Comparison of the Status of Interstitial Cells of Cajal in the Smooth Muscle of the Antrum and Pylorus in Diabetic Male and Female Patients with Severe Gastroparesis
Abstract
:1. Introduction
2. Materials and Methods
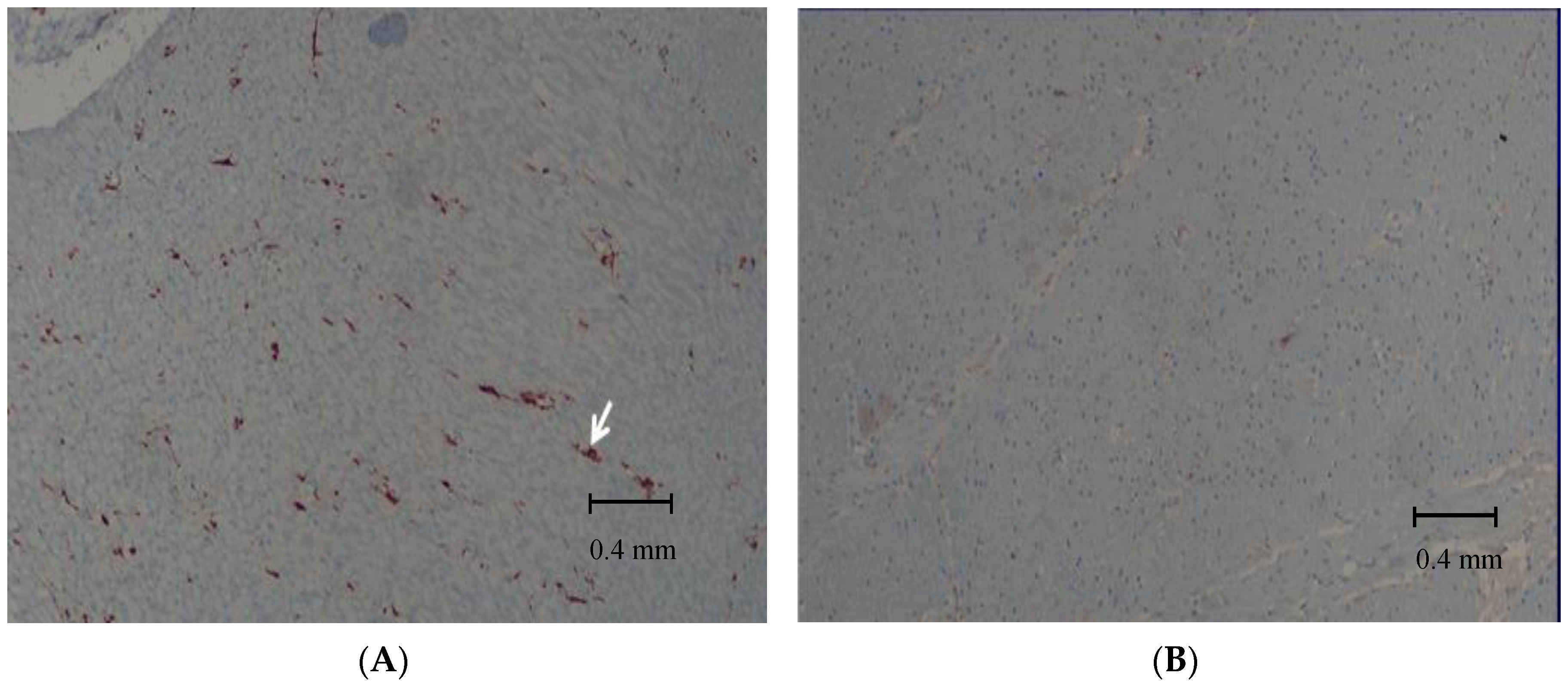
2.1. Histological Evaluation of the Biopsy Samples
2.2. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Parkman, H.P.; Hasler, W.L.; Fisher, R.S. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology 2004, 127, 1592–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revicki, D.A.; Rentz, A.M.; Dubois, D.; Kahrilas, P.; Stanghellini, V.; Talley, N.J.; Tack, J. Development and validation of a patient-assessed gastroparesis symptom severity measure: The Gastroparesis Cardinal Symptom Index. Aliment. Pharm. 2003, 18, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Gale, E.A.; Gillespie, K.M. Diabetes and Gender. Diabetologia 2001, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkman, H.P.; Yates, K.; Hasler, W.L.; Nguyen, L.; Pasricha, P.J.; Snape, W.J.; Farrugia, G.; Koch, K.L.; Calles, J.; Abell, T.L.; et al. Similarities and differences between diabetic and idiopathic gastroparesis. Clin. Gastroenterol. Hepatol. 2011, 9, 1056–1064; quiz e1133–1054. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.K.; Choung, R.S.; Locke, G.R., 3rd; Schleck, C.D.; Zinsmeister, A.R.; Szarka, L.A.; Mullan, B.; Talley, N.J. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology 2009, 136, 1225–1233. [Google Scholar] [CrossRef] [Green Version]
- Dudekula, A.; O’Connell, M.; Bielefeldt, K. Hospitalizations and testing in gastroparesis. J. Gastroenterol. Hepatol. 2011, 26, 1275–1282. [Google Scholar] [CrossRef]
- Parkman, H.P.; Yates, K.; Hasler, W.L.; Nguyen, L.; Pasricha, P.J.; Snape, W.J.; Farrugia, G.; Koch, K.L.; Abell, T.L.; McCallum, R.W.; et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology 2011, 140, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Soykan, I.; Sivri, B.; Sarosiek, I.; Kiernan, B.; McCallum, R.W. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig. Dis. Sci. 1998, 43, 2398–2404. [Google Scholar] [CrossRef]
- Camilleri, M.; Parkman, H.P.; Shafi, M.A.; Abell, T.L.; Gerson, L. Clinical guideline: Management of gastroparesis. Am. J. Gastroenterol. 2013, 108, 18–37; quiz 38. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Sarosiek, I.; Froster, J.; Damjanov, I.; Hou, Q.; McCallum, R.W. Association of the Status of Interstitial Cells of Cajal and Electrogastrogram Parameters, Gastric Emptying and Symptoms in Patients with Gastroparesis. Neurogastroenterol. Motil. 2010, 22, 56–61. [Google Scholar]
- Ördög, T.; Ward, S.M.; Sanders, K.M. Interstitial Cells of Cajal Generate Electrical Slow Waves in the Murine Stomach. J. Physiol. 1999, 518. [Google Scholar] [CrossRef]
