Multi-Analytical Assessment of Bodied Drying Oil Varnishes and Their Use as Binders in Armour Paints
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Overview
Application of Liquid Oils and Varnishes to Steel Sheet Substrate
2.2. Ageing Conditions
2.3. Investigations and Analytical Methods
2.3.1. Testing Chemical and Physical Properties of the Oils
2.3.2. Fourier-Transform Infrared Spectroscopy
2.3.3. Gas Chromatography–Mass Spectrometry
2.3.4. Water Immersion Test
3. Results and Discussion
3.1. Liquid Samples
3.1.1. Chemical and Physical Properties of Liquid Oils and Varnishes
3.1.2. FTIR
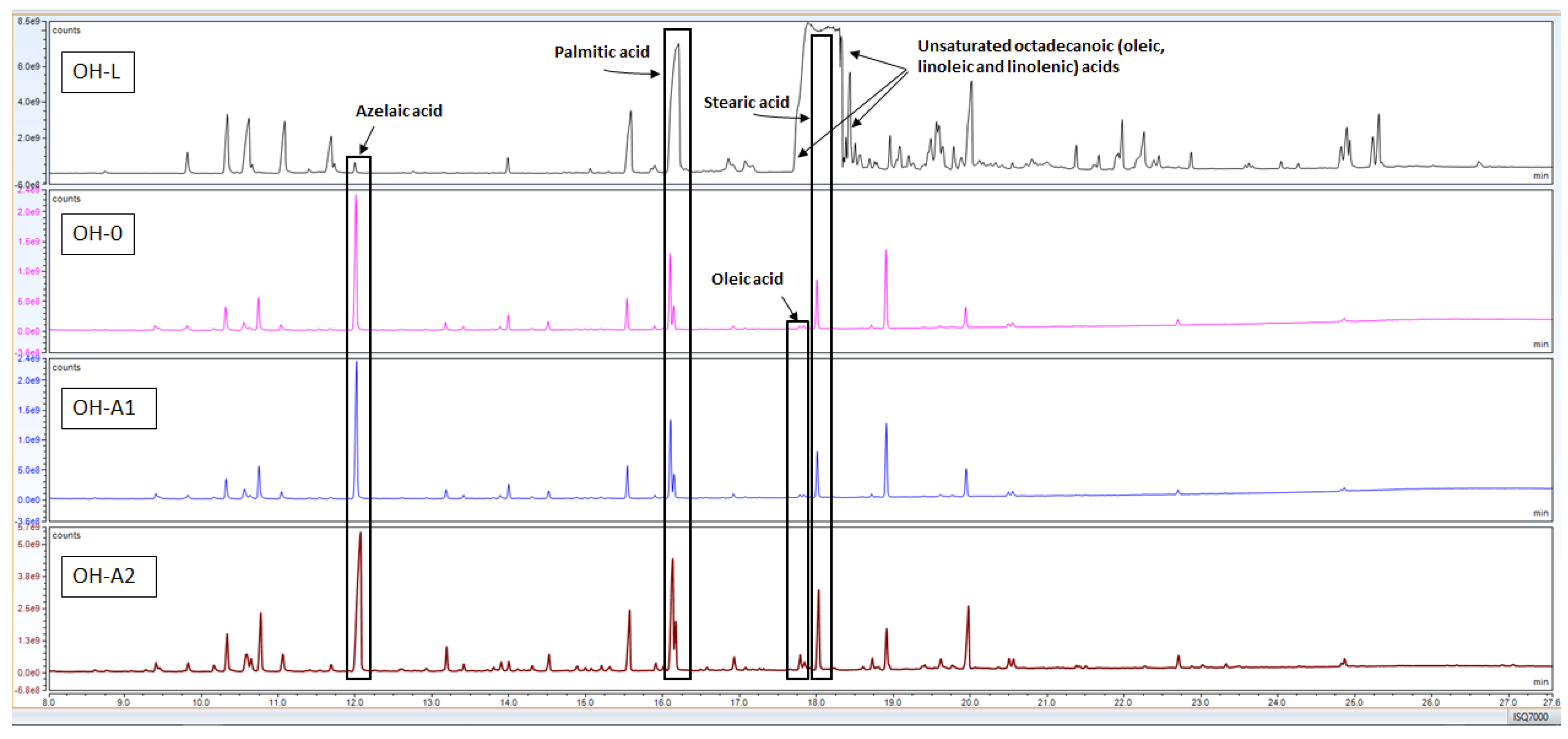
3.1.3. GC-MS
- -
- OH-L shows the typical fatty acid profile of linseed oil, corroborated by a P/S ratio of 1.7; all special fatty acids were found in this formulation;
- -
- WHT-L, which, similarly to stand oil, was found to contain cyclic compounds, also contained castor oil, as indicated by the identification of its marker (i.e., ricinoleic acid—mass spectrum in Figure 2c). This non-drying oil can be found in modern commercial artist oil formulations as an adulterant in linseed oil or added for rheological purposes [29]. Castor oil is made into a drying oil by dehydration through the formation of an unsaturated hydroxy fatty acid [6]. In raw and blown form it serves as a plasticiser in coatings. In hydrated form, castor oil improves the elasticity, water resistance, alkali resistance and colour retention of paint, similar to tung oil. Its presence likely influenced the final P/S ratio of the mixture, which was 1.2. Indeed, castor oil’s P/S ratio is generally around 1, while linseed oil has a P/S ratio ranging from 1.3 to 1.8 [30];
- -
