Polyphenol Extraction from Humulus lupulus (Hop) Using a Neoteric Glycerol/L-Alanine Deep Eutectic Solvent: Optimisation, Kinetics and the Effect of Ultrasound-Assisted Pretreatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material and Treatments
2.3. Synthesis of DES
2.4. Batch Stirred-Tank Extraction
2.5. Ultrasound-Assisted Pretreatment
2.6. Extraction Optimisation by Response Surface Methodology
2.7. Total Polyphenol (TP) Determination
2.8. Total Flavonoid (TFn) Determination
2.9. Total Flavanol (TF) Determination
2.10. Determination of the Antiradical Activity (AAR)
2.11. Determination of the Reducing Power (PR)
2.12. Statistical Analyses
3. Results and Discussion
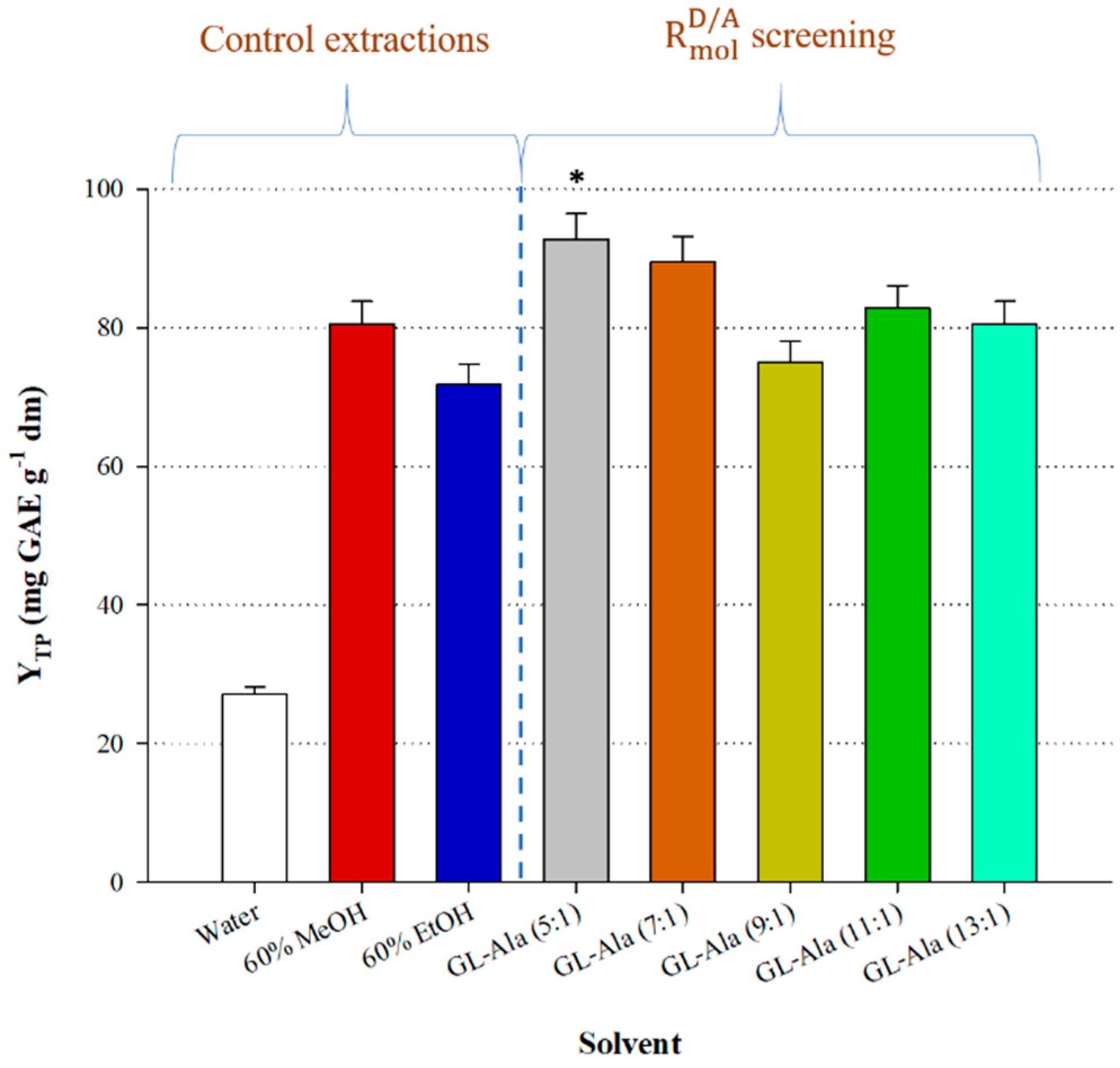
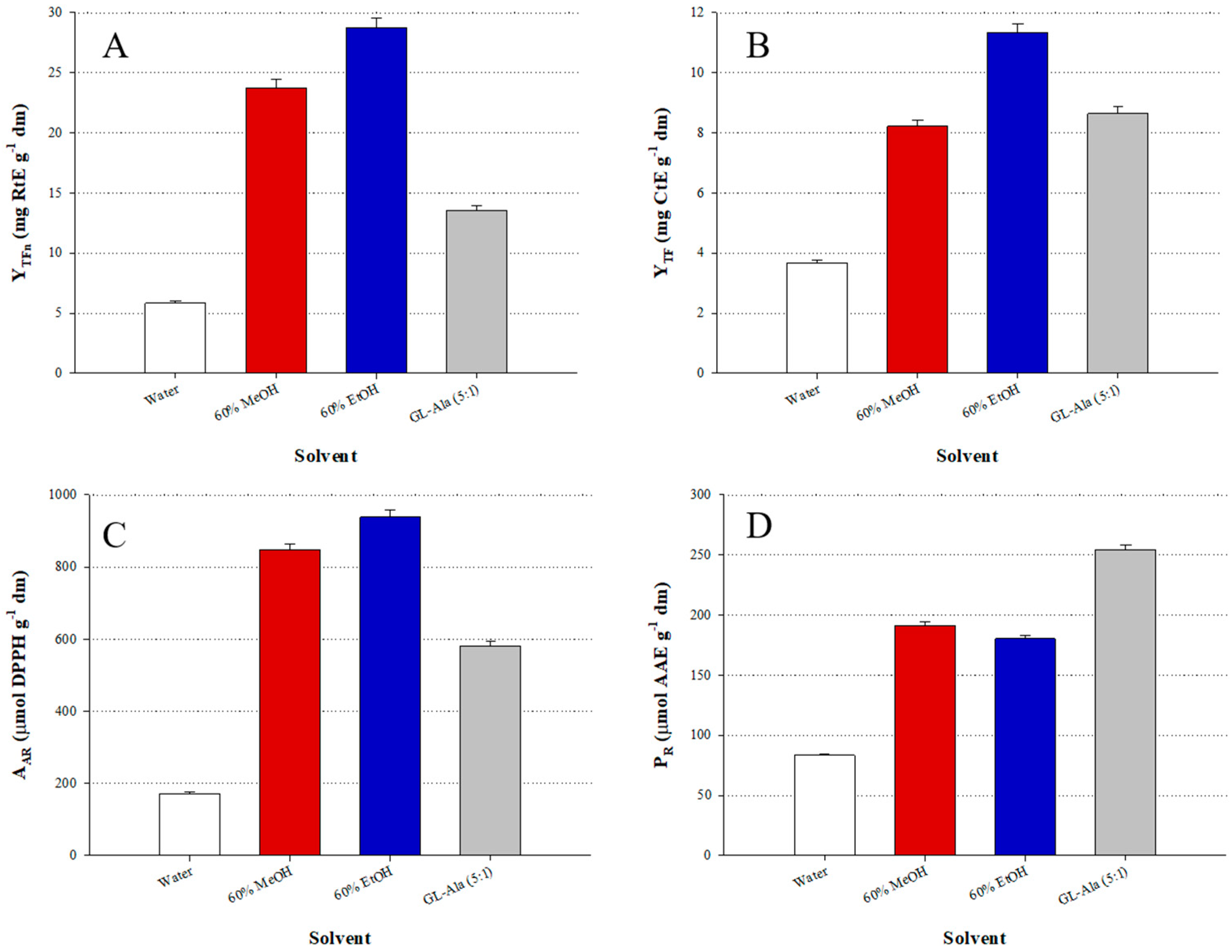
3.1. DES Synthesis and HBD:HBA Molar Ratio () Assay
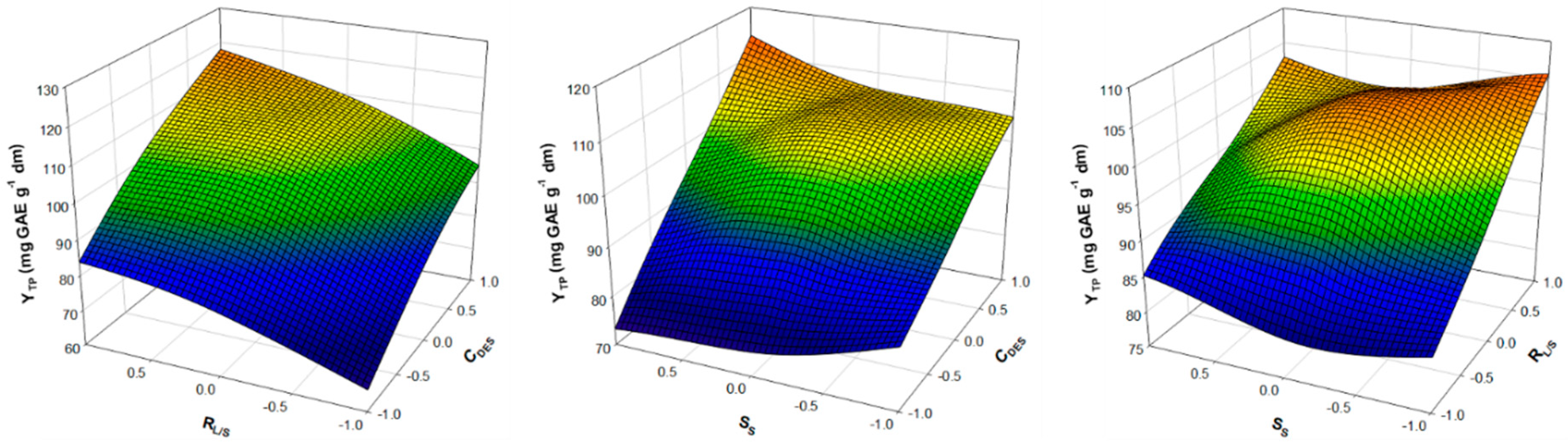
3.2. Optimisation of Extraction Performance
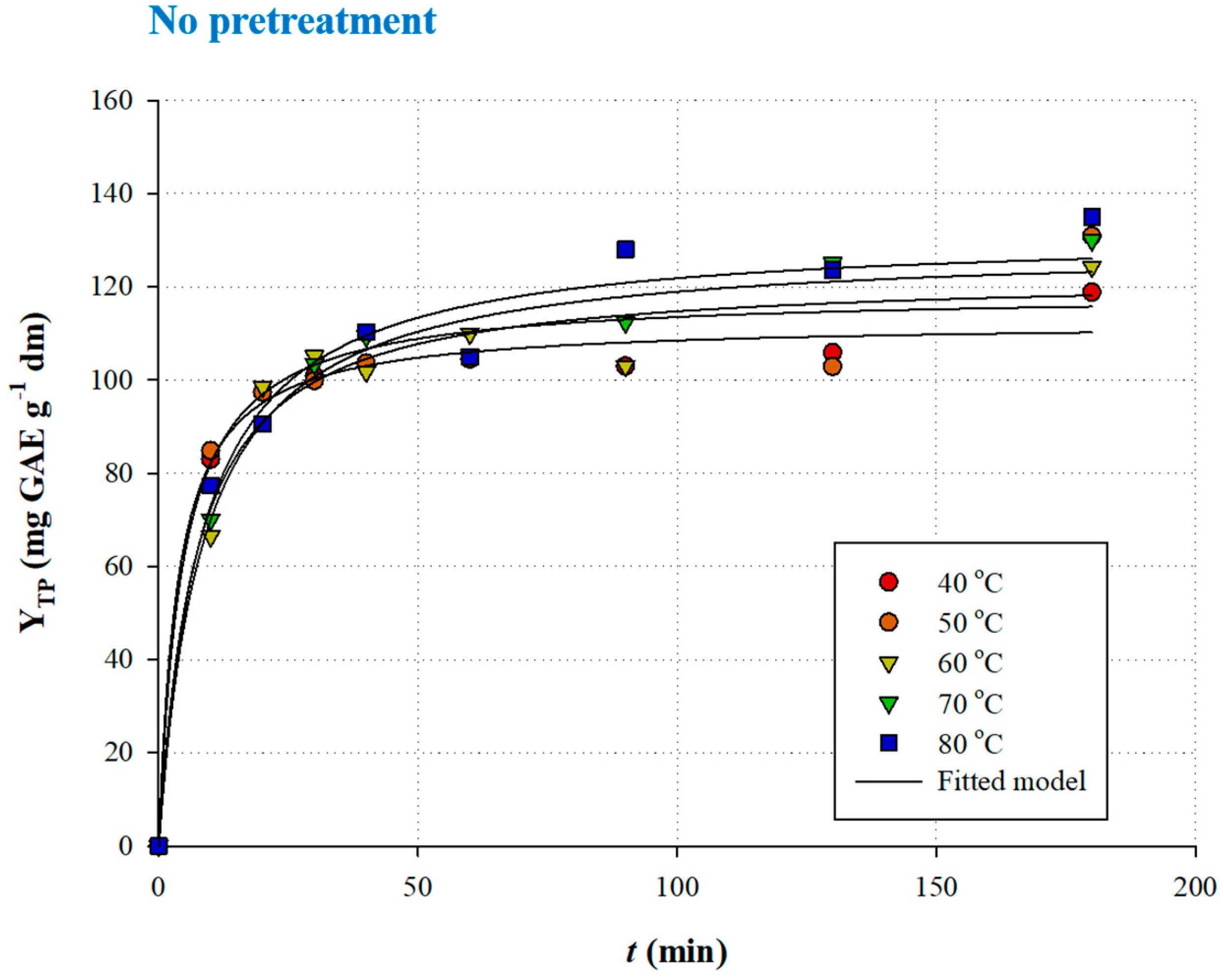