- Huizinga, J.D.; Thuneberg, L.; Kluppel, M.; Malysz, J.; Mikkelsen, H.B.; Bernstein, A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 1995, 373, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.M.; Burns, A.J.; Torihashi, S.; Sanders, K.M. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J. Physiol. 1994, 480, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, J.D.; Zarate, N.; Farrugia, G. Physiology, Injury, and Recovery of Interstitial Cells of Cajal: Basic and Clinical Science. Gastroenterology 2009, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, K.M. A Case for Interstitial Cells of Cajal as Pacemakers and Mediators of Neurotransmission in the Gastrointestinal Tract. Gastroenterology 1996, 111. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Farrugia, G.; Lurken, M.S.; Bernard, C.E.; Faussone-Pellegrini, M.S.; Smyrk, T.C.; Parkman, H.P.; Abell, T.L.; Snape, W.J.; Hasler, W.L.; et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology 2011, 140, 1575–1585. [Google Scholar] [CrossRef] [Green Version]
- Grover, M.; Bernard, C.E.; Pasricha, P.J.; Lurken, M.S.; Faussone-Pellegrini, M.S.; Smyrk, T.C.; Parkman, H.P.; Abell, T.L.; Snape, W.J.; Hasler, W.L.; et al. Clinical-histological associations in gastroparesis: Results from the Gastroparesis Clinical Research Consortium. NeuroGastroenterol. Motil. 2012, 24, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Wells, C.; O’Grady, G. Interstitial Cells of Cajal. Nature 2019, 267–274. [Google Scholar] [CrossRef]
- Bashashati, M.; McCallum, R.W. Is Interstitial Cells of Cajal–opathy Present in Gastroparesis? J. NeuroGastroenterol. Motil. 2015, 21, 486–493. [Google Scholar] [CrossRef]
- Moraveji, S.; Bashashati, M.; Elhanafi, S.; Sunny, J.; Sarosiek, I.; Davis, B.; Torabi, A.; McCallum, R.W. Depleted interstitial cells of Cajal and fibrosis in the pylorus: Novel features of gastroparesis. NeuroGastroenterol. Motil. 2016, 28, 1048–1054. [Google Scholar] [CrossRef]
- Bashashati, M.; McCallum, R.W. Motility: Is ’ICC-opathy’ Present in Gastroparesis-Like Syndrome? Nat. Rev. Gastroenterol. Hepatol. 2015, 12. [Google Scholar] [CrossRef]
- Angeli, T.R.; Cheng, L.K.; Du, P.; Wang, T.H.; Bernard, C.E.; Vannucchi, M.G.; Faussone-Pellegrini, M.S.; Lahr, C.; Vather, R.; Windsor, J.A.; et al. Loss of Interstitial Cells of Cajal and Patterns of Gastric Dysrhythmia in Patients With Chronic Unexplained Nausea and Vomiting. Gastroenterology 2015, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abell, T.L.; Camilleri, M.; Donohoe, K.; Hasler, W.L.; Lin, H.C.; Maurer, A.H.; McCallum, R.W.; Nowak, T.; Nusynowitz, M.L.; Parkman, H.P.; et al. Consensus recommendations for gastric emptying scintigraphy: A joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. J. Nucl. Med. Technol. 2008, 36, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Cutts, T.F.; Luo, J.; Starkebaum, W.; Rashed, H.; Abell, T.L. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? NeuroGastroenterol. Motil. 2005, 17, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Anand, C.; Al-Juburi, A.; Familoni, B.; Rashed, H.; Cutts, T.; Abidi, N.; Johnson, W.D.; Minocha, A.; Abell, T.L. Gastric electrical stimulation is safe and effective: A long-term study in patients with drug-refractory gastroparesis in three regional centers. Digestion 2007, 75, 83–89. [Google Scholar] [CrossRef]