- WS-L contains a special monounsaturated fatty acid, namely erucic acid (mass spectrum in Figure 2d), which is considered as the biomarker for seeds of the Brassicaceae family, such as rapeseed. Rapeseed oil is a semi-drying oil that was introduced in the 20th century as a lubricant and/or as an adulterant in linseed oils [29,31]. Again, the presence of a mixture of different oils could have slightly modified the P/S ratio, which was 1.2;
- -
- The mixture containing WHT-L and tung oil did not contain any additional oils and presented a P/S ratio of 1.2.
3.2. Solid Samples
3.2.1. Artificial Ageing
3.2.2. Water Immersion Test
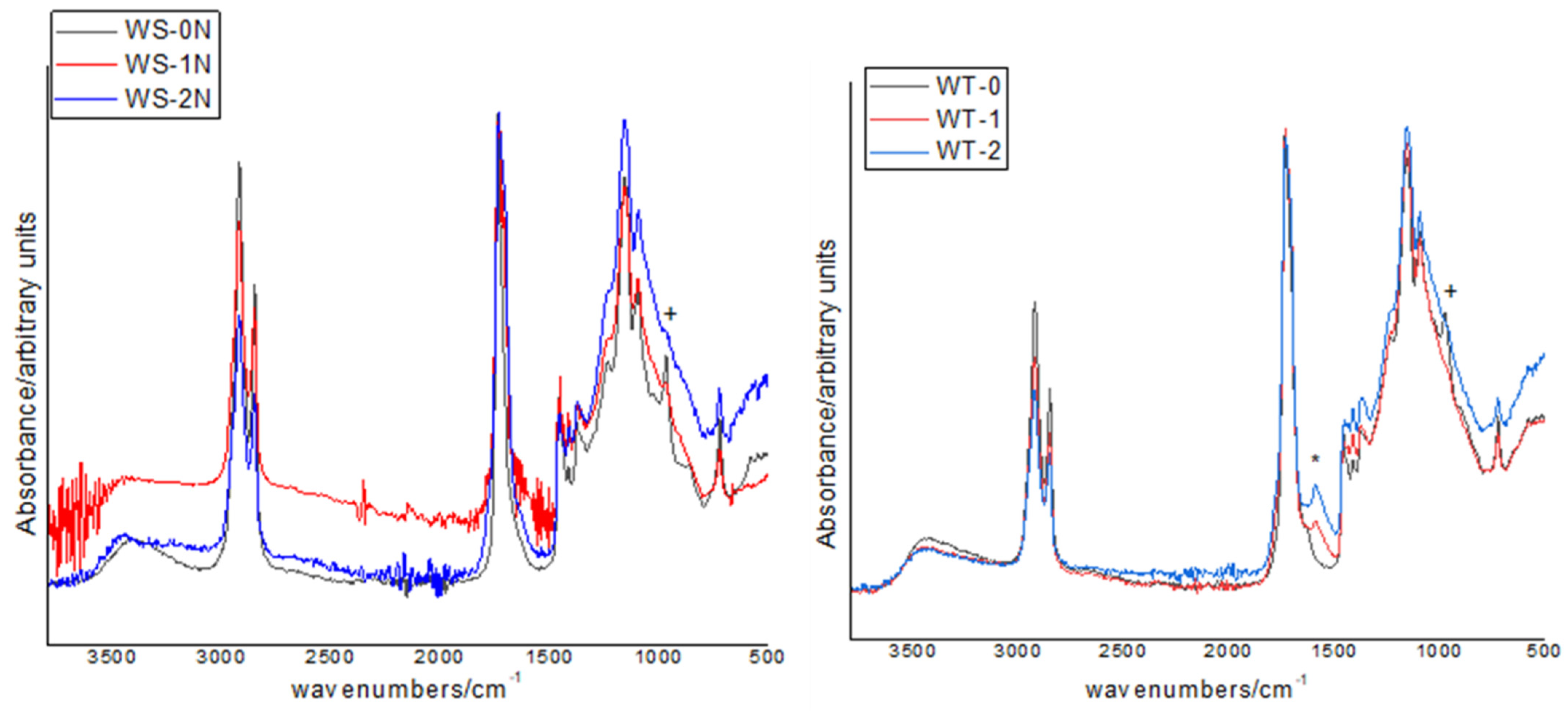
3.2.3. FTIR
3.2.4. GC-MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Chemical and Physical Properties of the Paint Binders
| Measured Property/Characteristic | Results on Commercial Binders | |||||||
| Methodological Details | OH-L | WHT-L | WS-L | T-L | ||||
| Method | Unit/at | At | Uncert., % | |||||
| Density | Electronic | g/cm3 | 20 °C | - | 0.9339 | 0.9370 | 0.9575 | 0.9391 |
| Refractive index | Automatic | - | 20 °C | ±0.01 | 1.4826 | 1.4823 | 1.4907 | 1.5194 |
| 60 °C | ±0.01 | 1.4682 | 1.4679 | 1.4763 | >1.5000 | |||
| Cold test | AAK method | - | 6 h | - | 2 | 2 | 2 | 2 |
| Insoluble impurities | AOCS Ca 3a-46 | % | 20 | <0.01 | 0.01 | <0.01 | <0.01 | |
| Alkaline impurities | EP 2.4.19 | Ml | - | 0.15 | <0.10 | <0.10 | <0.10 | |
| Water content | AOCS CA 2E-84 | % | ±15 | 0.06 | 0.1 | 0.03 | 0.04 | |
| Iodine value | AOCS Cd 1c-85 + ISO 3961 | mg iodine/100 g | - | 196 | 177 | 112 | 161 | |
| Saponification value | IUPAC 2.202 | mg KOH/g | ±2 | 188 | 191 | 192 | 192 | |
| Free fatty acids | IUPAC 2.201 (m) | - | 282 | ±3 | 0.94 | 1.7 | 3.1 | 1.7 |
| Acid value | IUPAC 2.201 (m) | mg KOH/g | ±3 | 1.87 | 3.41 | 6.24 | 3.3 | |
