part I: A review. Cem. Concr. Res. 2006, 36, 656–670. [Google Scholar] [CrossRef]
- Conjeaud, M.; Boyer, H. Some possibilities of Raman microprobe in cement chemistry. Cem. Concr. Res. 1980, 10, 61–70. [Google Scholar] [CrossRef]
- Handke, M. Vibrational spectra, force constants, and Si-O bond character in calcium silicate crystal structure. Appl. Spectrosc. 1986, 40, 871–877. [Google Scholar] [CrossRef]
- Fukuda, K.; Takeda, A.; Yamaguchi, A.; Hashimoto, S. Characterization of liquid exsolved by remelting reaction of belite. J. Am. Ceram. Soc. 2001, 84, 1155–1160. [Google Scholar] [CrossRef]
- Fujimori, H.; Komatsu, H.; Ioku, K.; Goto, S.; Watanabe, T. Vibrational spectra of Ca3SiO5: Ultraviolet Laser Raman Spectroscopy at high temperatures. J. Am. Ceram. Soc. 2005, 88, 1995–1998. [Google Scholar] [CrossRef]
- Ibáñez, J.; Artús, L.; Cuscó, R.; López, Á.; Menéndez, E.; Andrade, M. Hydration and carbonation of monoclinic C2S and C3S studied by Raman spectroscopy. J. Raman Spectrosc. 2007, 38, 61–67. [Google Scholar] [CrossRef]
- Dariz, P.; Schmid, T. Ferruginous phases in 19th century lime and cement mortars: A Raman microspectroscopic study. Mater. Charact. 2017, 129, 9–17. [Google Scholar] [CrossRef]
- Dariz, P.; Neubauer, J.; Götz-Neunhoeffer, F.; Schmid, T. Calcium aluminates in clinker remnants as marker phases for various types of 19th-century cement studied by Raman microspectroscopy. Eur. J. Mineral. 2016, 28, 907–914. [Google Scholar] [CrossRef]
- Torréns-Martín, D.; Fernández-Carrasco, L.; Martínez-Ramírez, S.; Ibáñez, J.; Artús, L.; Matschei, T. Raman spectroscopy of anhydrous and hydrated calcium aluminates and sulfoaluminates. J. Am. Ceram. Soc. 2013, 96, 3589–3595. [Google Scholar] [CrossRef]
- Torréns-Martín, D.; Fernández-Carrasco, L.; Martínez-Ramírez, S. Hydration of calcium aluminates and calcium sulfoaluminate studied by Raman spectroscopy. Cem. Concrete Res. 2013, 47, 43–50. [Google Scholar] [CrossRef]
- Higl, J.; Köhler, M.; Lindén, M. Confocal Raman microscopy as a non-destructive tool to study microstructure of hydrating cementitious materials. Cem. Concrete Res. 2016, 88, 136–143. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; W. De Gruyter: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- Hewlett, P. Lea’s Chemistry of Cement and Concrete, 4th ed.; Butterworth Heinemann: Oxford, UK, 2004. [Google Scholar]
- Casanova, E.; Pelé-Meziani, C.; Guilminot, É.; Mevellec, J.-Y.; Riquier-Bouclet, C.; Vinçotte, A.; Lemoine, G. The use of vibrational spectroscopy techniques as a tool for the discrimination and identification of the natural and synthetic organic compounds used in conservation. Anal. Methods 2016, 8, 8514–8527. [Google Scholar] [CrossRef]
- Tennent, N.; Caen, J.; Courtney, P.; Lozano Diz, E. In-situ Raman spectroscopic characterisation of polymers used in past conservation treatments. e-Preserv. Sci. 2009, 6, 107–111. [Google Scholar]
- Schmid, T.; Jungnickel, R.; Neuhaus, B.; Riedel, J.; Kneipp, J.; Lüter, C. Raman spectroscopy as a tool for the collection management of microscope slides. Zool. Anz. 2016, 265, 178–190. [Google Scholar] [CrossRef] [Green Version]
- Insley, H. Structural characteristics of some constituents of Portland cement clinker. J. Res. Natl. Bur. Stand. 1936, 17, 353–361. [Google Scholar] [CrossRef]
- Fukuda, K.; Maki, I. Orientation of β-Ca2SiO4 solid solution lamellae formed in the host α-phase. Cem. Concrete Res. 1989, 19, 913–918. [Google Scholar] [CrossRef]
- Fukuda, K.; Maki, I.; Ito, S. Transformation-induced microtextures in belites. In Proceedings of the 10th International Congress on the Chemistry of Cement, Gothenburg, Sweden, 2–6 June 1997; Justnes, H., Ed.; Amarkai AB and Congrex Göteborg AB: Gothenburg, Sweden, 1997; pp. 1–52. [Google Scholar]
- Remy, C.; Reynard, B.; Madon, M. Raman spectroscopic investigations of dicalcium silicate: Polymorphs and high-temperature phase transformations. J. Am. Ceram. Soc. 1997, 80, 413–423. [Google Scholar] [CrossRef]
