Fluorosilane Water-Repellent Coating for the Protection of Marble, Wood and Other Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
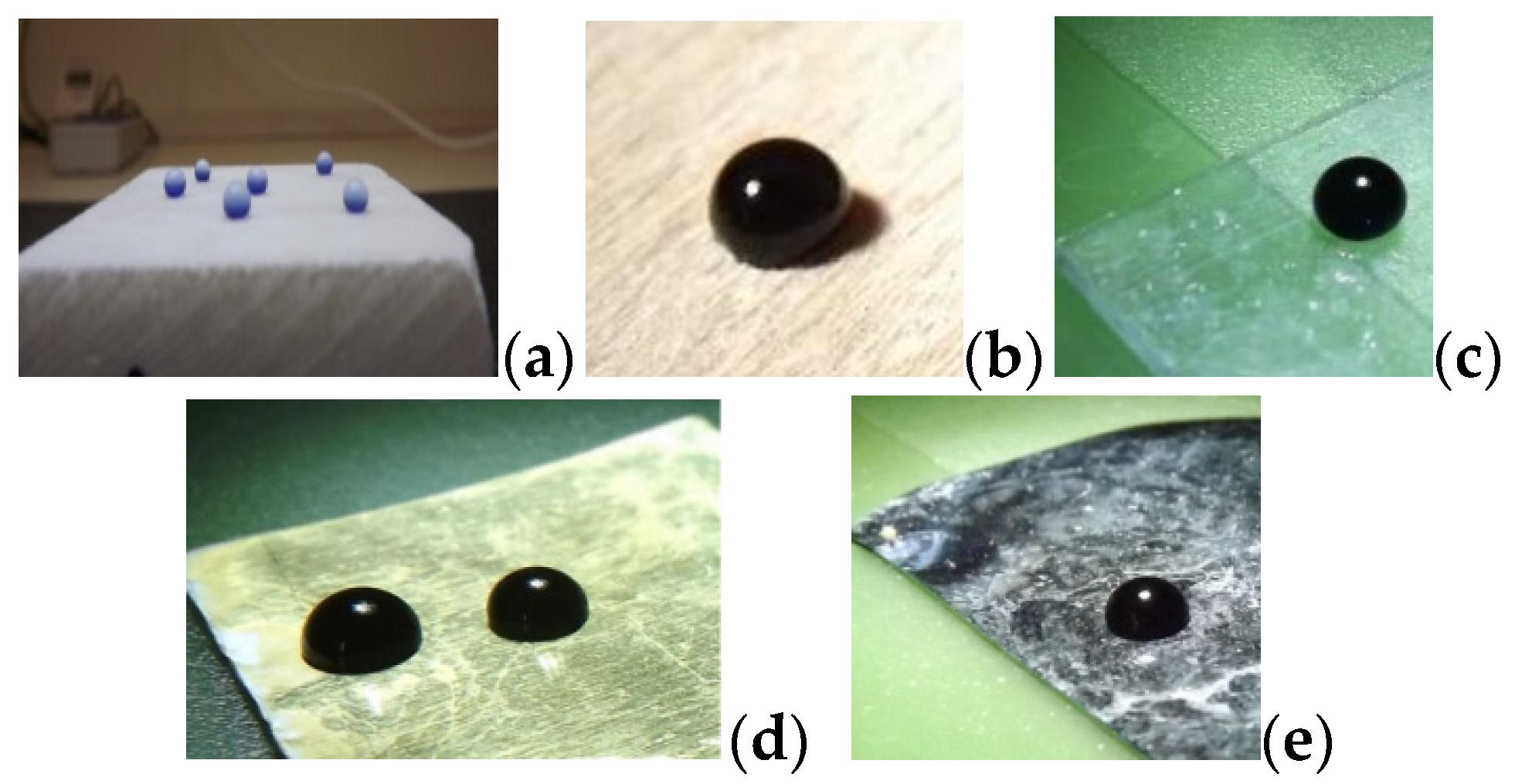
2.2. Instrumentation and Characterization Tests
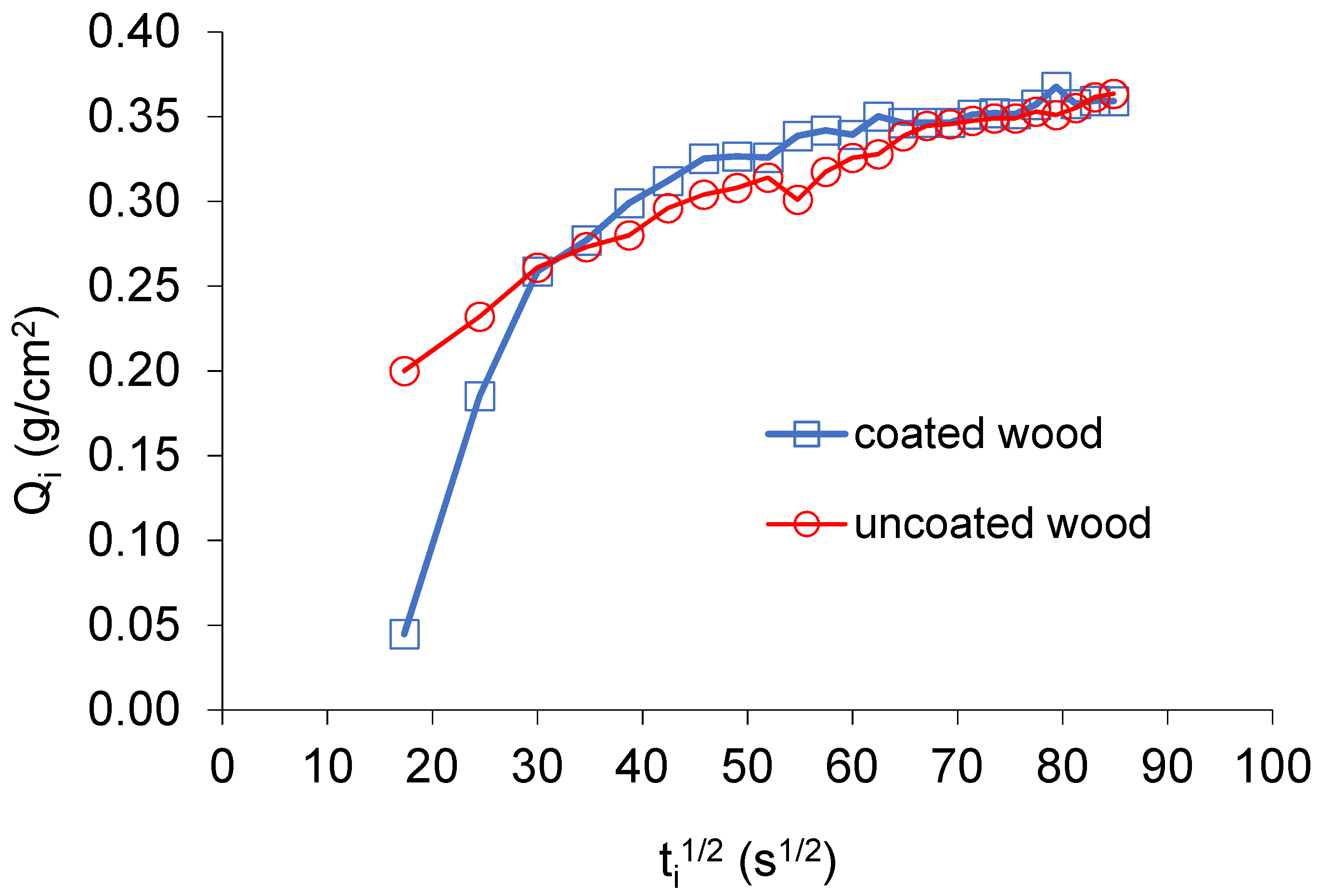
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lukaszewicz, J.W.; Lukaszewicz, J.P. Historic Heritage Protection as Part of Sustainable Growth. In Gulf Conference on Sustainable Built Environment; Springer International Publishing: Basel, Switzerland, 2020. [Google Scholar]
- Wheeler, G. Alkoxysilanes and the Consolidation of Stone; Getty Publications: Los Angeles, CA, USA, 2005. [Google Scholar]
- Delgado Rodrigues, J. Consolidation of decayed stones. A delicate problem with few practical solutions. In Historical Constructions; Lourenço, P.B., Roca, P., Eds.; University of Minho: Guimarães, Portugal, 2001. [Google Scholar]
- Karapanagiotis, I.; Pavlou, A.; Manoudis, P.N.; Aifantis, K.E. Water repellent ORMOSIL films for the protection of stone and other materials. Mater. Lett. 2014, 131, 276–279. [Google Scholar] [CrossRef]
- Adamopoulos, F.G.; Vouvoudi, E.C.; Pavlidou, E.; Achilias, D.S.; Karapanagiotis, I. TEOS-based superhydrophobic coating for the protection of stone-built cultural heritage. Coatings 2021, 11, 135. [Google Scholar] [CrossRef]
- Manoudis, P.; Papadopoulou, S.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Panayiotou, C. Polymer-Silica nanoparticles composite films as protective coatings for stone-based monuments. J. Phys. Conf. Ser. 2007, 61, 1361–1365. [Google Scholar] [CrossRef]
- Manoudis, P.; Tsakalof, A.; Karapanagiotis, I.; Zuburtikudis, I.; Panayiotou, C. Fabrication of super-hydrophobic surfaces for enhanced stone protection. Surf. Coat. Technol. 2009, 203, 1322–1328. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Kolinkeová, B.; Panayiotou, C. Superhydrophobic films for the protection of outdoor cultural heritage assets. Appl. Phys. A 2009, 97, 351–360. [Google Scholar] [CrossRef]