| Peroxide value | AOCS Cd 8-53 | meq/kg | ±15 | 2.6 | 6 | 3.3 | 5.6 | |
| Colour (Lovibond) | AOCS Cc 13j-97 | Yellow | ±25 | 130.0 | 140.0 | 40.0 | 60.0 | |
| Red | 20.0 | 14.0 | 3.0 | 7.0 | ||||
| Blue | 0.0 | 0.0 | 0.0 | 0.0 | ||||
| Total | 330.0 | 280.0 | 70.0 | 130.0 | ||||
- European Pharmacopeia Commission. Alkaline impurities in fatty oils. Methods of analysis EP 2.4.19; EU): European Pharmacopeia Commission: Strasbourg, Germany, 2005.
- International Organization for Standardization. (2018). Animal and vegetable fats and oils. Determination of iodine value. (ISO 3961:2018); International Organization for Standardization: Geneva, Switzerland, 2018
- IUPAC (1992). Determination of the saponification value (S.V). Standard Methods for the Analysis of Oils, Fats and Derivatives. IUPAC 2.202:1992; Oxford, United Kingdom: International Union of Pure and Applied Chemistry Commission on Oils, Fats and Derivatives, 1992.
- IUPAC (1992). Determination of the acidity of lecithins. Standard Methods for the Analysis of Oils, Fats and Derivatives. IUPAC 2.201/ (5.201); Oxford, United Kingdom: International Union of Pure and Applied Chemistry Commission on Oils, Fats and Derivatives, 1992.
- IUPAC (1992). Capillary column gas—liquid chromatography of fatty acid methyl esters. Standard Methods for the Analysis of Oils, Fats and Derivatives. IUAPAC 2.304:1992; Oxford, United Kingdom: International Union of Pure and Applied Chemistry Commission on Oils, Fats and Derivatives, 1992.
- AOCS (2017). Moisture. Karl Fischer Method. AOCS Official Method Ca 2e-84, revised 2017. Urbana, USA: The American Oil Chemists’ Society, 2017.
- AOCS (2017). Calculated Iodine Value. AOCS Official Method AOCS Cd 1c-85, revised 2017; Urbana, USA: The American Oil Chemists’ Society, 2017.