3.3. Ultrasound-Assisted Pretreatment: Effect on Extraction Kinetics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| AAR | antiradical activity (μmol DPPH g−1) |
| PR | reducing power (μmol AAE g−1) |
| RL/S | liquid-to-solid ratio (mL g−1) |
| molar HBD:HBA ratio (dimensionless) | |
| t | time (min) |
| T | temperature (°C) |
| YTF | yield in total flavanols (mg CtE g-1) |
| YTFn | yield in total flavonoids (mg RtE g-1) |
| YTP | yield in total polyphenols (mg GAE g−1) |
Abbreviations
| AAE | ascorbic acid equivalents |
| DES | deep eutectic solvents |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl radical |
| GAE | gallic acid equivalents |
| HBA | hydrogen bond acceptor |
| HBD | hydrogen bond donor |
| TPTZ | 2,4,6-tripyridyl-s-triazine |
| US | ultrasonication |
References
- Van Cleemput, M.; Cattoor, K.; De Bosscher, K.; Haegeman, G.; De Keukeleire, D.; Heyerick, A. Hop (Humulus lupulus)-derived bitter acids as multipotent bioactive compounds. J. Nat. Prod. 2009, 72, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Bocquet, L.; Sahpaz, S.; Hilbert, J.; Rambaud, C.; Rivière, C. Humulus