Fluoropolymer Film Formation by Electron Activated Vacuum Deposition
Abstract
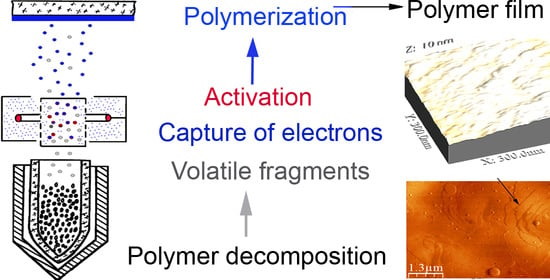
:1. Introduction
2. Materials and Methods
3. Results
3.1. Processes in Gas Phase
3.2. Structure of the Films by Infrared Spectroscopy
| Frequency, cm−1 | Vibration | Band Intensity, PTFE | Band Intensity, PHFP PCTFE | Reference | |||
|---|---|---|---|---|---|---|---|
| Thermal | EVD | EVD+RF | EVD | Thermal | |||
| 509 | CF2 bending | w | w | w | m | m | [40] |
| 526 | CF2 bending | m | m | m | m | w | [40] |
| 553/557 | CF2 deformation | m | m | m | m | w | [40] |
| 580 | CF2 wagging | - | - | - | - | w | [40] |
| 620 | CF2 wagging | m | m | m | m | - | [38] |
| 640/660 | Chain stretching | m | m | m | m | m | [40] |
| 703 | Amorphous | w | w | w | m | w | [38,41] |
| 721 | Amorphous | w | w | w | m | w | [38] |
| 738 | Amorphous | w | m | m | m | w | [38,41] |
| 780/778 | CF2 | w | w | w | m | w | [41] |
| 850 | Amorphous | - | - | - | w | - | [38] |
| 895 | CCl stretching | - | - | - | - | m | [45] |
| 972 | CCl stretching | - | - | - | - | s | [40] |
| 982/983 | CF3 | - | w | w | s | - | [42] |
| 1151/1156/1139 | CF2 sym stretch. | s | s | s | s | s | [40] |
| 1213/1216/1195 | CF2 asym stretch. | s | s | s | s | s | [40] |
| 1285 | CF stretching | - | - | - | - | m | [40] |
| 1340 | –C=CF2 | w | m | m | w | - | [18] |
| 1352/1350 | –C=C- | w | w | m | w | m | [18,42] |
| 1455 | CF2 sym stretch. | w | w | w | w | - | [40] |
3.3. AFM Studies of the Films Surface and Mechanical Properties
3.4. Optical Properties of the Films
3.5. Dye-Filled PTFE Films
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gritsenko, K.P.; Krasovsky, A.M. Thin film deposition of polymers by vacuum degradation. Chem. Rev. 2003, 103, 3607–3650. [Google Scholar] [CrossRef]
- Yaschchuk, V.M.; Gytsenko, K.P.; Schrader, S.; Navozenko, O. Applications of vacuum deposited perfluoropolymer films in OLED and OFET. Bull. Kyiv Univ. 2017, 2, 109–118. [Google Scholar]
- Shin, J.Y.; Kim, T.W.; Kim, G.Y.; Lee, S.M.; Hong, J.W. Performance of three-layered organic light-emitting diodes using the hole-transport and injection layer of TPD and Teflon-AF, and the electron-injection layer of Li2CO3 and LiF. Trans. Electr. Electron. Mater. 2017, 18, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Roh, J.; Cho, I.-T.; Shin, H.; Baek, G.W.; Hong, B.H.; Lee, J.-H.; Jin, S.H.; Lee, C. Fluorinated CYTOP passivation effects on the electrical reliability of multilayer MoS2 field effect transistors. Nanotechnology 2015, 26, 455201. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Gourisankar, S.V. Preparation and characterisarion of thin film surface coatings for biological environments. Biomaterials 1986, 7, 403–422. [Google Scholar] [CrossRef]