- Chatzigrigoriou, A.; Manoudis, P.N.; Karapanagiotis, I. Fabrication of water repellent coatings using waterborne resins for the protection of the cultural heritage. Macromol. Symp. 2013, 331–332, 158–165. [Google Scholar] [CrossRef]
- Cappelletti, G.; Fermo, P.; Camiloni, M. Smart hybrid coatings for natural stones conservation. Prog. Org. Coat. 2015, 78, 511–516. [Google Scholar] [CrossRef]
- Facio, D.S.; Carrascosa, L.A.M.; Mosquera, M.J. Producing lasting amphiphobic building surfaces with self-cleaning properties. Nanotechnology 2017, 28, 265601. [Google Scholar] [CrossRef]
- Helmi, F.M.; Hefni, Y.K. Using nanocomposites in the consolidation and protection of sandstone. Int. J. Conserv. Sci. 2016, 7, 29. [Google Scholar]
- Pino, F.; Fermo, P.; La Russa, M.; Ruffolo, S.; Comite, V.; Baghdachi, J.; Pecchioni, E.; Fratini, F.; Cappelletti, G. Advanced mortar coatings for cultural heritage protection. Durability towards prolonged UV and outdoor exposure. Environ. Sci. Pollut. Res. 2017, 24, 12608–12617. [Google Scholar] [CrossRef]
- Zarzuela, R.; Carbú, M.; Gil, M.L.A.; Cantoral, J.M.; Mosquera, M.J. Ormosils loaded with SiO2 nanoparticles functionalized with Ag as multifunctional superhydrophobic/biocidal/consolidant treatments for buildings conservation. Nanotechnology 2019, 30, 345701. [Google Scholar] [CrossRef]
- Chatzigrigoriou, A.; Karapanagiotis, I.; Poulios, I. Superhydrophobic coatings based on siloxane resin and calcium hydroxide nanoparticles for marble protection. Coatings 2020, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Karapanagiotis, I.; Manoudis, P. Superhydrophobic and water repellent polymer-nanoparticle composite films. In Industrial Applications for Intelligent Polymers and Coatings; Hosseini, M., Makhlouf, A.S.H., Eds.; Springer