- Bhagavantam, S. Effect of crystal orientation on the Raman spectrum of calcite. Proc. Ind. Acad. Soc. A 1940, 11, 62–71. [Google Scholar] [CrossRef]
- Hopkins, J.B.; Farrow, L.A. Raman microprobe determination of local crystal orientation. J. Appl. Phys. 1986, 59, 1103–1110. [Google Scholar] [CrossRef]
- Hayward, I.P.; Baldwin, K.J.; Hunter, D.M.; Batchelder, D.N.; Pitt, G.D. Direct imaging and confocal mapping of diamond films using luminescence and Raman scattering. Diam. Rel. Mater. 1995, 4, 617–621. [Google Scholar] [CrossRef]
- Loudon, R. The Raman effect in crystals. Adv. Phys. 1964, 13, 423–482. [Google Scholar] [CrossRef]
- Fukuda, K.; Maki, I.; Ito, S. Phase stability study on the remelting reaction in Ca2SiO4 solid solutions. J. Am. Ceram. Soc. 1995, 78, 3387–3389. [Google Scholar] [CrossRef]
- Fukuda, K.; Wakamatsu, N.; Ito, S.; Yoshida, H. Acceleration of early hydration in belite-rich cement by remelting reaction. J. Ceram. Soc. Jpn. 1999, 107, 901–906. [Google Scholar] [CrossRef]
- Schlütter, F.; Kaiser, W.; Juling, H. High fired gypsum mortar for screeds, terrazzo and masonry repair on historic monuments. Production, properties and sample applications. In Proceedings of the 2nd Historic Mortars Conference HMC2010 and RILEM TC 203-RHM Final Workshop, Prague, Czech Republic, 22–24 September 2010; Válek, J., Groot, C., Hughes, J., Eds.; RILEM Publications SARL: Bagneux, France, 2010; pp. 1169–1180. [Google Scholar]
- Schlütter, F. Mittelalterlicher Hochbrandgips. In 800 Jahre Kunststein—Vom Imitat Zum Kunstgut; für Denkmalpflege, B.L., Archäologisches Landesmuseum, A., Eds.; Wernersche Verlagsgesellschaft mbH: Worms, Germany, 2012; pp. 27–39. [Google Scholar]
- Schlütter, F.; Jakubek, M.; Juling, H. Charakterisierung und Eigenschaften historischer Gipsmörtel aus unterschiedlichen Epochen und Anwendungsgebieten. In Gips als Baugrund, Mörtel und Dekorationsmaterial; Institut für Steinkonservierung e.V., IFS: Mainz, Germany, 2012; pp. 49–59. [Google Scholar]
- Lenz, R.; Sobott, R. Beobachtungen zu Gefügen historischer Gipsmörtel. In Gipsmörtel im Historischen Mauerwerk und an Fassaden; Auras, M., Zier, H.-W., Eds.; WTA Publications: Munich, Germany, 2008; pp. 23–34. [Google Scholar]
- Dariz, P.; Jakob, C.; Ectors, D.; Neubauer, J.; Schmid, T. Measuring the burning temperatures of anhydrite micrograins in a high-fired medieval gypsum mortar. ChemistrySelect 2017, 2, 9153–9156. [Google Scholar] [CrossRef]
- Dariz, P.; Schmid, T. Phase composition and burning history of medieval high-fired gypsum mortars studied by Raman microspectroscopy. Mater. Charact. 2019, 151, 292–301. [Google Scholar] [CrossRef]
- Schmid, T.; Jungnickel, R.; Dariz, P. Raman band widths of anhydrite II reveal the burning history of high-fired medieval gypsum mortars. J. Raman Spectrosc. 2019, in press. [Google Scholar] [CrossRef]
















| Sample | Figures | Pixel Number | Area | Time |
|---|---|---|---|---|
| Baluster 1 | 6a, 9a, 10b, 12, 14a–b | 80 × 100 = 8000 | 72 µm × 90 µm | 8 h 53 min |
| Lion 1 | 5, 6b, 7d, 8, 9b, 11, 13, 14c–d | 80 × 100 = 8000 | 72 µm × 90 µm | 8 h 53 min |
| Chapel of the Holy Cross 2 | 16b–d | 75 × 44 = 3300 | 75 µm × 44 µm | 3 h 40 min |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmid, T.; Dariz, P. Raman Microspectroscopic Imaging of Binder Remnants in Historical Mortars Reveals Processing Conditions. Heritage 2019, 2, 1662-1683. https://0-doi-org.brum.beds.ac.uk/10.3390/heritage2020102
Schmid T, Dariz P. Raman Microspectroscopic Imaging of Binder Remnants in Historical Mortars Reveals Processing Conditions. Heritage. 2019; 2(2):1662-1683. https://0-doi-org.brum.beds.ac.uk/10.3390/heritage2020102
Chicago/Turabian StyleSchmid, Thomas, and Petra Dariz. 2019. "Raman Microspectroscopic Imaging of Binder Remnants in Historical Mortars Reveals Processing Conditions" Heritage 2, no. 2: 1662-1683. https://0-doi-org.brum.beds.ac.uk/10.3390/heritage2020102