- Grytsenko, K.P.; Kolomsarov, Y.V.; Belyaev, O.E.; Schrader, S. Protective applications of vacuum deposited perfluoropolymer films. Semicond. Phys. Quantum Electron. Optoelectron. 2016, 19, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Grytsenko, K.P. Design and Research of Thin Film Metal-Polymer Materials for Optical Information Carrier of Computer. Ph.D. Thesis, Metal-Polymer Research Institute of National Academy of Sciences of Belarus, Gomel, Belarus, 1997. [Google Scholar]
- Gritsenko, K.P.; Capobianchi, A.; Convertino, A.; Friedrich, J.; Schulze, R.D.; Ksensov, V.; Schrader, S. Polymer-Metal Composite Thin Film Prepared by Co-Evaporation in Vacuum. In Polymer Surface Modification and Polymer Coatings by Dry Process Technologies; Iwamori, S., Ed.; Research Signpost: Kerala, India, 2005; pp. 85–109. [Google Scholar]
- Takele, H.; Kulkarni, A.; Jebril, S.; Chakravadhanula, V.S.K.; Hanisch, C.; Strunskus, T.; Zaporojtchenko, V.; Faupel, F. Plasmonic properties of vapour-deposited polymer composites containing Ag nanoparticles and their changes upon annealing. J. Phys. D Appl. Phys. 2008, 41, 125409. [Google Scholar] [CrossRef]
- Shokouh, S.H.; Jeon, P.J.; Pezeshki, A.; Choi, K.; Lee, H.S.; Kim, J.S.; Park, E.Y.; Im, S. High-performance, air-stable, top-gate, p-channel WSe2 field-effect transistor with fluoropolymer buffer layer. Adv. Func. Mater. 2015, 25, 7208–7214. [Google Scholar] [CrossRef]
- Pandey, S.S.; Pandey, M.; Nagamatsu, S.; Kumari, N. Recent advances in orientation of conjugated polymers for organic field-effect transistors. J. Mater. Chem. C 2019, 7, 13323–13351. [Google Scholar] [CrossRef]
- Wang, B.; Ruud, C.J.; Price, J.S.; Kim, H.; Giebink, N.C. Graded-index fluoropolymer antireflection coatings for invisible plastic optics. Nano Lett. 2019, 19, 787–792. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, X.; Dang, Y.; Huang, S.; Chen, X.; Kang, B.; Silva, S.R.P. PTFE/MoO3 Anode bi-layer buffer layers for improved performance in PCDTBT:PC71BM blend organic solar cells. ACS Sustain. Chem. Eng. 2016, 4, 6473–6479. [Google Scholar] [CrossRef]
- Psarski, M.; Pawlak, D.; Grobelny, J.; Celichowski, G. Hydrophobic and superhydrophobic surfaces fabricated by plasma polymerization of perfluorohexane, perfluoro(2-methylpent-2-ene), and perfluoro(4-methylpent-2-ene). J. Adhes. Sci. Technol. 2015, 29, 2035–2048. [Google Scholar] [CrossRef]
- Yi, N.; Baj, S.; Zhou, H.; Xin, Y.; Huang, A.; Ma, Y.; Li, R.; Jin, P. Preparation of microstructure-controllable superhydrophobic polytetrafluoroethylene porous thin film by vacuum thermal evaporation. Front. Mater. Sci. 2016, 10, 320–327. [Google Scholar] [CrossRef]
- Karre, V. Direct Electron Patterning of Teflon-AF and Its Application to Optical Waveguides. Master’s Thesis, University of Kentucky, Lexington, KY, USA, 2009. [Google Scholar]
- Xu, W.; Zheng, H.; Liu, Y.; Zhou, X.; Zhang, C.; Song, Y.; Deng, X.; Leung, M.; Yang, Z.; Xu, R.X.; et al. A droplet-based electricity generator with high instantaneous power density. Nature 2020, 578, 392–396. [Google Scholar] [CrossRef]