- Jayanthi, N.V.; Dexter, S.P.; Sarela, A.I. Gastric electrical stimulation for treatment of clinically severe gastroparesis. J. Minim. Access. Surg. 2013, 9, 163–167. [Google Scholar] [CrossRef]
- Maranki, J.L.; Lytes, V.; Meilahn, J.E.; Harbison, S.; Friedenberg, F.K.; Fisher, R.S.; Parkman, H.P. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig. Dis. Sci. 2008, 53, 2072–2078. [Google Scholar] [CrossRef]
- Forster, J.; Sarosiek, I.; Lin, Z.; Durham, S.; Denton, S.; Roeser, K.; McCallum, R.W. Further Experience With Gastric Stimulation to Treat Drug Refractory Gastroparesis. Am. J. Surg. 2003, 186. [Google Scholar] [CrossRef]
- Lahr, C.J.; Griffith, J.; Subramony, C.; Halley, L.; Adams, K.; Paine, E.R.; Schmieg, R.; Islam, S.; Salameh, J.; Spree, D.; et al. Gastric Electrical Stimulation for Abdominal Pain in Patients With Symptoms of Gastroparesis. Am. Surg. 2013, 79, 457–464. [Google Scholar]
- Keller, D.S.; Parkman, H.P.; Boucek, D.O.; Sankineni, A.; Meilahn, J.E.; Gaughan, J.P.; Harbison, S. Surgical Outcomes After Gastric Electric Stimulator Placement for Refractory Gastroparesis. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2013, 17. [Google Scholar] [CrossRef]
- Davis, B.R.; Sarosiek, I.; Bashashati, M.; Alvarado, B.; McCallum, R.W. The Long-Term Efficacy and Safety of Pyloroplasty Combined with Gastric Electrical Stimulation Therapy in Gastroparesis. J. Gastrointest. Surg. 2017, 21, 222–227. [Google Scholar] [CrossRef]
- Bashashati, M.; Sarosiek, I.; Davis, B.R.; Diaz, J.R.; Padilla, O.; Espino, K.; McCallum, R.W. Mo1599—Combined Gastric Electrical Stimulation Pyloroplasty Improves Gastroparesis Symptoms and Gastric Emptying Without Affecting the Count of Antral Interstitial Cells of Cajal. Gastroenterology 2019, 156. [Google Scholar] [CrossRef]
- Toro, J.P.; Lytle, N.W.; Patel, A.D.; Davis, S.S., Jr.; Christie, J.A.; Waring, J.P.; Sweeney, J.F.; Lin, E. Efficacy of laparoscopic pyloroplasty for the treatment of gastroparesis. J. Am. Coll. Surg. 2014, 218, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Harberson, J.; Thomas, R.M.; Harbison, S.P.; Parkman, H.P. Gastric Neuromuscular Pathology in Gastroparesis: Analysis of Full-Thickness Antral Biopsies. Dig. Dis. Sci. 2010, 55, 359–370. [Google Scholar]
- Bashashati. Pathological Findings of the Antral and Pyloric Smooth Muscle in Patients with Gastroparesis-Like Syndrome Compared to Gastroparesis: Similarities and Differences. Available online: https://elpaso.ttuhsc.edu/ (accessed on 8 August 2020).
