Does Irritable Bowel Syndrome Exist? Identifiable and Treatable Causes of Associated Symptoms Suggest It May Not
Abstract
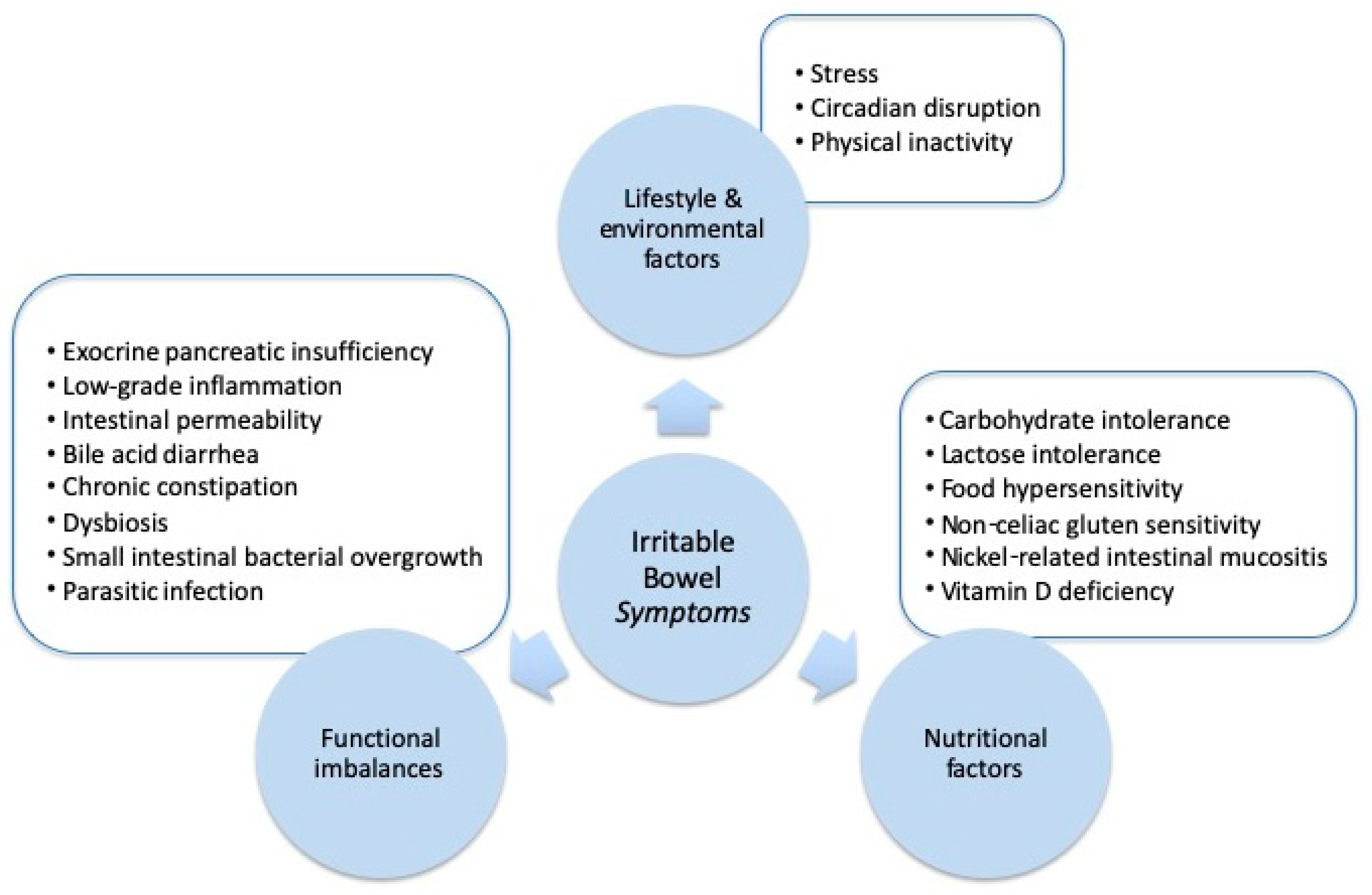
:1. Introduction
2. Lifestyle and Environmental Factors
2.1. Stress
2.2. Circadian Disruption
2.3. Physical Inactivity
3. Nutritional Factors
3.1. Carbohydrate Restriction
3.2. Lactose Intolerance
3.3. Food Hypersensitivity
3.4. Non-Celiac Gluten Sensitivity
3.5. Nickel-Related Intestinal Mucositis
3.6. Vitamin D Deficiency
4. Functional Imbalances
4.1. Exocrine Pancreatic Insufficiency
4.2. Low-Grade Inflammation
4.3. Intestinal Permeability
4.4. Bile Acid Diarrhea
4.5. Chronic Constipation
4.6. Dysbiosis
4.7. Small Intestinal Bacterial Overgrowth
4.8. Parasitic Infection
5. Discussion
Funding
Acknowledgments
Conflicts of Interest
References
- Lovell, R.M.; Ford, A.C. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721. [Google Scholar] [CrossRef]
- Spiegel, B.M. The burden of IBS: Looking at metrics. Curr. Gastroenterol. Rep. 2009, 11, 265–269. [Google Scholar] [CrossRef]
- Silk, D.B. Impact of irritable bowel syndrome on personal relationships and working practices. Eur. J. Gastroenterol. Hepatol. 2001, 13, 1327–1332. [Google Scholar] [CrossRef]
- Ford, A.C.; Moayyedi, P.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.; Quigley, E.M. Task Force on the Management of Functional Bowel Disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am. J. Gastroenterol. 2014, 109 (Suppl. 1), S2–S26. [Google Scholar] [CrossRef]
- Spiegel, B.M.; Farid, M.; Esrailian, E.; Talley, J.; Chang, L. Is irritable bowel syndrome a diagnosis of exclusion?: A survey of primary care providers, gastroenterologists, and IBS experts. Am. J. Gastroenterol. 2010, 105, 848–858. [Google Scholar] [CrossRef]
- Ford, A.C.; Lacy, B.E.; Talley, N.J. Irritable Bowel Syndrome. N. Engl. J. Med. 2017, 376, 2566–2578. [Google Scholar] [CrossRef] [Green Version]
- Sood, R.; Gracie, D.J.; Law, G.R.; Ford, A.C. Systematic review with meta-analysis: The accuracy of diagnosing irritable bowel syndrome with symptoms, biomarkers and/or psychological markers. Aliment. Pharmacol. Ther. 2015, 42, 491–503. [Google Scholar] [CrossRef]
- Cremonini, F.; Talley, N.J. Diagnostic and therapeutic strategies in the irritable bowel syndrome. Minerva Med. 2004, 95, 427–441. [Google Scholar]
- Tack, J.; Fried, M.; Houghton, L.A.; Spicak, J.; Fisher, G. Systematic review: The efficacy of treatments for irritable bowel syndrome—A European perspective. Aliment. Pharmacol. Ther. 2006, 24, 183–205. [Google Scholar] [CrossRef]
- Lembo, A. Irritable bowel syndrome medications side effects survey. J. Clin. Gastroenterol. 2004, 38, 776–781. [Google Scholar] [CrossRef]
- Ford, A.C.; Quigley, E.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.; Moayyedi, P. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: Systematic review and meta-analysis. Am. J. Gastroenterol. 2014, 109, 1547–1561. [Google Scholar] [CrossRef]
- Ford, A.C.; Quigley, E.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.; Moayyedi, P. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: Systematic review and meta-analysis. Am. J. Gastroenterol. 2014, 109, 1350–1365. [Google Scholar] [CrossRef]
- Bellini, M.; Gambaccini, D.; Stasi, C.; Urbano, M.T.; Marchi, S.; Usai-Satta, P. Irritable bowel syndrome: A disease still searching for pathogenesis, diagnosis and therapy. World J. Gastroenterol. 2014, 20, 8807–8820. [Google Scholar]
- Habba, S.F. Diarrhea Predominant Irritable Bowel Syndrome (IBS-D): Fact or fiction. Med. Hypotheses 2011, 76, 97–99. [Google Scholar] [CrossRef]
- Holtmann, G.J.; Ford, A.C.; Talley, N.J. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 2016, 1, 133–146. [Google Scholar] [CrossRef]
- Enck, P.; Aziz, Q.; Barbara, G.; Farmer, A.D.; Fukudo, S.; Mayer, E.A.; Niesler, B.; Quigley, E.M.; Rajilić-Stojanović, M.; Schemann, M.; et al. Irritable bowel syndrome. Nat. Rev. Dis. Primers 2016, 2, 16014. [Google Scholar] [CrossRef]
- Bland, J. Defining Function in the Functional Medicine Model. Integr. Med. Encinitas 2017, 16, 22–25. [Google Scholar]
- Goepp, J.; Fowler, E.; McBride, T.; Landis, D. Frequency of abnormal fecal biomarkers in irritable bowel syndrome. Glob. Adv. Health Med. 2014, 3, 9–15. [Google Scholar] [CrossRef]
- Emmanuel, A.; Landis, D.; Peucker, M.; Hungin, A.P. Faecal biomarker patterns in patients with symptoms of irritable bowel syndrome. Frontline Gastroenterol. 2016, 7, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Parsons, K.; Goepp, J.; Dechairo, B.; Fowler, E.; Markward, N.; Hanaway, P.; McBride, T.; Landis, D. Novel Testing Enhances Irritable Bowel Syndrome Medical Management: The IMMINENT Study. Glob. Adv. Health Med. 2014, 3, 25–32. [Google Scholar] [CrossRef]
- Quigley, E.M.; Shanahan, F. The language of medicine: Words as servants and scoundrels. Clin. Med. 2009, 9, 131–135. [Google Scholar] [CrossRef]
- Lee, C.; Doo, E.; Choi, J.M.; Jang, S.H.; Ryu, H.S.; Lee, J.Y.; Oh, J.H.; Park, J.H.; Kim, Y.S.; Brain-Gut Axis Research Group of Korean Society of Neurogastroenterology and Motility. The Increased Level of Depression and Anxiety in Irritable Bowel Syndrome Patients Compared with Healthy Controls: Systematic Review and Meta-analysis. J. Neurogastroenterol. Motil. 2017, 23, 349–362. [Google Scholar] [CrossRef] [Green Version]
- Koloski, N.A.; Jones, M.; Talley, N.J. Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: A 1-year population-based prospective study. Aliment. Pharmacol. Ther. 2016, 44, 592–600. [Google Scholar] [CrossRef]
- Bennett, E.J.; Tennant, C.C.; Piesse, C.; Badcock, C.A.; Kellow, J.E. Level of chronic life stress predicts clinical outcome in irritable bowel syndrome. Gut 1998, 43, 256–261. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Naliboff, B.D.; Shih, W.; Presson, A.P.; Videlock, E.J.; Ju, T.; Kilpatrick, L.; Gupta, A.; Mayer, E.A.; Chang, L. Resilience is decreased in irritable bowel syndrome and associated with symptoms and cortisol response. Neurogastroenterol. Motil. 2017. [Google Scholar] [CrossRef]
- Muscatello, M.R.; Bruno, A.; Scimeca, G.; Pandolfo, G.; Zoccali, R.A. Role of negative affects in pathophysiology and clinical expression of irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 7570–7586. [Google Scholar] [CrossRef]
- Konturek, P.C.; Brzozowski, T.; Konturek, S.J. Stress and the gut: Pathophysiology, clinical consequences, diagnostic approach and treatment options. J. Physiol. Pharmacol. 2011, 62, 591–599. [Google Scholar]
- Suárez-Hitz, K.A.; Otto, B.; Bidlingmaier, M.; Schwizer, W.; Fried, M.; Ehlert, U. Altered psychobiological responsiveness in women with irritable bowel syndrome. Psychosom. Med. 2012, 74, 221–231. [Google Scholar] [CrossRef]
- Stasi, C.; Bellini, M.; Gambaccini, D.; Duranti, E.; de Bortoli, N.; Fani, B.; Albano, E.; Russo, S.; Sudano, I.; Laffi, G.; et al. Neuroendocrine Dysregulation in Irritable Bowel Syndrome Patients: A Pilot Study. J. Neurogastroenterol. Motil. 2017, 23, 428–434. [Google Scholar] [CrossRef] [Green Version]
- Kohen, R.; Tracy, J.H.; Haugen, E.; Cain, K.C.; Jarrett, M.E.; Heitkemper, M.M. Rare Variants of the Serotonin Transporter Are Associated With Psychiatric Comorbidity in Irritable Bowel Syndrome. Biol. Res. Nurs. 2016, 18, 394–400. [Google Scholar] [CrossRef] [Green Version]
- Jin, D.C.; Cao, H.L.; Xu, M.Q.; Wang, S.N.; Wang, Y.M.; Yan, F.; Wang, B.M. Regulation of the serotonin transporter in the pathogenesis of irritable bowel syndrome. World J. Gastroenterol. 2016, 22, 8137–8148. [Google Scholar] [CrossRef] [PubMed]