- Zadorozhny, V.G. Physical-Chemical Processes During Polymer Deposition in Vacuum and Creation of the New Technologies. Ph.D. Thesis, Institute of High Molecular Compounds of National Academy of Sciences of Ukraine, Kyiv, Ukraine, 1989. (In Russian). [Google Scholar]
- Murakami, Y.; Shintani, T. Vacuum deposition of teflon-FEP. Thin Solid Films 1972, 9, 301–304. [Google Scholar] [CrossRef]
- Chen, R.; Gorelik, V.; Silverstain, M.S. Plasma polymerization of hexafluoropropylene: Film deposition and structure. J. Appl. Polym. Sci. 1995, 56, 615–623. [Google Scholar] [CrossRef]
- Garrison, M.D.; Luginbühl, R.; Overney, R.M.; Ratner, B.D. Glow discharge plasma deposited hexafuoropropylene films: Surface chemistry and interfacial materials properties. Thin Solid Films 1999, 352, 13–21. [Google Scholar] [CrossRef]
- Lau, K.K.S.; Caulfield, J.A.; Gleason, K.K. Variable angle spectroscopic ellipsometry of fluorocarbon films from hot filament chemical vapor deposition. J. Vac. Sci. Technol. A Vac. Surf. Films 2000, 18, 2404–2411. [Google Scholar] [CrossRef]
- Bruk, A.; Zhikharev, E.N.; Volegova, I.A.; Spirin, A.V.; Kozlova, N.V.; Teleshov, E.N.; Kal’nov, V.A. The mechanism of formation and some properties of thin fluorocarbon films deposited onto silicon plates by electron beam polymerization of hexafluoropropylene from the vapor phase. Polym. Sci. Ser. B 2010, 52, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Hallahan, J.R.; Wydeven, T.; Johnson, C.C. Combination moisture resistant and antireflection plasma polymerized thin films for optical coatings. Appl. Opt. 1974, 13, 1844–1849. [Google Scholar] [CrossRef]
- Martinu, L. Deposition and structure of gold containing plasma polymerized halocarbon films. Thin Solid Films 1986, 140, 307–319. [Google Scholar] [CrossRef]
- Tsuji, O.; Minaguchi, T.; Nakano, H.; Tatsuta, T. Surface using property of plasma deposited copolymer films chlorofluorocarbon with ethylene monomer. J. Photopolym. Sci. Technol. 1997, 10, 143–148. [Google Scholar] [CrossRef] [Green Version]
- Grytsenko, K.P.; Schrader, S. Nanoclusters in polymer matrices prepared by co-deposition from a gas phase. Adv. Colloid Interface Sci. 2005, 116, 263–276. [Google Scholar] [CrossRef]
- Grytsenko, K.P. Vacuum-evaporation deposited polytetrafluoroethylene films: Growth mechanism, properties and applications of vacuum-deposited PTFE films. Russ. J. Gen. Chem. 2009, 79, 642–656. [Google Scholar] [CrossRef]
- Luff, P.P.; White, M. Thermal degradation of polyethylene and polytetrafluoroethylene during vacuum evaporation. Vacuum 1968, 18, 437–440. [Google Scholar] [CrossRef]
- Collins, R.D.; Fiveash, P.; Holland, L. A mass spectrometry study of the evaporation and pyrolysis of polytetrafluorethylene. Vacuum 1969, 19, 113. [Google Scholar] [CrossRef]
