Paradigm Shift of Healthcare Cost for Patients with Inflammatory Bowel Diseases: A Claims Data-Based Analysis in Japan
Abstract
:1. Introduction
2. Results
2.1. Patient Summary
2.2. Estimation of Health Care Costs and Use of Anti-TNFα Agents
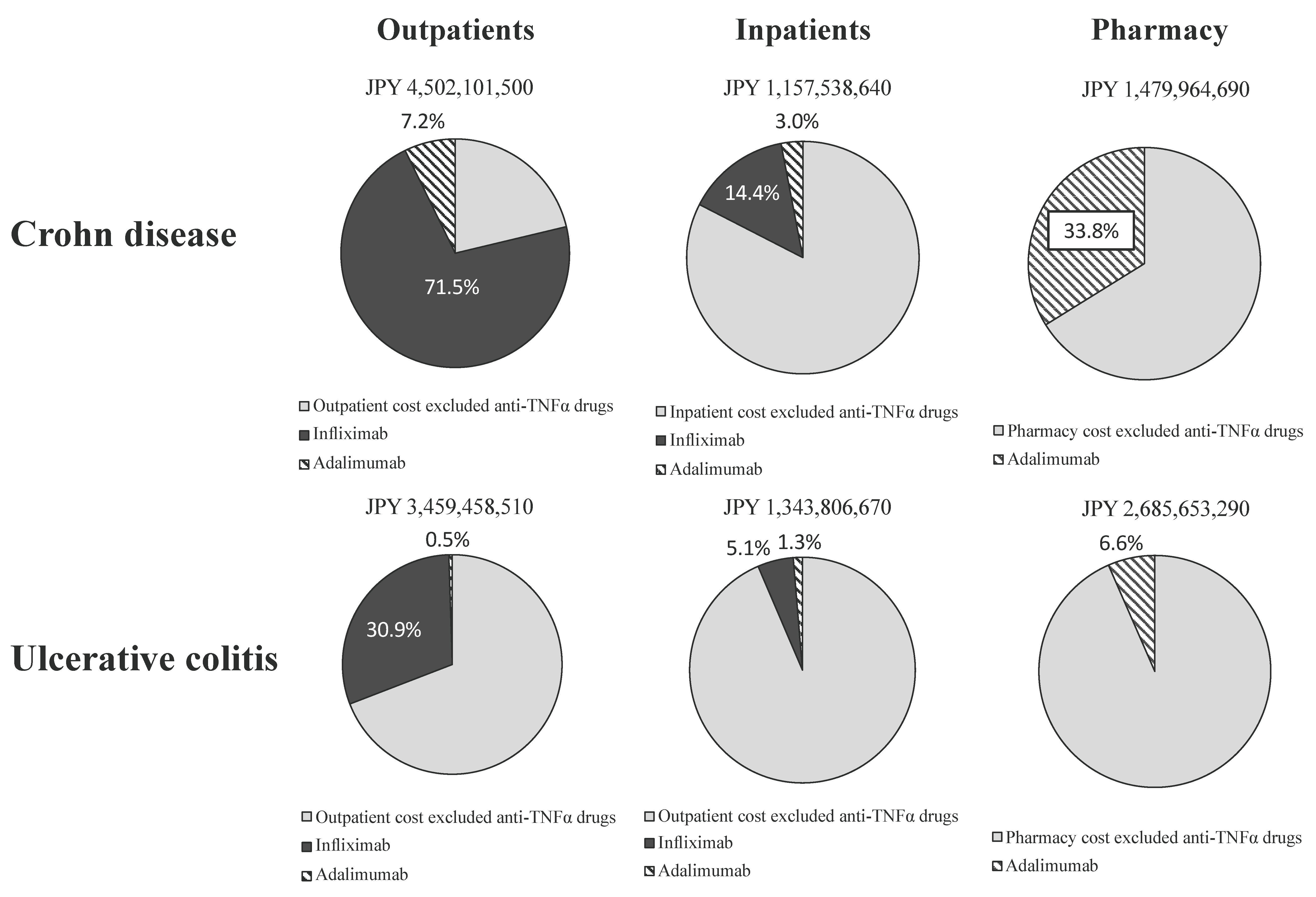
2.3. Share of Drug Costs Associated with Anti-TNFα Agents in Healthcare Costs
2.4. Number of Patients with IBD Who Received Biologics among PMPY Subgroups
3. Discussion
4. Materials and Methods
4.1. Overview of Claims Data
4.2. Patient Identification
4.3. Estimation of Reimbursement of Health Care Costs
4.4. Statistical Analysis
4.5. Ethical Considerations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matsuoka, K.; Kobayashi, T.; Ueno, F.; Matsui, T.; Hirai, F.; Inoue, N.; Kato, J.; Kobayashi, K.; Kobayashi, K.; Koganei, K.; et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J. Gastroenterol. 2018, 53, 305–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, F.; Nakayama, Y.; Hagiwara, E.; Kurimoto, S.; Hibi, T. Impact of inflammatory bowel disease on Japanese patients’ quality of life: Results of a patient questionnaire survey. J. Gastroenterol. 2017, 52, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Frolkis, A.D.; Dykeman, J.; Negrón, M.E.; deBruyn, J.; Jette, N.; Fiest, K.M.; Frolkis, T.; Barkema, H.W.; Rioux, K.P.; Panaccione, R.; et al. Risk of surgery for inflammatory bowel diseases has decreased over time: A systematic review and meta-analysis of population-based studies. Gastroenterology 2013, 145, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Di Sario, A.; Bendia, E.; Schiadà, L.; Sassaroli, P.; Benedetti, A. Biologic Drugs in Crohn’s Disease and Ulcerative Colitis: Safety Profile. Curr. Drug Saf. 2016, 11, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Mao, E.J.; Hazlewood, G.S.; Kaplan, G.G.; Peyrin-Biroulet, L.; Ananthakrishnan, A.N. Systematic review with meta-analysis: Comparative efficacy of immunosuppressants and biologics for reducing hospitalisation and surgery in Crohn’s disease and ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 45, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Park, K.T.; Colletti, R.B.; Rubin, D.T.; Sharma, B.K.; Thompson, A.; Krueger, A. Health Insurance Paid Costs and Drivers of Costs for Patients with Crohn’s Disease in the United States. Am. J. Gastroenterol. 2016, 111, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Van der Valk, M.E.; Mangen, M.-J.J.; Leenders, M.; Dijkstra, G.; van Bodegraven, A.A.; Fidder, H.H.; de Jong, D.J.; Pierik, M.; van der Woude, C.J.; Romberg-Camps, M.J.L.; et al. COIN study group and the Dutch Initiative on Crohn and Colitis. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: Results from the COIN study. Gut 2014, 63, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Niewiadomski, O.; Studd, C.; Hair, C.; Wilson, J.; McNeill, J.; Knight, R.; Prewett, E.; Dabkowski, P.; Dowling, D.; Alexander, S.; et al. Health Care Cost Analysis in a Population-based Inception Cohort of Inflammatory Bowel Disease Patients in the First Year of Diagnosis. J. Crohns Colitis 2015, 9, 988–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, S.; Sato, T.; Ikeda, S.; Noda, M.T.; Nakayama, T. Development of a database of health insurance claims: Standardization of disease classifications and anonymous record linkage. J. Epidemiol. 