International Publishing: Basel, Switzerland, 2016; pp. 205–221. [Google Scholar]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Yao, W.; Li, Y.; Huang, X. Fluorinated poly(meth)acrylate: Synthesis and properties. Polymers 2014, 55, 6197–6211. [Google Scholar] [CrossRef] [Green Version]
- Aslanidou, D.; Karapanagiotis, I.; Panayiotou, C. Tuning the wetting properties of siloxane-nanoparticle coatings to induce superhydrophobicity and superoleophobicity for stone protection. Mater. Des. 2016, 108, 736–744. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Lampakis, D. Waterborne superhydrophobic and superoleophobic coatings for the protection of marble and sandstone. Materials 2018, 11, 585. [Google Scholar] [CrossRef] [Green Version]
- Mosquera, M.J.; Carrascosa, L.A.M.; Badreldin, N. Producing superhydrophobic/oleophobic coatings on Cultural Heritage building materials. Pure Appl. Chem. 2018, 90, 551–561. [Google Scholar] [CrossRef]
- Colangiuli, D.; Calia, A.; Bianco, N. Novel multifunctional coatings with photocatalytic and hydrophobic properties for the preservation of the stone building heritage (review). Constr. Build. Mater. 2015, 93, 189. [Google Scholar] [CrossRef]
- Alessandrini, G.; Aglietto, M.; Castelvetro, V.; Ciardelli, F.; Peruzzi, R.; Toniolo, L. Comparative evaluation of fluorinated and unfluorinated acrylic copolymers as water-repellent coating materials for stone. J. Appl. Polym. Sci. 2000, 76, 962–977. [Google Scholar] [CrossRef]
- Tian, S.; Liu, S.; Gao, F.; Fan, M.; Ren, J. Preparation and assessment of superhydrophobic organic-inorganic hybrid coatings for conservation of Yungang grottoes. MRS Proc. 2011, 1319, 333. [Google Scholar] [CrossRef]