- Graupner, K.; Field, T.A.; Mayhew, C.A. Dissociative electron attachment to the highly reactive difluoromethylene molecule—Importance of CF2 for negative ion formation in fluorocarbon plasmas. New J. Phys. 2010, 12, 1–15. [Google Scholar] [CrossRef]
- Rozum, I.; Limao-Vieira, P.; Eden, S.; Tennyson, J.; Mason, N.J. Electron interaction cross sections for CF3I, C2F4, and CFx (x=1–3) radicals. J. Phys. Chem. Ref. Data 2006, 35, 267–282. [Google Scholar] [CrossRef] [Green Version]
- Simpson, M.J.; Tuckett, R.P.; Dunn, K.F.; Hunniford, C.A.; Latimer, C.J. Vacuum-UV negative photoion spectroscopy of CF3Cl, CF3Br, and CF3I. J. Chem. Phys. 2009, 130, 194302. [Google Scholar] [CrossRef] [Green Version]
- Mitterdorfer, C.; Edtbauer, A.; Karolczak, S.; Postler, J.; Gschliesser, D.; Denif, S.; Illenberger, E.; Scheier, P. Strong fragmentation processes driven by low energy electron attachment to various small perfluoroether molecules. Int. J. Mass Spectrom. 2011, 306, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Harvey, J.; Bodi, A.; Tuckett, R.P.; Sztaray, B. Dissociation dynamics of fluorinated ethane cations: From time bombs on a molecular level to double-regime dissociators. Phys. Chem. Chem. Phys. 2012, 14, 3935–4394. [Google Scholar] [CrossRef] [Green Version]
- Wijesundara, M.B.; Ji, Y.; Ni, B.; Sinott, S.B. Effect of polyatomic ion structure on thin-film growth: Experiments and molecular dynamics simulations. J. Appl. Phys. 2000, 88, 5004–5011. [Google Scholar] [CrossRef] [Green Version]
- Gritsenko, K. Mechanism of PTFE film growth in vacuum. Ukr. Chem. J. 1991, 57, 782–784. [Google Scholar]
- Moynihan, R.E. The molecular structure of perfluorocarbon polymers. Infrared studies on Polytetrafluoroethylene. J. Am. Chem. Soc. 1959, 81, 1045–1050. [Google Scholar] [CrossRef]
- Sianesi, D.; Caporiccio, G. Stereospecific polymerization of perfluoroolefins. Die Makromol. Chem. Macromol. Chem. Phys. 1963, 60, 213–222. [Google Scholar] [CrossRef]
- Liang, C.Y.; Krimm, S. Infrared spectra of high polymers. III. Polytetrafluoroethylene and polychlorotrifluoroethylene. J. Chem. Phys. 1956, 25, 563–571. [Google Scholar] [CrossRef] [Green Version]
- Lantoukh, G.V.; Gritsenko, K.P. Identification of the band 780 cm−1 of polytetrafluoroethylene infrared spectrum. J. Appl. Spectrosc. Beloruss. 1990, 52, 611–613. [Google Scholar]
- Dube, G.; Kriegsmann, M. Das infrarospectrum und die umwandlungspunkte von polytetrafluorathylen. Z. Chem. 1965, 5, 421–422. [Google Scholar] [CrossRef]
- Zhang, Y.; Katoh, T.; Endo, A. Deposition of highly oriented Teflon thin films by synchrotron radiation etching. J. Electron Spectrosc. Relat. Phenom. 2001, 119, 247–253. [Google Scholar] [CrossRef]
- DuPont FEP Film. Information Bulleting. Available online: https://fluorolab.com/wp-content/uploads/2018/02/Teflon-FEP-Film-Information-Bulletin.pdf. (accessed on 4 September 2018).