- Karling, P.; Danielsson, Å.; Wikgren, M.; Söderström, I.; Del-Favero, J.; Adolfsson, R.; Norrback, K.F. The relationship between the val158met catechol-O-methyltransferase (COMT) polymorphism and irritable bowel syndrome. PLoS ONE 2011, 6, e18035. [Google Scholar] [CrossRef] [PubMed]
- Han, C.J.; Kohen, R.; Jun, S.; Jarrett, M.E.; Cain, K.C.; Burr, R.; Heitkemper, M.M. COMT Val158Met Polymorphism and Symptom Improvement Following a Cognitively Focused Intervention for Irritable Bowel Syndrome. Nurs. Res. 2017, 66, 75–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballou, S.; Keefer, L. Psychological Interventions for Irritable Bowel Syndrome and Inflammatory Bowel Diseases. Clin. Transl. Gastroenterol. 2017, 8, e214. [Google Scholar] [CrossRef] [PubMed]
- Lowén, M.B.; Mayer, E.A.; Sjöberg, M.; Tillisch, K.; Naliboff, B.; Labus, J.; Lundberg, P.; Ström, M.; Engström, M.; Walter, S.A. Effect of hypnotherapy and educational intervention on brain response to visceral stimulus in the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2013, 37, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
- Konturek, P.C.; Brzozowski, T.; Konturek, S.J. Gut clock: Implication of circadian rhythms in the gastrointestinal tract. J. Physiol. Pharmacol. 2011, 62, 139–150. [Google Scholar] [PubMed]
- Rotem, A.Y.; Sperber, A.D.; Krugliak, P.; Freidman, B.; Tal, A.; Tarasiuk, A. Polysomnographic and actigraphic evidence of sleep fragmentation in patients with irritable bowel syndrome. Sleep 2003, 26, 747–752. [Google Scholar] [CrossRef]
- Buchanan, D.T.; Cain, K.; Heitkemper, M.; Burr, R.; Vitiello, M.V.; Zia, J.; Jarrett, M. Sleep measures predict next-day symptoms in women with irritable bowel syndrome. J. Clin. Sleep Med. 2014, 10, 1003–1009. [Google Scholar] [CrossRef]
- Patel, A.; Hasak, S.; Cassell, B.; Ciorba, M.A.; Vivio, E.E.; Kumar, M.; Gyawali, C.P.; Sayuk, G.S. Effects of disturbed sleep on gastrointestinal and somatic pain symptoms in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016, 44, 246–258. [Google Scholar] [CrossRef]
- Nojkov, B.; Rubenstein, J.H.; Chey, W.D.; Hoogerwerf, W.A. The impact of rotating shift work on the prevalence of irritable bowel syndrome in nurses. Am. J. Gastroenterol. 2010, 105, 842–847. [Google Scholar] [CrossRef]
- Wisniewska-Jarosinska, M.; Chojnacki, J.; Konturek, S.; Brzozowski, T.; Smigielski, J.; Chojnacki, C. Evaluation of urinary 6-hydroxymelatonin sulphate excretion in women at different age with irritable bowel syndrome. J. Physiol. Pharmacol. 2010, 61, 295–300. [Google Scholar] [PubMed]
- Radwan, P.; Skrzydlo-Radomanska, B.; Radwan-Kwiatek, K.; Burak-Czapiuk, B.; Strzemecka, J. Is melatonin involved in the irritable bowel syndrome? J. Physiol. Pharmacol. 2009, 60 (Suppl. 3), 67–70. [Google Scholar] [PubMed]
- Song, G.H.; Leng, P.H.; Gwee, K.A.; Moochhala, S.M.; Ho, K.Y. Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances: A randomised, double blind, placebo controlled study. Gut 2005, 54, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, S.; Rahimi, R.; Abdollahi, M. Implications of melatonin therapy in irritable bowel syndrome: A systematic review. Curr. Pharm. Des. 2010, 16, 3646–3655. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.G. Light-at-night, circadian disruption and breast cancer: Assessment of existing evidence. Int. J. Epidemiol. 2009, 38, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Saltin, B. Exercise as medicine-evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci Sports 2015, 25 (Suppl. 3), 1–72. [Google Scholar] [CrossRef] [PubMed]
- Dainese, R.; Serra, J.; Azpiroz, F.; Malagelada, J.R. Effects of physical activity on intestinal gas transit and evacuation in healthy subjects. Am. J. Med. 2004, 116, 536–539. [Google Scholar] [CrossRef]
- De Schryver, A.M.; Keulemans, Y.C.; Peters, H.P.; Akkermans, L.M.; Smout, A.J.; De Vries, W.R.; van Berge-Henegouwen, G.P. Effects of regular physical activity on defecation pattern in middle-aged patients complaining of chronic constipation. Scand. J. Gastroenterol. 2005, 40, 422–429. [Google Scholar] [CrossRef]
- Matsuzaki, J.; Suzuki, H.; Masaoka, T.; Tanaka, K.; Mori, H.; Kanai, T. Influence of regular exercise on gastric emptying in healthy men: A pilot study. J. Clin. Biochem. Nutr. 2016, 59, 130–133. [Google Scholar] [CrossRef]
- Johannesson, E.; Ringström, G.; Abrahamsson, H.; Sadik, R. Intervention to increase physical activity in irritable bowel syndrome shows long-term positive effects. World J. Gastroenterol. 2015, 21, 600–608. [Google Scholar] [CrossRef]
- Shahabi, L.; Naliboff, B.D.; Shapiro, D. Self-regulation evaluation of therapeutic yoga and walking for patients with irritable bowel syndrome: A pilot study. Psychol. Health Med. 2016, 21, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, W.S.; Skidmore, P.M.; O’Brien, L.; Wilkinson, T.J.; Gearry, R.B. Efficacy of the low FODMAP diet for treating irritable bowel syndrome: The evidence to date. Clin. Exp. Gastroenterol. 2016, 9, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.; Eslick, E.M.; Eslick, G.D. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur. J. Nutr. 2016, 55, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Cozma-Petruţ, A.; Loghin, F.; Miere, D.; Dumitraşcu, D.L. Diet in irritable bowel syndrome: What to recommend, not what to forbid to patients! World J. Gastroenterol. 2017, 23, 3771–3783. [Google Scholar] [CrossRef]
- Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Simrén, M. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: A randomized controlled trial. Gastroenterology 2015, 149, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Hurst, A.; Knott, F. Intestinal carbohydrate dyspepsia. QJM 1931, 94, 171–179. [Google Scholar] [CrossRef]
- Ledochowski, M.; Widner, B.; Bair, H.; Probst, T.; Fuchs, D. Fructose- and sorbitol-reduced diet improves mood and gastrointestinal disturbances in fructose malabsorbers. Scand. J. Gastroenterol. 2000, 35, 1048–1052. [Google Scholar] [CrossRef]
- Austin, G.L.; Dalton, C.B.; Hu, Y.; Morris, C.B.; Hankins, J.; Weinland, S.R.; Westman, E.C.; Yancy, W.S., Jr.; Drossman, D.A. A very low-carbohydrate diet improves symptoms and quality of life in diarrhea-predominant irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2009, 7, 706–708. [Google Scholar] [CrossRef]
- O’Dwyer, D.D.; Darville, R.L. Specific carbohydrate diet: Irritable bowel syndrome patient case study. Nutr. Food Sci. 2015, 45, 859–872. [Google Scholar] [CrossRef]
- Ierardi, E.; Losurdo, G.; Sorrentino, C.; Giorgio, F.; Rossi, G.; Marinaro, A.; Romagno, K.R.; Di Leo, A.; Principi, M. Macronutrient intakes in obese subjects with or without small intestinal bacterial overgrowth: An alimentary survey. Scand. J. Gastroenterol. 2016, 51, 277–280. [Google Scholar] [CrossRef]
- Parlesak, A.; Klein, B.; Schecher, K.; Bode, J.C.; Bode, C. Prevalence of small bowel bacterial overgrowth and its association with nutrition intake in nonhospitalized older adults. J. Am. Geriatr. Soc. 2003, 51, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.S. How to institute the low-FODMAP diet. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. 1), 8–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedewa, A.; Rao, S.S. Dietary fructose intolerance, fructan intolerance and FODMAPs. Curr. Gastroenterol. Rep. 2014, 16, 370. [Google Scholar] [CrossRef] [PubMed]