- Zia, J.K.; Heitkemper, M.M. Upper Gastrointestinal Tract Motility Disorders in Women, Gastroparesis, and Gastroesophageal Reflux Disease. Gastroenterol. Clin North. Am 2016, 45, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Knight, L.C.; Parkman, H.P.; Brown, K.L.; Miller, M.A.; Trate, D.M.; Maurer, A.H.; Fisher, R.S. Delayed gastric emptying and decreased antral contractility in normal premenopausal women compared with men. Am. J. Gastroenterol. 1997, 92, 968–975. [Google Scholar] [PubMed]
- Hennig, G.W.; Spencer, N.J.; Jokela-willis, S.; Bayguinov, P.O.; Lee, H.T.; Ritchie, L.A.; Ward, S.M.; Smith, T.K.; Sanders, K.M. ICC-MY Coordinate Smooth Muscle Electrical and Mechanical Activity in the Murine Small Intestine. NeuroGastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2010, 22. [Google Scholar] [CrossRef] [Green Version]
- Sanders, K.M. Interstitial Cells of Cajal at the Clinical and Scientific Interface. J. Physiol. 2006, 576. [Google Scholar] [CrossRef]
- Long, Q.L.; Fang, D.C.; Shi, H.T.; Luo, Y.H. Gastro-electric dysrhythm and lack of gastric interstitial cells of cajal. World J. Gastroenterol. 2004, 10, 1227–1230. [Google Scholar] [CrossRef]
- Gangula, P.R.; Maner, W.L.; Micci, M.A.; Garfield, R.E.; Pasricha, P.J. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G725–G733. [Google Scholar] [CrossRef] [Green Version]
- Datz, F.L.; Christian, P.E.; Moore, J. Gender-related differences in gastric emptying. J. Nucl. Med. 1987, 28, 1204–1207. [Google Scholar]
- Hutson, W.R.; Roehrkasse, R.L.; Wald, A. Influence of gender and menopause on gastric emptying and motility. Gastroenterology 1989, 96, 11–17. [Google Scholar] [CrossRef]
- Ravella, K.; Al-Hendy, A.; Sharan, C.; Hale, A.B.; Channon, K.M.; Srinivasan, S.; Gangula, P.R. Chronic estrogen deficiency causes gastroparesis by altering neuronal nitric oxide synthase function. Dig. Dis. Sci. 2013, 58, 1507–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilleri, M.; Iturrino, J.; Bharucha, A.E.; Burton, D.; Shin, A.; JEONG, I.D.; Zinsmeister, A.R. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. NeuroGastroenterol. Motil. 2012, 24, 1076–e1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petring, O.U.; Flachs, H. Inter- and intrasubject variability of gastric emptying in healthy volunteers measured by scintigraphy and paracetamol absorption. Br. J. Clin. Pharm. 1990, 29, 703–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madsen, J.L. Effects of gender, age, and body mass index on gastrointestinal transit times. Dig. Dis. Sci. 1992, 37, 1548–1553. [Google Scholar] [CrossRef]
- Jones, K.L.; Russo, A.; Stevens, J.E.; Wishart, J.M.; Berry, M.K.; Horowitz, M. Predictors of delayed gastric emptying in diabetes. Diabetes Care 2001, 24, 1264–1269. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Guo, Y.; He, J.; Zhang, F.; Sun, X.; Yang, S.; Dong, H. Estrogen and estrogen receptors in the modulation of gastrointestinal epithelial secretion. Oncotarget 2017, 8, 97683–97692. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.S.; Doong, M.L.; Chang, F.Y.; Lee, S.D.; Wang, P.S. Effects of sex steroid hormones on gastric emptying and gastrointestinal transit in rats. Am. J. Physiol. 1995, 268, G171–G176. [Google Scholar] [CrossRef]
- Hogan, A.M.; Collins, D.; Baird, A.W.; Winter, D.C. Estrogen and its role in gastrointestinal health and disease. Int. J. Colorectal Dis. 2009, 24, 1367–1375. [Google Scholar] [CrossRef]