- Boschet, F.; Ameduri, B. Copolymers of chlorotrifluoroethylene: Synthesis, properties, and applications. Chem. Rev. 2014, 114, 927–980. [Google Scholar] [CrossRef]
- Yua, Y.; Xua, X.-Q.; Lua, C.; Zhang, T.; Ma, Y. Investigation on the microstructural and mechanical properties of a polytetrafluoroethylene thin film by radio frequency plasma. Thin Solid Films 2020, 712, 138302. [Google Scholar] [CrossRef]
- French, R.H.; Rodríguez-Parada, J.M.; Yang, M.K.; Derryberry, R.A.; Lemon, M.F.; Brown, M.J.; Haeger, C.R.; Samuels, S.L.; Romano, E.C.; Richardson, R.E. Optical Properties of Materials for Concentrator Photovoltaic Systems. In Proceedings of the 34th IEEE Photovoltaic Specialists Conference (PVSC), Philadelphia, PA, USA, 7–12 June 2009. [Google Scholar]
- Grytsenko, K.P.; Machulin, V.F.; Ait, A.O.; Gorelik, A.M.; Kobeleva, O.I.; Valova, T.M.; Barachevsky, V.A. Photochromic films prepared by vacuum co-deposition of polymer and spiropyrans. Opt. Mem. Neural Netw. 2010, 19, 254–259. [Google Scholar] [CrossRef]
- Grytsenko, K.; Navozenko, O.; Kolomzarov, Y.; Kryuchin, A.; Tolmachev, O.; Slominsky, Y.; Kurdukov, V.; Ksianzou, V.; Schrader, S. Optical properties of dye-filled polymer films, deposited in vacuum. Data Rec. Storage Proc. 2012, 14, 3–9. [Google Scholar]
- Grytsenko, K.; Schrader, S.; Detert, H. Ultra-stable dye-filled polytetrafluoroethylene thin films. Nanosci. Technol. 2014, 1, 1–5. [Google Scholar] [CrossRef]
- Gorbov, I.V.; Kryuchyn, A.A.; Grytsenko, K.P.; Manko, D.Y.; Borodin, Y.O. High-density data recording via laser thermo-lithography and ion-beam etching. Semicond. Phys. Quantum Electron. Optoelectron. 2014, 17, 52–55. [Google Scholar] [CrossRef] [Green Version]
- Petrov, V.V.; Kryuchin, A.A.; Gorbov, I.V.; Borodin, Y.O.; Briks, J.L.; Kurdiukov, V.V.; Slominskii, Y.L.; Tolmachev, O.I.; Grytsenko, K.P. Photosensitive Material for Optical Recording. Patent of Ukraine No. 110143, 25 November 2015. [Google Scholar]
- Grytsenko, K.; Kolomzarov, Y.; Lytvyn, P.; Beyer, H.; Ksenzou, V.; Schrader, S.; Schulze, R.D.; Friedrich, J. Effect of magnetic field on film formation by means of polytetrafluoroethylene decomposition in vacuum. Mater. Technol. Tools 2011, 16, 56–59. [Google Scholar]
- Keathley, P.D.; Hastings, J.T. Optical properties of sputtered fluorinated ethylene propylene and its application to surface-plasmon resonance sensor fabrication. J. Vac. Sci. Technol. B 2008, 266, 2473–2477. [Google Scholar] [CrossRef]
- Isakov, K.; Kauppinen, C.; Franssila, S.; Lipsanen, H. Superhydrophobic antireflection coating on glass using grass-like alumina and fluoropolymer. ACS Appl. Mater. Interfaces 2020, 12, 49957–49962. [Google Scholar] [CrossRef] [PubMed]