- La Russa, M.F.; Rovella, N.; De Buergo, M.A.; Belfiore, C.M.; Pezzino, A.; Crisci, G.M.; Ruffolo, S.A. Nano-TiO2 coatings for cultural heritage protection: The role of the binder on hydrophobic and self cleaning efficacy. Prog. Org. Coat. 2016, 91, 1. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, A.F.; Jiang, L. Petal Effect: A superhydrophobic state with high adhesive force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Gemenetzis, D.; Karapanagiotis, I. A comparative study of the wetting properties of a superhydrophobic siloxane material and rose petal. Sci. Cult. 2017, 3, 7. [Google Scholar]
- Fürstner, R.; Barthlott, W.; Neinhuis, C.; Walzel, P. Wetting and self-cleaning properties of artificial superhydrophobic surfaces. Langmuir 2005, 21, 956–961. [Google Scholar] [CrossRef] [PubMed]
- CEN. EN15886, Conservation of Cultural Property-Test Methods—Colour Measurement of Surfaces; CEN: Brussels, Belgium, 2010. [Google Scholar]
- Murase, H.; Fujibayashi, T. Characterization of molecular interfaces in hydrophobic systems. Prog. Org. Coat. 1997, 31, 97–104. [Google Scholar] [CrossRef]
- Rios, P.F.; Dodiuk, H.; Kenig, S.; McCarthy, S.; Dotan, A. The effect of polymer surface on the wetting and adhesion of liquid systems. J. Adhes. Sci. Technol. 2007, 21, 227–241. [Google Scholar] [CrossRef]
- Samuel, B.; Zhao, H.; Law, K.-Y. Study of wetting and adhesion interactions between water and various polymer and superhydrophobic surfaces. J. Phys. Chem. C 2011, 115, 14852–14861. [Google Scholar] [CrossRef]
- Pellerite, M.J.; Wood, E.J.; Jones, V.W. Dynamic contact angle studies of self-assembled thin films from fluorinated alkyltrichlorosilanes. J. Phys. Chem. B 2002, 106, 4746. [Google Scholar] [CrossRef]
- Muschi, M.; Brudieu, B.; Teisseire, J.; Sauret, A. Drop impact dynamics on slippery liquid-infused porous surfaces: Influence of oil thickness. Soft Matter 2018, 14, 1100–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Substrate | Coating Method | Uptake (g Coating/cm2 Substrate) | |
|---|---|---|---|
| Marble | Brush | 0.0045 ± 0.0010 | |