- Nucera, G.; Gabrielli, M.; Lupascu, A.; Lauritano, E.C.; Santoliquido, A.; Cremonini, F.; Cammarota, G.; Tondi, P.; Pola, P.; Gasbarrini, G.; et al. Abnormal breath tests to lactose, fructose and sorbitol in irritable bowel syndrome may be explained by small intestinal bacterial overgrowth. Aliment. Pharmacol. Ther. 2005, 21, 1391–1395. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, L.; Rice, E.; Langland, J. Integrative Treatment of Chronic Abdominal Bloating and Pain Associated With Overgrowth of Small Intestinal Bacteria: A Case Report. Altern. Ther. Health Med. 2017, 23, 56–61. [Google Scholar]
- Henström, M.; Diekmann, L.; Bonfiglio, F.; Hadizadeh, F.; Kuech, E.M.; von Köckritz-Blickwede, M.; Thingholm, L.B.; Zheng, T.; Assadi, G.; Dierks, C.; et al. Functional variants in the sucrase-isomaltase gene associate with increased risk of irritable bowel sydrome. Gut 2018, 67, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Farup, P.G.; Monsbakken, K.W.; Vandvik, P.O. Lactose malabsorption in a population with irritable bowel syndrome: Prevalence and symptoms. A case-control study. Scand. J. Gastroenterol. 2004, 39, 645–649. [Google Scholar] [CrossRef]
- Gupta, D.; Ghoshal, U.C.; Misra, A.; Misra, A.; Choudhuri, G.; Singh, K. Lactose intolerance in patients with irritable bowel syndrome from northern India: A case-control study. J. Gastroenterol. Hepatol. 2007, 22, 2261–2265. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, Y.; Gong, X.; Chen, M. Prevalence of lactose intolerance in patients with diarrhea-predominant irritable bowel syndrome: Data from a tertiary center in southern China. J. Health Popul. Nutr. 2017, 36, 38. [Google Scholar] [CrossRef]
- Varjú, P.; Gede, N.; Szakács, Z.; Hegyi, P.; Cazacu, I.M.; Pécsi, D.; Fábián, A.; Szepes, Z.; Vincze, Á.; Tenk, J.; et al. Lactose intolerance but not lactose maldigestion is more frequent in patients with irritable bowel syndrome than in healthy controls: A meta-analysis. Neurogastroenterol. Motil. 2019, 31, e13527. [Google Scholar] [CrossRef]
- Misselwitz, B.; Pohl, D.; Frühauf, H.; Fried, M.; Vavricka, S.R.; Fox, M. Lactos malabsorption and intolerance: Pathogenesis, diagnosis and treatment. United Eur. Gastroenterol. J. 2013, 1, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Tolliver, B.A.; Jackson, M.S.; Jackson, K.L.; Barnett, E.D.; Chastang, J.F.; DiPalma, J.A. Does lactose maldigestion really play a role in the irritable bowel? J. Clin. Gastroenterol. 1996, 23, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Suarez, F.L.; Savaiano, D.A.; Levitt, M.D. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance. N. Engl. J. Med. 1995, 333, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lisker, R.; Solomons, N.W.; Pérez Briceño, R.; Ramírez Mata, M. Lactase and placebo in the management of the irritable bowel syndrome: A double-blind, cross-over study. Am. J. Gastroenterol. 1989, 84, 756–762. [Google Scholar] [PubMed]
- Moritz, K.; Hemmer, W.; Jung, P.; Sesztak-Greinecker, G.; Götz, M.; Jarisch, R.; Wantke, F. Effect of a fructose and lactose elimination diet in patients with irritable bowel syndrome: A randomized double-blind placebo-controlled study. J. Gastroenterol. Hepatol. Res. 2013, 2, 833–839. [Google Scholar]
- Vernia, P.; Ricciardi, M.R.; Frandina, C.; Bilotta, T.; Frieri, G. Lactose malabsorption and irritable bowel syndrome. Effect of a long-term lactose-free diet. Ital. J. Gastroenterol. 1995, 27, 117–121. [Google Scholar] [PubMed]
- Böhmer, C.J.; Tuynman, H.A. The clinical relevance of lactose malabsorption inirritable bowel syndrome. Eur. J. Gastroenterol. Hepatol. 1996, 8, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Parker, T.J.; Woolner, J.T.; Prevost, A.T.; Tuffnell, Q.; Shorthouse, M.; Hunter, J.O. Irritable bowel syndrome: Is the search for lactose intolerance justified? Eur. J. Gastroenterol. Hepatol. 2001, 13, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fox, M.; Cong, Y.; Chu, H.; Zheng, X.; Long, Y.; Fried, M.; Dai, N. Lactose intolerance in irritable bowel syndrome patients with diarrhoea: The roles of anxiety, activation of the innate mucosal immune system and visceral sensitivity. Aliment. Pharmacol. Ther. 2014, 39, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Misselwitz, B.; Dai, N.; Fox, M. Lactose Intolerance in Adults: Biological Mechanism and Dietary Management. Nutrients 2015, 7, 8020–8035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansueto, P.; D’Alcamo, A.; Seidita, A.; Carroccio, A. Food allergy in irritable bowel syndrome: The case of non-celiac wheat sensitivity. World J. Gastroenterol. 2015, 21, 7089–7109. [Google Scholar] [CrossRef] [PubMed]
- Choung, R.S.; Talley, N.J. Food Allergy and Intolerance in IBS. Gastroenterol. Hepatol. 2006, 2, 756–760. [Google Scholar]
- Nanda, R.; James, R.; Smith, H.; Dudley, C.R.; Jewell, D.P. Food intolerance and the irritable bowel syndrome. Gut 1989, 30, 1099–1104. [Google Scholar] [CrossRef]
- Jones, V.A.; McLaughlan, P.; Shorthouse, M.; Workman, E.; Hunter, J.O. Food intolerance: A major factor in the pathogenesis of irritable bowel syndrome. Lancet 1982, 2, 1115–1117. [Google Scholar] [CrossRef]
- Bentley, S.J.; Pearson, D.J.; Rix, K.J. Food hypersensitivity in irritable bowel syndrome. Lancet 1983, 2, 295–297. [Google Scholar] [CrossRef]
- McKee, A.M.; Prior, A.; Whorwell, P.J. Exclusion diets in irritable bowel syndrome: Are they worthwhile? J. Clin. Gastroenterol. 1987, 9, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Niec, A.M.; Frankum, B.; Talley, N.J. Are adverse food reactions linked to irritable bowel syndrome? Am. J. Gastroenterol. 1998, 93, 2184–2190. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Salem, A.; Nanavati, J.; Mullin, G.E. The Role of Diet in the Treatment of Irritable Bowel Syndrome: A Systematic Review. Gastroenterol. Clin. North. Am. 2018, 47, 107–137. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, W.; Sheldon, T.A.; Shaath, N.; Whorwell, P.J. Food elimination based on IgG antibodies in irritable bowel syndrome: A randomised controlled trial. Gut 2004, 53, 1459–1464. [Google Scholar] [CrossRef]
- Zar, S.; Mincher, L.; Benson, M.J.; Kumar, D. Food-specific IgG4 antibody-guided exclusion diet improves symptoms and rectal compliance in irritable bowel syndrome. Scand. J. Gastroenterol. 2005, 40, 800–807. [Google Scholar] [CrossRef]
- Drisko, J.; Bischoff, B.; Hall, M.; McCallum, R. Treating irritable bowel syndrome with a food elimination diet followed by food challenge and probiotics. J. Am. Coll. Nutr. 2006, 25, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Li, Y.Q. The therapeutic effects of eliminating allergic foods according to food-specific IgG antibodies in irritable bowel syndrome. Zhonghua Nei Ke Za Zhi 2007, 46, 641–643. (In Chinese) [Google Scholar] [PubMed]
- Guo, H.; Jiang, T.; Wang, J.; Chang, Y.; Guo, H.; Zhang, W. The value of eliminating foods according to food-specific immunoglobulin G antibodies in irritable bowel syndrome with diarrhoea. J. Int. Med. Res. 2012, 40, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Aydinlar, E.I.; Dikmen, P.Y.; Tiftikci, A.; Saruc, M.; Aksu, M.; Gunsoy, H.G.; Tozun, N. IgG-based elimination diet in migraine plus irritable bowel syndrome. Headache 2013, 53, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Zwetchkenbaum, J.; Burakoff, R. The irritable bowel syndrome and food hypersensitivity. Ann. Allergy. 1988, 61, 47–49. [Google Scholar] [PubMed]
- Zar, S.; Benson, M.J.; Kumar, D. Food-specific serum IgG4 and IgE titers to common food antigens in irritable bowel syndrome. Am. J. Gastroenterol. 2005, 100, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.L.; Li, Y.Q.; Li, W.J.; Guo, Y.T.; Lu, X.F.; Li, J.M.; Desmond, P.V. Alterations of food antigen-specific serum immunoglobulins G and E antibodies in patients with irritable bowel syndrome and functional dyspepsia. Clin. Exp. Allergy 2007, 37, 823–830. [Google Scholar] [CrossRef]