- AOCS (2003). Peroxide Value—Acetic Acid-Chloroform Method. AOCS Official Method AOCS Cd 8-53 (1953/revised 2003); Urbana, USA: The American Oil Chemists’ Society, 2003.
- AOCS (2017). Insoluble Impurities in Fats and Oil. AOCS Official Method AOCS Ca 3a-46, revised 2017; Urbana, USA:. The American Oil Chemists’ Society, 2017.
- AOCS (2017). Color of Fats and Oils, Automated Method. AOCS Official Method AOCS Cc 13j-97, revised 2017; Urbana, USA. The American Oil Chemists’ Society, 2017.
Appendix B. GC-MS Results
| RT (min) | Identified Compound | OH | WHT | WS | WT | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OH-L | OH-0 | OH-1 | OH-2 | WHT-L | WHT-0 | WHT-1 | WHT-2 | WS-L | WS-0 | WS-1 | WS-2 | WT-L | WT-0 | WT-1 | WT-2 | ||
| 9.412 | Pimelic acid, dimethyl ester | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| 9.784 | Glycerol derivative | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 10.165 | Octanoic acid, 8-hydroxy-, methyl ester | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| 10.342 | Glycerol derivative | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 10.580 | Glycerol derivative | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 10.652 | Nonanoic acid, 9-oxo-, methyl ester | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| 10.767 | Suberic acid, dimethyl ester | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| 11.053 | Glycerol derivative | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 11.692 | Decanoic acid, 9-oxo-, methyl ester | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| 12.063 | Azelaic acid, dimethyl ester | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| 13.196 | Sebacic acid, dimethyl ester | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| 14.005 | Myristic acid methyl ester | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 15.573 | Glycerol derivative | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 16.131 | Palmitic acid, methyl ester | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 16.172 | Glycerol derivative | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 16.934 | Undecanedioic acid, 4-oxo-, dimethyl ester | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| 17.801 | Oleic acid methyl ester | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 18.036 | Stearic acid methyl ester | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||
| 18.206 | Linoleic acid methyl ester | √ | √ | √ | √ | √ | |||||||||||
| 18.399 | Linolenic acid methyl ester | √ | √ | √ | |||||||||||||
| 18.920 | Nonadecanoic acid, methyl ester (internal standard) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 19.210 | Alpha-eleostearic acid methyl ester | √ | |||||||||||||||
| 19.570 | Ricinoleic acid methyl ester | √ | √ | √ | √ | ||||||||||||
| 19.624 | Octadecanoic acid, 10-oxo-, methyl ester | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| 19.767 | Arachidic acid methyl ester | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| 19.803 | 7-(o-pentylphenyl)-heptanoic acid methyl ester | √ | √ | √ | |||||||||||||
| 19.883 | 9-(o-propylphenyl)-nonanoic acid methyl ester | √ | √ | √ | |||||||||||||
| 19.944 | Glycerol (main) | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| 21.196 | Erucid acid methyl ester | √ | √ | √ | √ | ||||||||||||