2010, 20, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Pillai, N.; Dusheiko, M.; Burnand, B.; Pittet, V. A systematic review of cost-effectiveness studies comparing conventional, biological and surgical interventions for inflammatory bowel disease. PLoS ONE 2017, 12, e0185500. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; MacIsaac, D.; Wong, J.J.; Sellers, Z.M.; Wren, A.A.; Bensen, R.; Kin, C.; Park, K.T. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment. Pharmacol. Ther. 2018, 47, 364–370. [Google Scholar] [CrossRef] [PubMed]
- The Japanese Society of Gastroenterology. Clinical Practice Guideline for Inflammatory Bowel Disease; Nankodo: Tokyo, Japan, 2016. (In Japanese) [Google Scholar]
- Blonski, W.; Buchner, A.M.; Lichtenstein, G.R. Inflammatory bowel disease therapy: Current state-of-the-art. Curr. Opin. Gastroenterol. 2011, 27, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Amezaga, A.J.; Van Assche, G. Practical Approaches to “Top-Down” Therapies for Crohn’s Disease. Curr. Gastroenterol. Rep. 2016, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Bonovas, S.; Peyrin-Biroulet, L. Biosimilars in IBD: From theory to practice. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Brodszky, V.; Baji, P.; Balogh, O.; Péntek, M. Budget impact analysis of biosimilar infliximab (CT-P13) for the treatment of rheumatoid arthritis in six Central and Eastern European countries. Eur. J. Health Econ. 2014, 15, 65–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, A.; Upton, A.; Dunlop, W.C.; Akehurst, R. The Budget Impact of Biosimilar Infliximab (Remsima®) for the Treatment of Autoimmune Diseases in Five European Countries. Adv. Ther. 2015, 32, 742–756. [Google Scholar] [CrossRef] [PubMed]
- Mahlich, J.; Matsuoka, K.; Nakamura, Y.; Sruamsiri, R. The relationship between socio-demographic factors, health status, treatment type, and employment outcome in patients with inflammatory bowel disease in Japan. BMC Public Health 2017, 7, 623. [Google Scholar] [CrossRef] [PubMed]
- Bähler, C.; Vavricka, S.R.; Schoepfer, A.M.; Brüngger, B.; Reich, O. Trends in prevalence, mortality, health care utilization and health care costs of Swiss IBD patients: A claims data based study of the years 2010, 2012 and 2014. BMC Gastroenterol. 2017, 17, 138. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Lee, C.K.; Rhee, S.Y.; Oh, C.H.; Shim, J.-J.; Kim, H.J. Trends in health-care costs and utilization for inflammatory bowel disease from 2010 to 2014 in Korea: A nationwide population-based study. J. Gastroenterol. Hepatol. 2018, 33, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.C. Differences in the public medical insurance systems for inflammatory bowel disease treatment in Asian countries. Intest. Res. 2016, 14, 218–223. [Google Scholar] [CrossRef] [PubMed]

| Item | Subgroup | CD | ||||
|---|---|---|---|---|---|---|
| n | % | PMPY (JPY) | Anti-TNFα Agents Used (95% CI) | |||
| Median | Interquartile Range | |||||
| N | All | 1405 | 1,957,320 | 383,220–2,996,340 | 0.552 (0.526–0.578) | |