- Kennedy, D.A.; Lewis, E.; Cooley, K.; Fitz, H. An exploratory comparative investigation of Food Allergy/Sensitivity Testing in IBS (The FAST Study): A comparison between various laboratory methods and an elimination diet. Adv. Integr. Med. 2014, 1, 124–130. [Google Scholar] [CrossRef]
- Ligaarden, S.C.; Lydersen, S.; Farup, P.G. IgG and IgG4 antibodies in subjects with irritable bowel syndrome: A case control study in the general population. BMC Gastroenterol. 2012, 12, 166. [Google Scholar] [CrossRef]
- Mullin, G.E.; Swift, K.M.; Lipski, L.; Turnbull, L.K.; Rampertab, S.D. Testing for food reactions: The good, the bad, and the ugly. Nutr. Clin. Pract. 2010, 25, 192–198. [Google Scholar] [CrossRef]
- Catassi, C.; Bai, J.C.; Bonaz, B.; Bouma, G.; Calabrò, A.; Carroccio, A.; Castillejo, G.; Ciacci, C.; Cristofori, F.; Dolinsek, J.; et al. Non-Celiac Gluten sensitivity: The new frontier of gluten related disorders. Nutrients 2013, 5, 3839–3853. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Pinto-Sanchez, M.I.; Boschetti, E.; Caio, G.; De Giorgio, R.; Verdu, E.F. Dietary Triggers in Irritable Bowel Syndrome: Is There a Role for Gluten? J. Neurogastroenterol. Motil. 2016, 22, 547–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biesiekierski, J.R.; Newnham, E.D.; Irving, P.M.; Barrett, J.S.; Haines, M.; Doecke, J.D.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Gluten causes gastrointestinal symptoms in subjects without celiac disease: A double-blind randomized placebo-controlled trial. Am. J. Gastroenterol. 2011, 106, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Mansueto, P.; Iacono, G.; Soresi, M.; D’Alcamo, A.; Cavataio, F.; Brusca, I.; Florena, A.M.; Ambrosiano, G.; Seidita, A.; et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: Exploring a new clinical entity. Am. J. Gastroenterol. 2012, 107, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Roque, M.I.; Camilleri, M.; Smyrk, T.; Murray, J.A.; Marietta, E.; O’Neill, J.; Carlson, P.; Lamsam, J.; Janzow, D.; Eckert, D.; et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: Effects on bowel frequency and intestinal function. Gastroenterology 2013, 144, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Trott, N.; Briggs, R.; North, J.R.; Hadjivassiliou, M.; Sanders, D.S. Efficacy of a Gluten-Free Diet in Subjects with Irritable Bowel Syndrome-Diarrhea Unaware of Their HLA-DQ2/8 Genotype. Clin. Gastroenterol. Hepatol. 2016, 14, 696–703. [Google Scholar] [CrossRef]
- Zanwar, V.G.; Pawar, S.V.; Gambhire, P.A.; Jain, S.S.; Surude, R.G.; Shah, V.B.; Contractor, Q.Q.; Rathi, P.M. Symptomatic improvement with gluten restriction in irritable bowel syndrome: A prospective, randomized, double blinded placebo controlled trial. Intest. Res. 2016, 14, 343–350. [Google Scholar] [CrossRef]
- Shahbazkhani, B.; Sadeghi, A.; Malekzadeh, R.; Khatavi, F.; Etemadi, M.; Kalantri, E.; Rostami-Nejad, M.; Rostami, K. Non-Celiac Gluten Sensitivity Has Narrowed the Spectrum of Irritable Bowel Syndrome: A Double-Blind Randomized Placebo-Controlled Trial. Nutrients 2015, 7, 4542–4554. [Google Scholar] [CrossRef] [Green Version]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of non-celiac gluten sensitivity (NCGS): The salerno experts’ criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef]
- Rizzi, A.; Nucera, E.; Laterza, L.; Gaetani, E.; Valenza, V.; Corbo, G.M.; Inchingolo, R.; Buonomo, A.; Schiavino, D.; Gasbarrini, A. Irritable Bowel Syndrome and Nickel Allergy: What Is the Role of the Low Nickel Diet? J. Neurogastroenterol. Motil. 2017, 23, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Minelli, M.; Schiavino, D.; Musca, F.; Bruno, M.E.; Falagiani, P.; Mistrello, G.; Riva, G.; Braga, M.; Turi, M.C.; Di Rienzo, V.; et al. Oral hyposensitization to nickel induces clinical improvement and a decrease in TH1 and TH2 cytokines in patients with systemic nickel allergy syndrome. Int. J. Immunopathol. Pharmacol. 2010, 23, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Picarelli, A.; Di Tola, M.; Vallecoccia, A.; Libanori, V.; Magrelli, M.; Carlesimo, M.; Rossi, A. Oral mucosa patch test: A new tool to recognize and study the adverse effects of dietary nickel exposure. Biol. Trace Elem. Res. 2011, 139, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Borghini, R.; Puzzono, M.; Rosato, E.; Di Tola, M.; Marino, M.; Greco, F.; Picarelli, A. Nickel-Related Intestinal Mucositis in IBS-Like Patients: Laser Doppler Perfusion Imaging and Oral Mucosa Patch Test in Use. Biol. Trace Elem. Res. 2016, 173, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Borghini, R.; Donato, G.; Alvaro, D.; Picarelli, A. New insights in IBS-like disorders: Pandora’s box has been opened; a review. Gastroenterol. Hepatol. Bed Bench 2017, 10, 79–89. [Google Scholar] [PubMed]
- Khayyat, Y.; Attar, S. Vitamin D Deficiency in Patients with Irritable Bowel Syndrome: Does it Exist? Oman Med. J. 2015, 30, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, B.U.; Maranda, L.; Candela, N. Vitamin D status in pediatric irritable bowel syndrome. PLoS ONE 2017, 12, e0172183. [Google Scholar]
- Li, Y.C.; Chen, Y.; Du, J. Critical roles of intestinal epithelial vitamin D receptor signaling in controlling gut mucosal inflammation. J. Steroid Biochem. Mol. Biol. 2015, 148, 179–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantorna, M.T.; McDaniel, K.; Bora, S.; Chen, J.; James, J. Vitamin D, immune regulation, the microbiota, and inflammatory bowel disease. Exp. Biol. Med. 2014, 239, 1524–1530. [Google Scholar] [CrossRef] [Green Version]
- Bashir, M.; Prietl, B.; Tauschmann, M.; Mautner, S.I.; Kump, P.K.; Treiber, G.; Wurm, P.; Gorkiewicz, G.; Högenauer, C.; Pieber, T.R. Effects of high doses of vitamin D3 on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. Eur. J. Nutr. 2016, 55, 1479–1489. [Google Scholar] [CrossRef]
- Sprake, E.F.; Grant, V.A.; Corfe, B.M. Vitamin D3 as a novel treatment for irritable bowel syndrome: Single case leads to critical analysis of patient-centred data. BMJ Case Rep. 2012, 2012. [Google Scholar] [CrossRef]
- Abbasnezhad, A.; Amani, R.; Hajiani, E.; Alavinejad, P.; Cheraghian, B.; Ghadiri, A. Effect of vitamin D on gastrointestinal symptoms and health-related quality of life in irritable bowel syndrome patients: A randomized double-blind clinical trial. Neurogastroenterol. Motil. 2016. [Google Scholar] [CrossRef] [PubMed]
- Leeds, J.S.; Hopper, A.D.; Sidhu, R.; Simmonette, A.; Azadbakht, N.; Hoggard, N.; Morley, S.; Sanders, D.S. Some patients with irritable bowel syndrome may have exocrine pancreatic insufficiency. Clin. Gastroenterol. Hepatol. 2010, 8, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Money, M.E.; Walkowiak, J.; Virgilio, C.; Talley, N.J. Pilot study: A randomised, double blind, placebo controlled trial of pancrealipase for the treatment of postprandial irritable bowel syndrome-diarrhoea. Frontline Gastroenterol. 2011, 2, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Tuck, C.J.; Taylor, K.M.; Gibson, P.R.; Barrett, J.S.; Muir, J.G. Increasing Symptoms in Irritable Bowel Symptoms With Ingestion of Galacto-Oligosaccharides Are Mitigated by α-Galactosidase Treatment. Am. J. Gastroenterol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Menees, S.B.; Powell, C.; Kurlander, J.; Goel, A.; Chey, W.D. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am. J. Gastroenterol. 2015, 110, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Martin-Viñas, J.J.; Quigley, E.M. Immune response in irritable bowel syndrome: A systematic review of systemic and mucosal inflammatory mediators. J. Dig. Dis. 2016, 17, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Spiller, R. Irritable bowel syndrome: New insights into symptom mechanisms and advances in treatment. F1000Research 2016, 5. [Google Scholar] [CrossRef]