| 21.386 | Behenic acid, methyl ester | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| 22.712 | Octadecanoic acid, 9,10-epoxy | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| 22.885 | Tetracosanoic acid, methyl ester | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| 24.287 | Hexacosanoic acid, methyl ester | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| 24.817 | β-Monolinolein | √ | √ | √ | |||||||||||||
| 24.875 | Octadecanoic acid, 9,10-oxo-, methyl ester | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| 24.889 | Linolenic acid 1,3-dimethoxypropan-2-yl ester | √ | √ | √ | |||||||||||||
| 24.930 | Linolenic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester (Z,Z,Z)- | √ | √ | √ | |||||||||||||
| 25.561 | Sitosterol | √ | |||||||||||||||
References
- Källbom, A. The Concept of Anticorrosive Aluminium-Pigmented Armour Paint, for Sustainable Maintenance of Ferrous Heritage. Int. J. Archit. Herit. 2021, 14, 1–19. [Google Scholar] [CrossRef]
- Källbom, A.; Izzo, F.; Nevin, A. Multi-analytical assessment of Armour Paints: The Ageing Characteristics of Historic Drying Oil Varnish Paints for Protection of Steel and Iron Surfaces in Sweden. Heritage 2021, 4, 63. [Google Scholar] [CrossRef]
- Standeven, H. House Paints, 1900–1960: History and Use; Getty Conservation Institute: Los Angeles, CA, USA, 2011. [Google Scholar]
- Magnusson, K.H. Linoljans framställning och förädling. [The Manufacturing and Refinement of Linseed Oil]. In Tekniska Samfundets i Göteborg. Avdelningen för Kemi och Fysik. 1914–1939; Göteborgslitografen: Göteborg, Sweden, 1939; pp. 253–266. [Google Scholar]
- Sabin, A.H. Industrial and Artistic Technology of Paint and Varnish, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1927. [Google Scholar]
- Singer, E. Fundamentals of Paints, Varnish and Lacquer Technology; The American Paint Journal Company: Washington, DC, USA, 1957. [Google Scholar]
- Lazzari, M.; Chiantore, O. Drying and Oxidative Degradation of Linseed Oil. Polym. Degrad. Stab. 1999, 65, 303–313. [Google Scholar] [CrossRef]
- Scalarone, D.; Lazzari, M.; Chiantore, O. Thermally Assisted Hydrolysis and Methylation-pyrolysis-gas chromatography/mass spectrometry of Light-aged Linseed Oil. J. Anal. Appl. Pyrolysis 2001, 58–59, 503–512. [Google Scholar] [CrossRef]
- Stern, E. Farbenbindemittel, Farbkörper und Anstrichtstoffe. In Kolloidchemische Technologie; Ein Handbuch Kolloidchemischer Betrachtungsweise in der Chemischen Industrie und Technik; Lesegang, R.E., Ed.; Springer: Berlin, Germany, 1932. [Google Scholar]
- Auer, L. Colloidal Chemistry of Drying Oils. Ind. Eng. Chem. 1938, 30, 466–472. [Google Scholar] [CrossRef]
- Dietemann, P.; Neugebauer, W.; Lutz, L.; Beil, C.; Fiedler, I.; Baumer, U. A Colloidal Description of Tempera and Oil Paints, based on a Case Study of Arnold Böcklin´s Painting Villa am Meer (1865). e-PRESERVATIONSCIENCE 2014, 11, 29–46. [Google Scholar]
- Berg, J. Introduction to Interfaces and Colloids; World Scientific Publishing Company: Singapore, 2009. [Google Scholar]
- International Organization for Standardization. ISO 16474-2:2013. Paints and Varnishes—Methods of Exposure to Laboratory Light Sources. Part 2: Xenon-Arc Lamps; International Organization for Standardization: Geneva, Switzerland, 2013. [Google Scholar]