- Liu, C.Y.; Chen, L.B.; Liu, P.Y.; Xie, D.P.; Wang, P.S. Effects of progesterone on gastric emptying and intestinal transit in male rats. World J. Gastroenterol. 2002, 8, 338–341. [Google Scholar] [CrossRef]
- Iino, S.; Horiguchi, S.; Horiguchi, K. Interstitial cells of Cajal in the gastrointestinal musculature of W(jic) c-kit mutant mice. J. Smooth Muscle Res. Nihon Heikatsukin Gakkai Kikanshi 2011, 47, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Mones, J.; Carrio, I.; Calabuig, R.; Estorch, M.; Sainz, S.; Berna, L.; Vilardell, F. Influence of the menstrual cycle and of menopause on the gastric emptying rate of solids in female volunteers. Eur. J. Nucl. Med. 1993, 20, 600–602. [Google Scholar] [CrossRef] [PubMed]
- Avalos, D.N.P.; McCallum, R.W. Understanding the Etiology and Spectrum of Idiopathic Gastroparesis. Pract. Gastroenterol. 2017, 42, 38–50. [Google Scholar]
- Gangula, P.R.; Sekhar, K.R.; Mukhopadhyay, S. Gender bias in gastroparesis: Is nitric oxide the answer? Dig. Dis. Sci. 2011, 56, 2520–2527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, T. Pathophysiological Significance of Neuronal Nitric Oxide Synthase in the Gastrointestinal Tract. J. Gastroenterol. 2003, 38. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2012, 33. [Google Scholar] [CrossRef] [Green Version]
- Lekontseva, O.; Chakrabarti, S.; Jiang, Y.; Cheung, C.C.; Davidge, S.T. Role of Neuronal Nitric-Oxide Synthase in Estrogen-Induced Relaxation in Rat Resistance Arteries. J. Pharmacol. Exp. Ther. 2011, 339. [Google Scholar] [CrossRef] [Green Version]
- Showkat Ali, M.; Tiscareno-Grejada, I.; Locovei, S.; Smiley, R.; Collins, T.; Sarosiek, J.; McCallum, R. Gender and estradiol as major factors in the expression and dimerization of nNOSalpha in rats with experimental diabetic gastroparesis. Dig. Dis. Sci. 2012, 57, 2814–2825. [Google Scholar] [CrossRef]


| Mean Number of ICC/HPF in the Antrum vs. Pylorus | ||||
|---|---|---|---|---|
| Antrum: Mean 11.5 (±5.3) Range (3–10) | Pylorus: Mean 7.4 (±4.7) Range (1–9) | p < 0.01 | ||
| ICC | Female | Male | p Value | |
| Antrum F n = 25 (66%) M n = 13 (34%) | Mean # of ICC for | 11.1 (±4.3) | 12.5 (±7.0) | n.s. |
| ICC less < 10 cells/HPF | 10 (40%) | 5 (38%) | n.s. | |
| PYLORUS F n = 19 (65%) M n = 10 (35%) | Mean # of ICC for | 7.9 (±4.4) | 6.4 (±5.4) | n.s. |
| ICC less < 10 cells/HPF | 13 (68%) | 8 (80%) | n.s. | |
| ICC less< 10 cells/HPF in both regions | 6 (32%) | 4 (40%) | n.s. | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez, Z.; Sarosiek, I.; Bashashati, M.; Davis, B.; Padilla, O.; McCallum, R. Comparison of the Status of Interstitial Cells of Cajal in the Smooth Muscle of the Antrum and Pylorus in Diabetic Male and Female Patients with Severe Gastroparesis. Gastrointest. Disord. 2020, 2, 236-245. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord2030023
Gonzalez Z, Sarosiek I, Bashashati M, Davis B, Padilla O, McCallum R. Comparison of the Status of Interstitial Cells of Cajal in the Smooth Muscle of the Antrum and Pylorus in Diabetic Male and Female Patients with Severe Gastroparesis. Gastrointestinal Disorders. 2020; 2(3):236-245. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord2030023
Chicago/Turabian StyleGonzalez, Zorisadday, Irene Sarosiek, Mohammad Bashashati, Brian Davis, Osvaldo Padilla, and Richard McCallum. 2020. "Comparison of the Status of Interstitial Cells of Cajal in the Smooth Muscle of the Antrum and Pylorus in Diabetic Male and Female Patients with Severe Gastroparesis" Gastrointestinal Disorders 2, no. 3: 236-245. https://0-doi-org.brum.beds.ac.uk/10.3390/gidisord2030023