- Melchior, C.; Aziz, M.; Aubry, T.; Gourcerol, G.; Quillard, M.; Zalar, A.; Coëffier, M.; Dechelotte, P.; Leroi, A.M.; Ducrotté, P. Does calprotectin level identify a subgroup among patients suffering from irritable bowel syndrome? Results of a prospective study. United Eur. Gastroenterol. J. 2017, 5, 261–269. [Google Scholar] [CrossRef]
- Barbara, G.; Cremon, C.; Annese, V.; Basilisco, G.; Bazzoli, F.; Bellini, M.; Benedetti, A.; Benini, L.; Bossa, F.; Buldrini, P.; et al. Randomised controlled trial of mesalazine in IBS. Gut 2016, 65, 82–90. [Google Scholar] [CrossRef]
- Lam, C.; Tan, W.; Leighton, M.; Hastings, M.; Lingaya, M.; Falcone, Y.; Zhou, X.; Xu, L.; Whorwell, P.; Walls, A.F.; et al. A mechanistic multicentre, parallel group, randomised placebo-controlled trial of mesalazine for the treatment of IBS with diarrhoea (IBS-D). Gut 2016, 65, 91–99. [Google Scholar] [CrossRef]
- Wouters, M.M.; Balemans, D.; Van Wanrooy, S.; Dooley, J.; Cibert-Goton, V.; Alpizar, Y.A.; Valdez-Morales, E.E.; Nasser, Y.; Van Veldhoven, P.P.; Vanbrabant, W.; et al. Histamine Receptor H1-Mediated Sensitization of TRPV1 Mediates Visceral Hypersensitivity and Symptoms in Patients with Irritable Bowel Syndrome. Gastroenterology 2016, 150, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Gizzi, G.; Pellegrini, L.; Corsi, M.; Dugall, M.; Cacchio, M.; Feragalli, B.; Togni, S.; Riva, A.; Eggenhoffner, R.; et al. Supplementation with a lecithin-based delivery form of Boswellia serrata extract (Casperome®) controls symptoms of mild irritable bowel syndrome. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2249–2254. [Google Scholar] [PubMed]
- Fritscher-Ravens, A.; Schuppan, D.; Ellrichmann, M.; Schoch, S.; Röcken, C.; Brasch, J.; Bethge, J.; Böttner, M.; Klose, J.; Milla, P.J. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2014, 147, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Piche, T.; Barbara, G.; Aubert, P.; Bruley des Varannes, S.; Dainese, R.; Nano, J.L.; Cremon, C.; Stanghellini, V.; De Giorgio, R.; Galmiche, J.P.; et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: Involvement of soluble mediators. Gut 2009, 58, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, B.; Verne, G.N. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 2009, 146, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Roque, M.I.; Camilleri, M.; Smyrk, T.; Murray, J.A.; O’Neill, J.; Carlson, P.; Lamsam, J.; Eckert, D.; Janzow, D.; Burton, D.; et al. Association of HLA-DQ gene with bowel transit, barrier function, and inflammation in irritable bowel syndrome with diarrhea. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 303, G1262–G1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shulman, R.J.; Jarrett, M.E.; Cain, K.C.; Broussard, E.K.; Heitkemper, M.M. Associations among gut permeability, infl ammatory markers, and symptoms in patients with irritable bowel syndrome. J. Gastroenterol. 2014, 49, 1467–1476. [Google Scholar] [CrossRef]
- Camilleri, M.; Lasch, K.; Zhou, W. Irritable bowel syndrome: Methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 303, G775–G785. [Google Scholar] [CrossRef]
- Barbara, G.; Zecchi, L.; Barbaro, R.; Cremon, C.; Bellacosa, L.; Marcellini, M.; De Giorgio, R.; Corinaldesi, R.; Stanghellini, V. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J. Clin. Gastroenterol. 2012, 46, S52–S55. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Ahrne, S.; Hagslatt, M.L. Effect of lactobacilli on paracellular permeability in the gut. Nutrients 2011, 3, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Michielan, A.; D’Incà, R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediat. Inflamm. 2015, 2015, 628157. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Li, Y.Q.; Zuo, X.L.; Zhen, Y.B.; Yang, J.; Liu, C.H. Clinical trial: Effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2008, 28, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Costinean, S.; Croce, C.M.; Brasier, A.R.; Merwat, S.; Larson, S.A.; Basra, S.; Verne, G.N. MicroRNA 29 targets nuclear factor-κB-repressing factor and Claudin 1 to increase intestinal permeability. Gastroenterology 2015, 148, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Verne, M.L.; Fields, J.Z.; Lefante, J.J.; Basra, S.; Salameh, H.; Verne, G.N. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut. 2019, 68, 996–1002. [Google Scholar]
- Camilleri, M. Bile Acid diarrhea: Prevalence, pathogenesis, and therapy. Gut Liver 2015, 9, 332–339. [Google Scholar] [CrossRef]
- Slattery, S.A.; Niaz, O.; Aziz, Q.; Ford, A.C.; Farmer, A.D. Systematic review with meta-analysis: The prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment. Pharmacol. Ther. 2015, 42, 3–11. [Google Scholar] [CrossRef]
- Peleman, C.; Camilleri, M.; Busciglio, I.; Burton, D.; Donato, L.; Zinsmeister, A.R. Colonic Transit and Bile Acid Synthesis or Excretion in Patients with Irritable Bowel Syndrome-Diarrhea Without Bile Acid Malabsorption. Clin. Gastroenterol. Hepatol. 2017, 15, 720–727. [Google Scholar] [CrossRef]
- Camilleri, M. Advances in understanding of bile acid diarrhea. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 49–61. [Google Scholar] [CrossRef]
- Camilleri, M.; Shin, A.; Busciglio, I.; Carlson, P.; Acosta, A.; Bharucha, A.E.; Burton, D.; Lamsam, J.; Lueke, A.; Donato, L.J.; et al. Genetic variation in GPBAR1 predisposes to quantitative changes in colonic transit and bile acid excretion. Am. J. Physiol.-Gastrointest. Liver Physiol. 2014, 307, G508–G516. [Google Scholar] [CrossRef] [Green Version]
- Money, M.E.; Camilleri, M. Review: Management of postprandial diarrhea syndrome. Am. J. Med. 2012, 125, 538–544. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, S.J. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.A.; Gahan, C.G. Bile Acid Modifications at the Microbe-Host Interface: Potential for Nutraceutical and Pharmaceutical Interventions in Host Health. Annu. Rev. Food Sci. Technol. 2016, 7, 313–333. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, C.; Monaghan, P.J.; Morris, J.; Issa, B.; Whorwell, P.J.; Keevil, B.; Houghton, L.A. Rome III functional constipation and irritable bowel syndrome with constipation are similar disorders within the spectrum of sensitization, regulated by serotonin. Gastroenterology 2013, 145, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Raahave, D.; Loud, F.B. Additional faecal reservoirs or hidden constipation: A link between functional and organic bowel disease. Dan. Med. Bull. 2004, 51, 422–425. [Google Scholar] [PubMed]
- Raahave, D.; Christensen, E.; Loud, F.B.; Knudsen, L.L. Correlation of bowel symptoms with colonic transit, length, and faecal load in functional faecal retention. Dan. Med. Bull. 2009, 56, 83–88. [Google Scholar]
- Wiesner, M.; Naylor, S.J.; Copping, A.; Furlong, A.; Lynch, A.G.; Parkes, M.; Hunter, J.O. Symptom classification in irritable bowel syndrome as a guide to treatment. Scand. J. Gastroenterol. 2009, 44, 796–803. [Google Scholar] [CrossRef]
- Simrén, M.; Barbara, G.; Flint, H.J.; Spiegel, B.M.; Spiller, R.C.; Vanner, S.; Verdu, E.F.; Whorwell, P.J.; Zoetendal, E.G.; Rome Foundation Committee. Intestinal microbiota in functional bowel disorders: A Rome foundation report. Gut 2013, 62, 159–176. [Google Scholar]
- Fan, W.T.; Ding, C.; Xu, N.N.; Zong, S.; Ma, P.; Gu, B. Close association between intestinal microbiota and irritable bowel syndrome. Eur. J. Clin. Microbiol. Infect. Dis. 2017. [Google Scholar] [CrossRef]