- Fuster-López, L.; Izzo, F.C.; Piovesan, M.; Sperni, L.; Zendri, E. Study of the chemical composition and the mechanical behaviour of 20th century commercial artists’ oil paints containing manganese-based pigments. Microchem. J. 2016, 124, 962–973. [Google Scholar] [CrossRef] [Green Version]
- Izzo, F.C.; Zanin, C.; van Keulen, H.; Da Roit, C. From pigments to paints: Studying original materials from the atelier of the artist Mariano Fortuny y Madrazo. Int. J. Conserv. Sci. 2017, 8, 547–564. [Google Scholar]
- Caravá, S.; García, C.R.; de Agredos-Pascual, M.L.V.; Mascarós, S.M.; Izzo, F. Investigation of modern oil paints through a physico-chemical integrated approach. Emblematic cases from Valencia, Spain. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2020, 240, 118633. [Google Scholar] [CrossRef]
- Fuster-López, L.; Izzo, F.; Damato, V.; Yusà-Marco, D.; Zendri, E. An insight into the mechanical properties of selected commercial oil and alkyd paint films containing cobalt blue. J. Cult. Herit. 2019, 35, 225–234. [Google Scholar] [CrossRef]
- Fuster-López, L.; Izzo, F.C.; Andersen, C.K.; Murray, A.; Vila, A.; Picollo, M.; Stefani, L.; Jiménez, R.; Aguado-Guardiola, E. Picasso’s 1917 paint materials and their influence on the condition of four paintings. SN Appl. Sci. 2020, 2, 1–14. [Google Scholar] [CrossRef]
- Hermans, J.J.; Keune, K.; Van Loon, A.; Iedema, P.D. Toward a complete molecular model for the formation of metal soaps in oil paints. In Metal Soaps in Art; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Izzo, F. 20th Century Artists’ Oil Paints: A Chemical–Physical Survey. Ph.D. Thesis, University “Ca’ Foscari”, Venice, Italy, 2011. [Google Scholar]
- Izzo, F.C.; van den Berg, K.J.; van Keulen, H.; Ferriani, B.; Zendri, E. Modern Oil Paints—Formulations, Organic Additives and Degradation: Some Case Studies. In Issues in Contemporary Oil Paint; van den Berg, K.J., Burnstock, A., de Keijzer, M., Krueger, J., Learner, T., Tagle, A., Heydenreich, d., Eds.; Springer: Cham, Switzerland, 2014. [Google Scholar]
- International Organization for Standardization. Raw, Refined and Boiled Linseed Oil for Paints and Varnishes—Specifications and Methods of Test. ISO 150:2006; ISO International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- International Organization for Standardization. Raw, Refined and Boiled Linseed Oil for Paints and Varnishes—Specifications and Methods of Test. ISO 150:2018; ISO International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- Edwards, J.D. Aluminum Paint and Powders, 1st ed.; Reinhold Publishing Corporation: New York, NY, USA, 1936. [Google Scholar]
- Gupta, M. Practical Guide to Vegetable Oil Processing, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Schönemann, A.; Edwards, H. Raman and FTIR microspectroscopic study of the alteration of Chinese tung oil and related drying oils during ageing. Anal. Bioanal. Chem. 2011, 400, 1173–1180. [Google Scholar] [CrossRef]
- Learner, T.; Getty Conservation Institute. Analysis of Modern Paints; Getty Conservation Institute: Los Angeles, CA, USA, 2004. [Google Scholar]
- Sebedio, J.L.; Grandgirard, A. Cyclic fatty acids: Natural sources, formation during heat treatment, synthesis and biological properties. Prog. Lipid Res. 1989, 28, 303–336. [Google Scholar] [CrossRef]