| By sex | Woman | 303 | 21.6% | 1,446,480 | 318,120–2,525,520 | 0.502 (0.446–0.558) |
| Man | 1102 | 78.4% | 2,053,680 | 406,620–3,103,830 | 0.566 (0.537–0.595) | |
| By age group (years) | 0–9 | 6 | 0.4% | 1,129,380 | 835,800–7,063,740 | 0.167 (0.030–0.563) |
| 10–19 | 82 | 5.8% | 2,292,240 | 557,250–3,180,630 | 0.634 (0.526–0.730) | |
| 20–29 | 285 | 20.3% | 2,173,800 | 697,860–3,071,940 | 0.670 (0.614–0.722) | |
| 30–39 | 389 | 27.7% | 2,056,080 | 383,100–3,103,500 | 0.584 (0.534–0.631) | |
| 40–49 | 377 | 26.8% | 1,930,800 | 363,840–3,105,480 | 0.538 (0.488–0.588) | |
| 50–59 | 207 | 14.7% | 1,341,720 | 306,840–2,718,000 | 0.440 (0.374–0.508) | |
| 60–69 | 52 | 3.7% | 373,560 | 231,660–1,745,340 | 0.216 (0.122–0.340) | |
| 70+ | 7 | 0.5% | 161,040 | 69,240–293,640 | 0 (NA) | |
| Item | Subgroup | UC | ||||
|---|---|---|---|---|---|---|
| n | % | PMPY (JPY) | Anti-TNFα Agents Used (95% CI) | |||
| Median | Interquartile Range | |||||
| N | All | 5771 | 278,760 | 160,560–456,720 | 0.075 (0.069–0.082) | |
| By sex | Woman | 2134 | 37.0% | 279,240 | 158,820–460,620 | 0.075 (0.065–0.087) |
| Man | 3637 | 63.0% | 278,280 | 161,760–453,540 | 0.075 (0.067–0.084) | |
| By age group (years) | 0–9 | 12 | 0.2% | 903,240 | 293,040–4,186,050 | 0.083 (0.015–0.354) |
| 10–19 | 170 | 2.9% | 464,100 | 239,730–1,303,200 | 0.200 (0.147–0.266) | |
| 20–29 | 704 | 12.2% | 301,800 | 172,530–553,470 | 0.117 (0.095–0.142) | |
| 30–39 | 1324 | 22.9% | 271,560 | 158,220–443,670 | 0.085 (0.071–0.101) | |
| 40–49 | 1825 | 31.6% | 258,960 | 148,740–417,960 | 0.066 (0.055–0.078) | |
| 50–59 | 1237 | 21.4% | 276,120 | 163,920–434,220 | 0.051 (0.040–0.065) | |
| 60–69 | 440 | 7.6% | 291,900 | 162,630–504,990 | 0.048 (0.031–0.072) | |
| 70+ | 59 | 1.0% | 332,880 | 213,240–623,280 | 0 (NA) | |
| PMPY Subgroup (JPY) | |||||
|---|---|---|---|---|---|
| Minimal Subgroup <1 Million | Low Subgroup 1 Million to 2 Million | Moderate Subgroup 2 Million to 3 Million | High Subgroup >3 Million | p-Value * | |
| CD | |||||
| Biologics | 23 | 85 | 326 | 342 | <0.001 |
| No biologics | 533 | 68 | 19 | 9 | |
| Total (n = 1405) | 556 | 153 | 345 | 351 | |
| UC | |||||
| Biologics | 46 | 115 | 165 | 107 | <0.001 |
| No biologics | 5060 | 190 | 60 | 28 | |
| Total (n = 5771) | 5106 | 305 | 225 | 135 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, S.; Nakazawa, K.; Suzuki, K.; Ishikawa, T.; Akazawa, K. Paradigm Shift of Healthcare Cost for Patients with Inflammatory Bowel Diseases: A Claims Data-Based Analysis in Japan. Gastrointest. Disord. 2019, 1, 120-128. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord1010009
Saito S, Nakazawa K, Suzuki K, Ishikawa T, Akazawa K. Paradigm Shift of Healthcare Cost for Patients with Inflammatory Bowel Diseases: A Claims Data-Based Analysis in Japan. Gastrointestinal Disorders. 2019; 1(1):120-128. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord1010009
Chicago/Turabian StyleSaito, Shota, Kyoko Nakazawa, Kenji Suzuki, Takashi Ishikawa, and Kouhei Akazawa. 2019. "Paradigm Shift of Healthcare Cost for Patients with Inflammatory Bowel Diseases: A Claims Data-Based Analysis in Japan" Gastrointestinal Disorders 1, no. 1: 120-128. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord1010009