- Lyra, A.; Rinttilä, T.; Nikkilä, J.; Krogius-Kurikka, L.; Kajander, K.; Malinen, E.; Mättö, J.; Mäkelä, L.; Palva, A. Diarrhoea-predominant irritable bowel syndrome distinguishable by 16S rRNA gene phylotype quantification. World J. Gastroenterol. 2009, 15, 5936–5945. [Google Scholar] [CrossRef]
- Liu, H.N.; Wu, H.; Chen, Y.Z.; Chen, Y.J.; Shen, X.Z.; Liu, T.T. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: A systematic review and meta-analysis. Dig. Liver Dis. 2017, 49, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, I.B.; Quigley, E.M.; Öhman, L.; Simrén, M.; O’Toole, P.W. The microbiota link to irritable bowel syndrome: An emerging story. Gut Microbes 2012, 3, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkes, G.C.; Rayment, N.B.; Hudspith, B.N.; Petrovska, L.; Lomer, M.C.; Brostoff, J.; Whelan, K.; Sanderson, J.D. Distinct microbial populations exist in the mucosa-associated microbiota of sub-groups of irritable bowel syndrome. Neurogastroenterol. Motil. 2012, 24, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M. Do patients with functional gastrointestinal disorders have an altered gut flora? Ther. Adv. Gastroenterol. 2009, 2, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Jalanka-Tuovinen, J.; Salonen, A.; Nikkil, J.; Immonen, O.; Kekkonen, R.; Lahti, L.; Palva, A.; de Vos, W.M. Intestinal microbiota in healthy adults: Temporal analysis reveals individual and common core and relation to intestinal symptoms. PLoS ONE 2011, 6, e23035. [Google Scholar] [CrossRef]
- Rajilic-Sotjanovic, M.; Biagi, E.; Heilig, H.G.; Kajander, K.; Kekkonen, R.A.; Tims, S.; de Vos, W.M. Global and deep molecular analysis of microbiota signatures in faecal samples from patients with irritable bowel syndrome. Gastroenterology 2011, 141, 1737–1801. [Google Scholar]
- Silk, D.B.; Davis, A.; Vulevic, J.; Tzortzis, G.; Gibson, G.R. Clinical trial: The effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2009, 29, 508–518. [Google Scholar] [CrossRef]
- Paineau, D.; Payen, F.; Panserieu, S.; Coulombier, G.; Sobaszek, A.; Lartigau, I.; Brabet, M.; Galmiche, J.P.; Tripodi, D.; Sacher-Huvelin, S.; et al. The effects of regular consumption of short-chain fructo-oligosaccharides on digestive comfort of subjects with minor functional bowel disorders. Br. J. Nutr. 2008, 99, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Azpiroz, F.; Dubray, C.; Bernalier-Donadille, A.; Cardot, J.M.; Accarino, A.; Serra, J.; Wagner, A.; Respondek, F.; Dapoigny, M. Effects of scFOS on the composition of fecal microbiota and anxiety in patients with irritable bowel syndrome: A randomized, double blind, placebo controlled study. Neurogastroenterol. Motil. 2017, 29, e12911. [Google Scholar] [CrossRef]
- Giannini, E.G.; Mansi, C.; Dulbecco, P.; Savarino, V. Role of partially hydrolyzed guar gum in the treatment of irritable bowel syndrome. Nutrition 2006, 22, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Andreozzi, P.; Zito, F.P.; Vozzella, L.; Savino, I.G.; Sarnelli, G.; Cuomo, R. Partially hydrolyzed guar gum in the treatment of irritable bowel syndrome with constipation: Effects of gender, age, and body mass index. Saudi J. Gastroenterol. 2015, 21, 104–110. [Google Scholar] [PubMed]
- Niv, E.; Halak, A.; Tiommny, E.; Yanai, H.; Strul, H.; Naftali, T.; Vaisman, N. Randomized clinical study: Partially hydrolyzed guar gum (PHGG) versus placebo in the treatment of patients with irritable bowel syndrome. Nutr. Metab. 2016, 13, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, N.B.; Bryrup, T.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Pedersen, O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: A systematic review of randomized controlled trials. Genome Med. 2016, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Sanders, S.E. Probiotics and microbiota composition. BMC Med. 2016, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, Y.A.; Alder, A.; Anderson, W.; Wills, A.; Goddard, L.; Gulia, P.; Jankovich, E.; Mutch, P.; Reeves, L.B.; Singer, A.; et al. British Dietetic Association evidence-based guidelines for the dietary management of irritable bowel syndrome in adults. J. Hum. Nutr. Diet. 2012, 25, 260–274. [Google Scholar] [CrossRef]
- Allen, A.P.; Clarke, G.; Cryan, J.F.; Quigley, E.M.M.; Dinan, T.G. Bifidobacterium infantis 35624 and other probiotics in the management of irritable bowel syndrome. Strain specificity, symptoms, and mechanisms. Curr. Med. Res. Opin. 2017, 33, 1349–1351. [Google Scholar] [CrossRef]
- Nobutani, K.; Sawada, D.; Fujiwara, S.; Kuwano, Y.; Nishida, K.; Nakayama, J.; Kutsumi, H.; Azuma, T.; Rokutan, K. The effects of administration of the Lactobacillus gasseri strain CP2305 on quality of life, clinical symptoms and changes in gene expression in patients with irritable bowel syndrome. J. Appl. Microbiol. 2017, 122, 212–224. [Google Scholar] [CrossRef]
- Power, S.E.; O’Toole, P.W.; Stanton, C.; Ross, R.P.; Fitzgerald, G.F. Intestinal microbiota, diet and health. Br. J. Nutr. 2014, 111, 387–402. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Lomer, M.C.; Anderson, J.L.; Barrett, J.S.; Muir, J.G.; Irving, P.M.; Whelan, K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J. Nutr. 2012, 142, 1510–1518. [Google Scholar] [CrossRef]
- Hill, P.; Muir, J.G.; Gibson, P.R. Controversies and Recent Developments of the Low-FODMAP Diet. Gastroenterol. Hepatol. 2017, 13, 36–45. [Google Scholar]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs alter symptoms and the metabolome of patients with IBS: A randomised controlled trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Choi, J.K.; Ryu, H.S.; Choi, C.H.; Kang, E.H.; Park, K.S.; Min, Y.W.; Hong, K.S. Therapeutic Modulation of Gut Microbiota in Functional Bowel Disorders. J. Neurogastroenterol. Motil. 2017, 23, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachdev, A.H.; Pimentel, M. Gastrointestinal bacterial overgrowth: Pathogenesis and clinical significance. Ther. Adv. Chronic Dis. 2013, 4, 223–231. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Shukla, R.; Ghoshal, U. Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome: A Bridge between Functional Organic Dichotomy. Gut Liver 2017, 11, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M. Review article: Potential mechanisms of action of rifaximin in the management of irritable bowel syndrome with diarrhoea. Aliment. Pharmacol. Ther. 2016, 43 (Suppl. 1), 37–49. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Törnblom, H.; Simrén, M. Small intestinal bacterial overgrowth as a cause for irritable bowel syndrome: Guilty or not guilty? Curr. Opin. Gastroenterol. 2017, 33, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Day, L.W.; Somsouk, M.; Sewell, J.L. Meta-analysis: Antibiotic therapy for small intestinal bacterial overgrowth. Aliment. Pharmacol. Ther. 2013, 38, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Enko, D.; Halwachs-Baumann, G.; Stolba, R.; Mangge, H.; Kriegshäuser, G. Refining small intestinal bacterial overgrowth diagnosis by means of carbohydrate specificity: A proof-of-concept study. Ther. Adv. Gastroenterol. 2016, 9, 265–272. [Google Scholar] [CrossRef]
- Rezaie, A.; Pimentel, M.; Rao, S.S. How to Test and Treat Small Intestinal Bacterial Overgrowth: An Evidence-Based Approach. Curr. Gastroenterol. Rep. 2016, 18, 8. [Google Scholar] [CrossRef]
- Ciampa, B.P.; Reyes Ramos, E.; Borum, M.; Doman, D.B. The Emerging Therapeutic Role of Medical Foods for Gastrointestinal Disorders. Gastroenterol. Hepatol. 2017, 13, 104–115. [Google Scholar]