- Izzo, F.C.; Ferriani, B.; Van Den Berg, K.J.; Van Keulen, H.; Zendri, E. 20th Century Artists’ Oil Paints: The Case of the Olii by Lucio Fontana. J. Cult. Herit. 2014, 15, 557–563. [Google Scholar] [CrossRef]
- Colombini, M.P.; Modugno, F. (Eds.) Organic Mass Spectrometry in Art and Archaeology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Van Keulen, H. Slow-drying oil additives in modern oil paints, and application in conservation treatments. An analytical sstudy in technical historical perspective. In ICOM Committee for Conservation 17th Triennial Meeting Melbourne Australia 19–23 September 2014; ICOM Committee for Conservation: Melbourne, Australia, 2014. [Google Scholar]
- Brambilla, L.; Riedo, C.; Baraldi, C.; Nevin, A.; Gamberini, M.C.; D’Andrea, C.; Chiantore, O.; Goidanich, S.; Toniolo, L. Characterization of fresh and aged natural ingredients used in historical ointments by molecular spectroscopic techniques: IR, Raman and fluorescence. Anal. Bioanal. Chem. 2011, 401, 1827. [Google Scholar] [CrossRef] [PubMed]
- Van der Weerd, J.; van Loon, A.; Boon, J.J. FTIR Studies of the Effects of Pigments on the Aging of Oil. Stud. Conserv. 2005, 50, 3–22. [Google Scholar] [CrossRef]
- Izzo, F.C.; Kratter, M.; Nevin, A.; Zendri, E. A Critical Review on the Analysis of Metal Soaps in Oil Paintings. ChemistryOpen 2021, 10, 904–921. [Google Scholar] [CrossRef]
- Wexler, H. Polymerization of drying oils. Chem. Rev. 1964, 64, 591–611. [Google Scholar] [CrossRef]
- Van den Berg, J.D.; Van den Berg, K.J.; Boon, J.J. Identification of non-cross-linked compounds in methanolic extracts of cured and aged linseed oil-based paint films using gas chromatography–mass spectrometry. J. Chromatogr. A 2002, 950, 195–211. [Google Scholar] [CrossRef]
- Araujo, W.S.; Margarit, I.C.P.; Mattos, O.R.; Fragata, F.L.; de Lima-Neto, P. Corrosion Aspects of Alkyd Paints Modified with Linseed and Soy Oils. Electrochim. Acta 2010, 55, 6204–6211. [Google Scholar] [CrossRef]
- Perrotta, A.; García, S.J.; Creatore, M. Ellipsometric Porosimetry and Electrochemical Impedance Spectroscopy Characterization for Moisture Permeation Barrier Layers. Plasma Process. Polym. 2015, 12, 968–979. [Google Scholar] [CrossRef]




| Typology (Drying Oil Used) | Paint Name | Description | Exposure |
|---|---|---|---|
| OH Air-blown linseed oil varnish (heated to 130–150 °C, drier: Co-Zr ethyl hexane/octate/proprionate). | OH-L | Liquid | Fresh oil |
| OH-0 | Solid film—unaged | 4 weeks naturally aged | |
| OH-A1 | Solid film—aged 1 | 64 kJ | |
| OH-A2 | Solid film—aged 2 | 97 kJ | |
| WHT Heat-bodied manganese compound varnish (boiled at high temperature, 280 °C). | WHT-L | Liquid | Fresh oil |
| WHT-0 | Solid film—unaged | ||
| WHT-A1 | Solid film—aged 1 | 64 kJ | |
| WHT-A2 | Solid film—aged 2 | 97 kJ | |
| WS Stand oil—linseed stand oil, 50 dPa·s | WS-L | Liquid | Fresh oil |
| WS-0 | Solid film—unaged | ||
| WS-A1 | Solid film—aged 1 | 64 kJ | |
| WS-A2 | Solid film—aged 2 | 97 kJ | |
| WT 80 wt% WHT + 20 wt% bodied tung oil | WT-L | Liquid | Fresh oil |
| WT-0 | Solid film—unaged | ||
| WT-A1 | Solid film—aged 1 | 64 kJ | |
| WT-A2 | Solid film—aged 2 | 97 kJ | |
| T Bodied tung oil | T | Liquid | For chemical and physical quantification only |