- Rotter, P.; Lechner, B.; Morherr, A.; Chisnall, D.; Ward, D.J.; Jardine, A.P.; Ellis, J.; Allison, W.; Eckhardt, B.; Witte, G. Coupling between diffusion and orientation of pentacene molecules on an organic surface. Nat. Mater. 2016, 15, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glazirin, N.P.; Tolstopyatov, E.M. Quasiperiodic effects of plasmachemical formation of fluoropolymer coatings in a long reactor exploiting longitudinal electrical discharge. Polym. Mater. Technol. Beloruss. 2017, 2, 34–40. [Google Scholar]









| Polymer | Density, g/cm3 | Melting T, °C | Viscosity, Poise/at T, °C | Decomposition T at Evaporation, °C | Deposition Rate, nm/s |
|---|---|---|---|---|---|
| PTFE | 2.2 | 327 | 1012/380 | 420–450 | 0.5–1.5 |
| PHFP | 2.1 | 260 | 104/270 | 300–320 | 0.4–1 |
| PCTFE | 2.1 | 215 | 1011/230 | 400–420 | 1–1.5 |
| Amu | Ionized Fragment | Intensity, %, PTFE | Intensity, %, PHFP | Intens., %, PCTFE | |||
|---|---|---|---|---|---|---|---|
| Thermal | Q_Closed | EVD | Thermal | EVD | Thermal/EVD | ||
| 12 | C+ | 14 | 6 | 4 | 4 | 3 | 7 |
| 24 | C2+ | 11 | 3 | 2 | 3 | 4 | 3 |
| 31 | CF+ | 100 | 96 | 45 | 100 | 40 | 100 |
| 35.5 | Cl+ | - | - | - | - | - | 16 |
| 47.5 | CCl+ | - | - | - | - | - | 23 |
| 50 | CF2+ | 39 | 46 | 16 | 19 | 14 | 7 |
| 62 | C2F2+ | 7 | 5 | 0 | 2 | 2 | 3 |
| 66.5 | CFCl+ | - | - | - | - | - | 13 |
| 69 | CF3+ | 9 | 39 | 69 | 84 | 100 | 33 |
| 85.5 | CF2Cl+ | - | - | - | 9 | ||
| 81 | C2F3+ | 43 | 32 | 34 | 30 | 12 | 0 |
| 93 | C3F3+ | 0 | 3 | 6 | 7 | 7 | 0 |
| 97.5 | C2F2Cl+ | - | - | - | - | - | 5 |
| 100 | C2F4+ | 20 | 40 | 6 | 23 | 15 | 3 |
| 112 | C3F4+ | 0 | 0 | 4 | 2 | 2 | 4 |
| 119 | C2F5+ | 0 | 0 | 9 | 4 | 10 | 2 |
| 131 | C3F5+ | 6 | 4 | 54 | 69 | 40 | 1 |
| 138 | C2F6+ | 0 | 5 | 0 | 1 | 2 | 0 |
| 143 | C4F5+ | 0 | 0 | 4 | 3 | 5 | 0 |
| 147.5 | C3F4Cl+ | - | - | - | - | - | 29 |
| 150 | C3F6+ | 0 | 0 | 3 | 9 | 5 | 0 |
| 169 | C3F7+/C3F5Cl+ | - | - | - | 0,5 | 3 | 11 |
| 174 | C5F6+ | - | - | - | 3 | 2 | - |
| 181 | C4F7+/C3F4Cl2+ | - | - | 4 | 2 | 8 | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grytsenko, K.; Ksianzou, V.; Kolomzarov, Y.; Lytvyn, P.; Dietzel, B.; Schrader, S. Fluoropolymer Film Formation by Electron Activated Vacuum Deposition. Surfaces 2021, 4, 66-80. https://0-doi-org.brum.beds.ac.uk/10.3390/surfaces4010009
Grytsenko K, Ksianzou V, Kolomzarov Y, Lytvyn P, Dietzel B, Schrader S. Fluoropolymer Film Formation by Electron Activated Vacuum Deposition. Surfaces. 2021; 4(1):66-80. https://0-doi-org.brum.beds.ac.uk/10.3390/surfaces4010009
Chicago/Turabian StyleGrytsenko, Kostyantyn, Viachaslau Ksianzou, Yurii Kolomzarov, Peter Lytvyn, Birgit Dietzel, and Sigurd Schrader. 2021. "Fluoropolymer Film Formation by Electron Activated Vacuum Deposition" Surfaces 4, no. 1: 66-80. https://0-doi-org.brum.beds.ac.uk/10.3390/surfaces4010009