- Pimentel, M.; Constantino, T.; Kong, Y.; Bajwa, M.; Rezaei, A.; Park, S. A 14-day elemental diet is highly effective in normalizing the lactulose breath test. Dig. Dis. Sci. 2004, 49, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J. Elemental diet and the nutritional treatment of Crohn’s disease. Gastroenterol. Hepatol. Bed Bench 2015, 8, 4–5. [Google Scholar] [PubMed]
- Logan, A.C.; Beaulne, T.M. The treatment of small intestinal bacterial overgrowth with enteric-coated peppermint oil: A case report. Altern. Med. Rev. 2002, 7, 410–417. [Google Scholar] [PubMed]
- Chedid, V.; Dhalla, S.; Clarke, J.O.; Roland, B.C.; Dunbar, K.B.; Koh, J.; Justino, E.; Tomakin, E.; Mullin, G.E. Herbal therapy is equivalent to rifaximin for the treatment of small intestinal bacterial overgrowth. Glob. Adv. Health Med. 2014, 3, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Scott-Hoy, B.; Jennings, L. Efficacy of a Quebracho, Conker Tree, and M. balsamea Willd blended extract in patients with irritable bowel syndrome with constipation. J. Gasterenterol. Hepatol. Res. 2015, 4, 1762–1767. [Google Scholar] [CrossRef]
- Brown, K.; Scott-Hoy, B.; Jennings, L.W. Response of irritable bowel syndrome with constipation patients administered a combined quebracho/conker tree/M. balsamea Willd extract. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 463–468. [Google Scholar] [CrossRef]
- Zhong, C.; Qu, C.; Wang, B.; Liang, S.; Zeng, B. Probiotics for Preventing and Treating Small Intestinal Bacterial Overgrowth: A Meta-Analysis and Systematic Review of Current Evidence. J. Clin. Gastroenterol. 2017, 51, 300–311. [Google Scholar] [CrossRef]
- Khalighi, A.R.; Khalighi, M.R.; Behdani, R.; Jamali, J.; Khosravi, A.; Kouhestani, S.H.; Radmanesh, H.; Esmaeelzadeh, S.; Khalighi, N. Evaluating the efficacy of probiotic on treatment in patients with small intestinal bacterial overgrowth (SIBO)—A pilot study. Indian J. Med. Res. 2014, 140, 604–608. [Google Scholar]
- Poirier, P.; Wawrzyniak, I.; Vivarès, C.P.; Delbac, F.; El Alaoui, H. New insights into Blastocystis spp.: A potential link with irritable bowel syndrome. PLoS Pathog. 2012, 8, e1002545. [Google Scholar] [CrossRef]
- Yakoob, J.; Jafri, W.; Jafri, N.; Khan, R.; Islam, M.; Beg, M.A.; Zaman, V. Irritable bowel syndrome: In search of an etiology: Role of Blastocystis hominis. Am. J. Trop. Med. Hyg. 2004, 70, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-González, D.E.; Martínez-Flores, W.A.; Reyes-Gordillo, J.; Ramírez-Miranda, M.E.; Arroyo-Escalante, S.; Romero-Valdovinos, M.; Stark, D.; Souza-Saldivar, V.; Martinez-Hernandez, F.; Flisser, A.; et al. Blastocystis infection is associated with irritable bowel síndrome in a Mexican patient population. Parasitol. Res. 2012, 110, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Nourrisson, C.; Scanzi, J.; Pereira, B.; NkoudMongo, C.; Wawrzyniak, I.; Cian, A.; Viscogliosi, E.; Livrelli, V.; Delbac, F.; Dapoigny, M.; et al. Blastocystis is associated with decrease of fecal microbiota protective bacteria: Comparative analysis between patients with irritable bowel syndrome and control subjects. PLoS ONE 2014, 9, e111868. [Google Scholar] [CrossRef] [PubMed]
- Krogsgaard, L.R.; Engsbro, A.L.; Stensvold, C.R.; Nielsen, H.V.; Bytzer, P. The prevalence of intestinal parasites is not greater among individuals with irritable bowel syndrome: A population-based case-control study. Clin. Gastroenterol. Hepatol. 2015, 13, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Ragavan, N.D.; Govind, S.K.; Chye, T.T.; Mahadeva, S. Phenotypic variation in Blastocystis sp. ST3. Parasites Vectors 2014, 7, 404. [Google Scholar] [CrossRef]
- Vargas-Sanchez, G.B.; Romero-Valdovinos, M.; Ramirez-Guerrero, C.; Vargas-Hernandez, I.; Ramirez-Miranda, M.E.; Martinez-Ocaña, J.; Valadez, A.; Ximenez, C.; Lopez-Escamilla, E.; Hernandez-Campos, M.E.; et al. Blastocystis Isolates from Patients with Irritable Bowel Syndrome and from Asymptomatic Carriers Exhibit Similar Parasitological Loads, but Significantly Different Generation Times and Genetic Variability across Multiple Subtypes. PLoS ONE 2015, 10, e0124006. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Riahi, S.M.; Haghighi, A.; Saber, V.; Armon, B.; Seyyedtabaei, S.J. The role of Blastocystis sp. and Dientamoeba fragilis in irritable bowel syndrome: A systematic review and meta-analysis. Parasitol. Res. 2017. [Google Scholar] [CrossRef]
- Coyle, C.M.; Varughese, J.; Weiss, L.M.; Tanowitz, H.B. Blastocystis: To treat or not to treat... Clin. Infect. Dis. 2012, 54, 105–110. [Google Scholar] [CrossRef]
- Stensvold, C.R.; Smith, H.V.; Nagel, R.; Olsen, K.E.; Traub, R.J. Eradication of Blastocystis carriage with antimicrobials: Reality or delusion? J. Clin. Gastroenterol. 2010, 44, 85–90. [Google Scholar] [CrossRef]
- Nagel, R.; Bielefeldt-Ohmann, H.; Traub, R. Clinical pilot study: Efficacy of triple antibiotic therapy in Blastocystis positive irritable bowel syndrome patients. Gut Pathog. 2014, 6, 34. [Google Scholar] [CrossRef]
- Force, M.; Sparks, W.S.; Ronzio, R.A. Inhibition of enteric parasites by emulsified oil of oregano in vivo. Phytother. Res. 2000, 14, 213–214. [Google Scholar] [CrossRef]
- Dinleyici, E.C.; Eren, M.; Dogan, N.; Reyhanioglu, S.; Yargic, Z.A.; Vandenplas, Y. Clinical efficacy of Saccharomyces boulardii or metronidazole in symptomatic nchildren with Blastocystis hominis infection. Parasitol. Res. 2011, 108, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Shariati, A.; Fallah, F.; Pormohammad, A.; Taghipour, A.; Safari, H.; Chirani, A.S.; Sabour, S.; Alizadeh-Sani, M.; Azimi, T. The possible role of bacteria, viruses, and parasites in initiation and exacerbation of irritable bowel syndrome. J. Cell. Physiol. 2019, 234, 8550–8569. [Google Scholar] [CrossRef] [PubMed]
- Lagacé-Wiens, P.R.; VanCaeseele, P.G.; Koschik, C. Dientamoeba fragilis: An emerging role in intestinal disease. CMAJ 2006, 175, 468–469. [Google Scholar] [CrossRef] [PubMed]
- Borody, T.; Warren, A.; Wettstein, G.; Robertson, G.; Recabarren, P.; Fontela, A.; Herdman, K.; Surace, R. Eradication of Dientamoeba fragilis can resolve IBS-like symptoms. J. Gastroenterol. Hepatol. 2002, 17, A103. [Google Scholar]
- Ali, S.; Khetpal, N.; Khan, M.T.; Rasheed, M.; Asad-Ur-Rahman, F.; Echeverria-Beltran, K. A Mexican Honeymoon Marred by Gastrointestinal Upset: A Case of Dientamoeba fragilis Causing Post-infectious Irritable Bowel Syndrome. Cureus 2017, 9, e1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engsbro, A.L.; Stensvold, C.R.; Nielsen, H.V.; Bytzer, P. Treatment of Dientamoeba fragilis in patients with irritable bowel syndrome. Am. J. Trop. Med. Hyg. 2012, 87, 1046–1052. [Google Scholar] [CrossRef]
- Stark, D.; Barratt, J.; Chan, D.; Ellis, J.T. Dientamoeba fragilis, the Neglected Trichomonad of the Human Bowel. Clin. Microbiol. Rev. 2016, 29, 553–580. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.S.; Liu, T.T.; Wen, S.H.; Wang, C.C.; Yi, C.H.; Chen, J.H.; Lei, W.Y.; Orr, W.C.; Fabio, P.; Chen, C.L. Clinical, metabolic, and psychological characteristics in patients with gastroesophageal reflux disease overlap with irritable bowel syndrome. Eur. J. Gastroenterol. Hepatol. 2015, 27, 516–522. [Google Scholar] [CrossRef]
- De Bortoli, N.; Tolone, S.; Frazzoni, M.; Martinucci, I.; Sgherri, G.; Albano, E.; Ceccarelli, L.; Stasi, C.; Bellini, M.; Savarino, V.; et al. Gastroesophageal reflux disease, functional dyspepsia and irritable bowel syndrome: Common overlapping gastrointestinal disorders. Ann. Gastroenterol. 2018, 31, 639–648. [Google Scholar] [CrossRef]