| L | L-0 | Unaged | Litharge varnish * |
| P | P-0 | Unaged | Pyrolusite varnish * |
| MB | MB-0 | Unaged | Manganese borate varnish * |
| OHT | OHT-0 | Unaged | OH with 20 wt% tung oil * |
| Assignment | Linseed Oils | OH-L | WHT-L | WS-L | Tung Oils | T-L |
|---|---|---|---|---|---|---|
| ν (O-H) | ||||||
| 3067 | 3451 | |||||
| -/3340 | 3047 | |||||
| ν (C-H) in C=C-H | 3008/- | 3010 | 3010 | 3012 | 3013 | |
| 3006 | ||||||
| ν (C-H) | 2954 sh/overlapped | 2956 | 2954 | 2953 | ||
| ν (C-H) | 2924 | 2924 | 2923 | 2922 | 2932/2927 | 2924 |
| ν (C-H) | 2852 | 2853 | 2853 | 2853 | ||
| ν (C=O) | 1744/1740 | 1743 | 1742 | 1742 | 1745/1740 | 1741 |
| ν (C=C) | 1652/1634 sh | 1654 | 1654 | 1656 | 1642 | 1642 |
| 1587 | 1586 | |||||
| 1521 | ||||||
| δ (C-H) in methyl | 1461/1462 | 1460 | 1460 | 1459 | 1464/1461 | 1459 |
| δ (C-H) in methylene | 1418/1416 | 1418 | 1418 | 1418 | 1416/1415 | |
| δ (C-H) in methyl | 1375 | 1376 | 1376 | 1376 | 1376/1377 | 1377 |
| ν (C-O) in C-O-C in esters | 1238/1240 | 1237 | 1238 | 1235 | 1238/1240 | 1237 |
| ν (C-O) in C-O-C in esters | 1163/1165 | 1160 | 1160 | 1159 | 1159 | |
| 1119 | ||||||
| ν (C-O) in C-O-C in esters | 1099 | 1099 | 1098 | 1099 | 1100 | 1099 |
| 1069 | 1069 | |||||
| 1028 | 1029 | |||||
| 992 | 990 | |||||
| 987 | 987 | 975 | ||||
| ω (CH) in CH=CH wagging isolated trans | -/971 | 969 | 970 | 967 | 965 | 964 |
| 914 | 914 | |||||
| 872 | ||||||
| 852 | ||||||
| 807 | ||||||
| 866 | 867 | |||||
| 791 | 792 | |||||
| δ/CH2) rocking | -/720 | 721 | 721 | 722 | 728 | 726 |
| OH-0 | WHT-0 | WS-0 | WT-0 | OHT-0 | L-0 | P-0 | MB-0 | |
|---|---|---|---|---|---|---|---|---|
| Milky time | 1.5 h | 1.5 h | >24 h | >24 h | >24 h | >24 h | 1.5 h | ~16 h |
| Comments | White | White | * | * | * | * | White | Somewhat frosty appearance |
| Molar Ratios | OH | WHT | WS | WT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OH-0 | OH-A1 | OH-A2 | WHT-0 | WHT-A1 | WHT-A2 | WS-0 | WS-A1 | WS-A2 | WT-0 | WT-A1 | WT-A2 | |
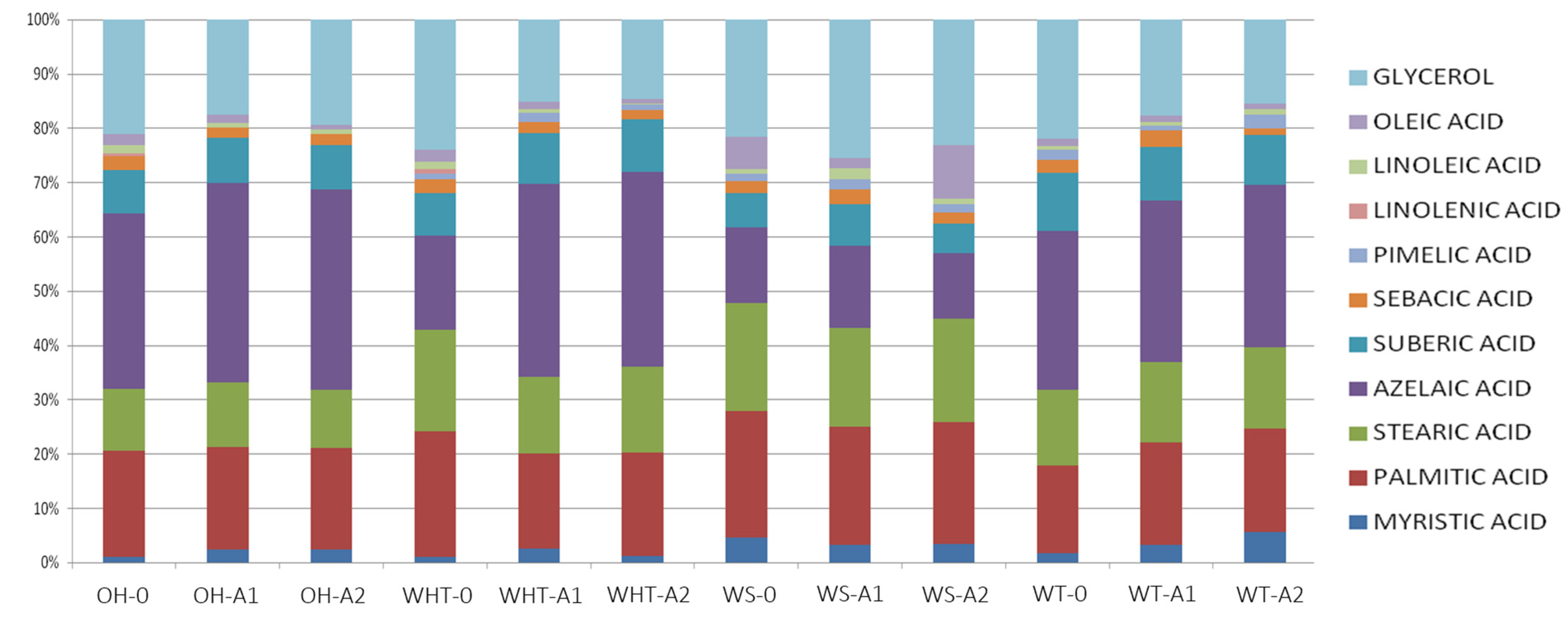
| P/S | 1.73 | 1.70 | 1.73 | 1.24 | 1.24 | 1.20 | 1.17 | 1.19 | 1.18 | 1.15 | 1.26 | 1.24 |
| A/P | 1.64 | 1.90 | 1.97 | 0.76 | 2.03 | 1.50 | 0.60 | 0.70 | 0.54 | 1.82 | 1.60 | 1.62 |
| D/P | 2.18 | 2.47 | 2.52 | 1.21 | 2.67 | 1.92 | 0.97 | 1.18 | 0.87 | 2.63 | 2.30 | 2.26 |
| O/S | 0.17 | 0.12 | 0.08 | 0.12 | 0.09 | 0.05 | 0.30 | 0.10 | 0.52 | 0.09 | 0.08 | 0.07 |
| %D | 42.86 | 47.03 | 47.30 | 28.85 | 48.63 | 47.33 | 23.82 | 27.46 | 21.09 | 44.26 | 43.74 | 43.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izzo, F.C.; Källbom, A.; Nevin, A. Multi-Analytical Assessment of Bodied Drying Oil Varnishes and Their Use as Binders in Armour Paints. Heritage 2021, 4, 3402-3420. https://0-doi-org.brum.beds.ac.uk/10.3390/heritage4040189
Izzo FC, Källbom A, Nevin A. Multi-Analytical Assessment of Bodied Drying Oil Varnishes and Their Use as Binders in Armour Paints. Heritage. 2021; 4(4):3402-3420. https://0-doi-org.brum.beds.ac.uk/10.3390/heritage4040189
Chicago/Turabian StyleIzzo, Francesca Caterina, Arja Källbom, and Austin Nevin. 2021. "Multi-Analytical Assessment of Bodied Drying Oil Varnishes and Their Use as Binders in Armour Paints" Heritage 4, no. 4: 3402-3420. https://0-doi-org.brum.beds.ac.uk/10.3390/heritage4040189