| Wood | Dip coating | 0.0572 ± 0.0050 | |
| Glass | Brush | 0.0375 ± 0.0010 | |
| Si wafer | Brush | 0.0500 ± 0.0020 | |
| Brass | Brush | 0.0042 ± 0.0020 | |
| Paper | Brush | 0.0011 ± 0.0002 | |
| Silk | Brush | 0.0002 ± 0.0001 |
| Substrate | Coating Method | Contact Angle (°) | Sliding Angle (°) |
|---|---|---|---|
| Marble | Brush | 155.0 ± 2.4 | 2.7 ± 0.2 |
| Wood | Dip coating | 133.6 ± 1.3 | 1.6 ± 0.2 |
| Glass | Brush | 114.8 ± 2.1 | 1.9 ± 0.2 |
| Si wafer | Brush | 110.7 ± 2.6 | 2.3 ± 0.1 |
| Brass | Brush | 99.1 ± 1.1 | 5.3 ± 0.1 |
| Paper | Brush | <90° | |
| Silk | Brush | <90° |
| Substrate | Coating Method | ΔL* | Δa* | Δb* | ΔE* |
|---|---|---|---|---|---|
| Marble | Brush | 0.07 | 0.22 | 0.26 | 0.30 ± 0.05 |
| Wood | Dip coating | 3.04 | 0.55 | 0.64 | 3.20 ± 1.47 |
| Glass | Brush | 5.02 | 0.81 | 1.59 | 5.30 ± 0.10 |
| Si wafer | Brush | 19.37 | 0.09 | 4.00 | 19.77 ± 0.50 |
| Brass | Brush | 8.75 | 0.17 | 12.06 | 14.90 ± 0.60 |
| Paper | Brush | 0.46 | 0.05 | 0.05 | 0.46 ± 0.10 |
| Silk | Brush | 13.14 | 0.72 | −1.09 | 13.20 ± 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamopoulos, F.G.; Vouvoudi, E.C.; Achilias, D.S.; Karapanagiotis, I. Fluorosilane Water-Repellent Coating for the Protection of Marble, Wood and Other Materials. Heritage 2021, 4, 2668-2675. https://0-doi-org.brum.beds.ac.uk/10.3390/heritage4040150
Adamopoulos FG, Vouvoudi EC, Achilias DS, Karapanagiotis I. Fluorosilane Water-Repellent Coating for the Protection of Marble, Wood and Other Materials. Heritage. 2021; 4(4):2668-2675. https://0-doi-org.brum.beds.ac.uk/10.3390/heritage4040150
Chicago/Turabian StyleAdamopoulos, Fotios G., Evangelia C. Vouvoudi, Dimitris S. Achilias, and Ioannis Karapanagiotis. 2021. "Fluorosilane Water-Repellent Coating for the Protection of Marble, Wood and Other Materials" Heritage 4, no. 4: 2668-2675. https://0-doi-org.brum.beds.ac.uk/10.3390/heritage4040150