- Kountouras, J.; Doulberis, M.; Papaefthimiou, A.; Polyzos, S.A. Gastroesophageal reflux disease, irritable bowel syndrome and functional dyspepsia as overlapping conditions: Focus on effect of trimebutine. Ann. Gastroenterol. 2019, 32, 318. [Google Scholar] [CrossRef] [PubMed]
- Davis, S. Reversal of Irritable Bowel Syndrome, Sleep Disturbance, and Fatigue with an Elimination Diet, Lifestyle Modification, and Dietary Supplements: A Case Report. Integr. Med. Encinitas 2016, 15, 60–66. [Google Scholar] [PubMed]
| Contributing Factor | Mechanisms | Clinical and Biochemical Investigations | Potential Management and Treatment Approaches |
|---|---|---|---|
| Stress | Dysregulation of the brain–gut axis can influence the main pathophysiological features of IBS, including visceral hypersensitivity, colonic motility and alterations in the gut microbiota. | Clinical symptom severity. Salivary diurnal and/or waking cortisol. Serum neuropeptide Y. Serotonin transporter gene variants. Catechol-O-methyltransferase Val158Met gene variants. | Mind–body therapies. Cognitive behavioral therapy. Hypnotherapy. Educational interventions. |
| Circadian disruption | Circadian rhythm disruption can affect several aspects of gastrointestinal function including gastrointestinal motility, visceral sensitivity, immunological function, and barrier integrity. | Questioning around sleep patterns and electric light-at-night exposure. Salivary melatonin. | Extending the dark period. Regular sleep patterns. |
| Physical inactivity | Clinical studies have found that increasing physical activity can improve gastric emptying, intestinal gas transit and reduce abdominal distension. | Tracking physical activity levels. Activity tracker step count. | Exercise counselling. Yoga. Walking. |
| Carbohydrate intolerance | Fermentable carbohydrates may increase osmotic load and gas production in the distal small bowel and the proximal colon. | Breath testing for lactose and fructose intolerance. Breath testing for small intestinal bacterial overgrowth. Genetic testing for sucrase-isomaltase gene variants. | Reduce intake of refined sugar and carbohydrates. Low FODMAP diet. |
| Lactose intolerance | Unabsorbed lactose in the intestine can increase osmotic load and intestinal water content in addition to undergoing fermentation to produce short chain fatty acids and gas including hydrogen and methane, which may cause or aggravate symptoms in susceptible individuals. | Positive test of lactose malabsorption (genetic, biopsy, or H2-breath test) and subsequent open-label lactose challenge. | Lactase enzyme therapy. Low-lactose or lactose-free diet. |
| Food hypersensitivity | Immunologically mediated food hypersensitivity may play a role in the development of gastrointestinal symptoms via a local and limited IgE-mediated reaction in the intestinal mucosa and/or IgE-independent mechanisms. | Dietary elimination and re-challenge. IgG antibodies to foods. | Elimination and re-challenge diet. IgG antibody-guided exclusion diet. |
| Non-celiac gluten sensitivity | People with NCGS have evidence of immune activation (measured with increased serum levels of soluble CD14), leaky gut (lipopolysaccharide-binding protein and antibody reactivity to bacterial LPS and flagellin) and intestinal damage (fatty acid-binding protein 2). | Clinical rating scale, response to a gluten-free diet, and double-placebo controlled gluten challenge. HLA-DQ2/8 gene variants. | Gluten-free diet. |
| Nickel-related intestinal mucositis | In nickel sensitive people, exposure results in elevated levels of IFNy, IL-5, and IL-13 in the supernatants of peripheral blood mononuclear cell cultures stimulated with nickel, suggesting a pro-inflammatory effect. | Positive nickel patch test. | Low nickel diet. |
| Vitamin D deficiency | Vitamin D deficiency could contribute to mucosal inflammation, impaired epithelial cell integrity, and the alterations in the composition of the gut microbiome. | Serum 25-hydroxy vitamin D. | Sunlight exposure. Vitamin D3 supplementation. |
| Exocrine pancreatic insufficiency | Impaired digestion of food may increase antigenic load and/or contribute to postprandial osmotic diarrhoea. | Clinical symptoms of postprandial osmotic diarrhoea, or galacto-oligosaccharide intolerance. Fecal elastase-1 (FE-1). | Digestive enzyme therapy. |
| Low-grade inflammation | Low-grade intestinal inflammation contributes to altered permeability, hypersensitivity of enteric nerves and changes in serotonin signaling. | Fecal calprotectin. | Low antigenic/elimination diet. Anti-inflammatory diet. Anti-inflammatory herbal medicines, such as Boswellia serrata. |
| Intestinal permeability | Intestinal permeability contributes to low-grade intestinal inflammation and increased visceral and pain sensitivity. | Intestinal permeability (lactulose/mannitol). Serum lipopolysaccharide binding protein. | Probiotics. Glutamine. |
| Bile acid malabsorption | An increase in the fecal excretion and change in the proportion of the various bile acids in stool contributes to intestinal permeability, water and electrolyte secretion, and increased colonic transit. | Therapeutic trial of bile acid binders. Serum 7α-hydroxy-4-cholesten-3-one. Selenium homotaurocholic acid test. KLB rs17618244 and GPBAR1rs11554825 gene variants. | Bile acid binders. Pancreatic enzyme therapy. Diet that minimizes bile acid production and/or excretion. Probiotics. Prebiotics. |
| Chronic constipation | Functional constipation is associated with visceral sensitivity and alterations in serotonin signaling. | The Bristol Stool Scale. | Laxatives. Fiber therapy. |
| Dysbiosis | Alterations in the gastrointestinal microbiota activate mucosal innate immune responses which increase epithelial permeability, initiate nociceptive sensory pathways and dysregulate the enteric nervous system. | Comprehensive stool microbiology. Breath testing for small intestinal bacterial overgrowth. | Gut-microbiota-targeted dietary interventions. Probiotics. Prebiotics. |
| Small intestinal bacterial overgrowth | Colonic gram-negative aerobes and anaerobic species overgrow in the small intestine where they ferment carbohydrate and contribute to digestive symptoms. | Breath testing for small intestinal bacterial overgrowth. | Antibiotics. Medical food-based elemental diet. Herbal medicine. Probiotics. |
| Parasitic infection or overgrowth | Specific subtypes of Blastocystis species may contribute to dysbiosis, upregulate pro-inflammatory cytokines, and degrade tight junction proteins and increase intestinal permeability. D. fragilis-infection may result in inflammatory changes and intestinal permeability in the gut mucosa. | Comprehensive stool parasitology, including Blastocystis species and D. fragilis. | Antibiotic therapy. Emulsified oil of Oregano. Saccharomyces boulardii. |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, B.I. Does Irritable Bowel Syndrome Exist? Identifiable and Treatable Causes of Associated Symptoms Suggest It May Not. Gastrointest. Disord. 2019, 1, 314-340. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord1030027
Brown BI. Does Irritable Bowel Syndrome Exist? Identifiable and Treatable Causes of Associated Symptoms Suggest It May Not. Gastrointestinal Disorders. 2019; 1(3):314-340. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord1030027
Chicago/Turabian StyleBrown, Benjamin I. 2019. "Does Irritable Bowel Syndrome Exist? Identifiable and Treatable Causes of Associated Symptoms Suggest It May Not" Gastrointestinal Disorders 1, no. 3: 314-340. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord1030027