lupulus L., a very popular beer ingredient and medicinal plant: Overview of its phytochemistry, its bioactivity, and its biotechnology. Phytochem. Rev. 2018, 17, 1047–1090. [Google Scholar] [CrossRef]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Karabín, M.; Hudcová, T.; Jelínek, L.; Dostálek, P. Biologically active compounds from hops and prospects for their use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567. [Google Scholar] [CrossRef]
- Dostálek, P.; Karabín, M.; Jelínek, L. Hop phytochemicals and their potential role in metabolic syndrome prevention and therapy. Molecules 2017, 22, 1761. [Google Scholar] [CrossRef]
- Luca, S.V.; Macoveia, I.; Bujora, A.; Mirona, A.; Skalicka-Wozniakb, K.; Aprotosoaiea, A.K.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2019, 7, 1–34. [Google Scholar] [CrossRef]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Herrero, M.; Ibañez, E. Green extraction processes, biorefineries and sustainability: Recovery of high added-value products from natural sources. J. Supercrit. Fluids 2018, 134, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and sustainable solvents in chemical processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- De los Ángeles Fernández, M.; Boiteux, J.; Espino, M.; Gomez, F.V.; Silva, M.F. Natural deep eutectic solvents-mediated extractions: The way forward for sustainable analytical developments. Anal. Chim. Acta 2018, 1038, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bubalo, M.C.; Vidović, S.; Redovniković, I.R.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Kottaras, P.; Koulianos, M.; Makris, D.P. Low-Transition temperature mixtures (LTTMs) made of bioorganic molecules: Enhanced extraction of antioxidant phenolics from industrial cereal solid wastes. Recycling 2017, 2, 3. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Karakashov, B.; Grigorakis, S.; Loupassaki, S.; Mourtzinos, I.; Makris, D.P. Optimisation of organic solvent-free polyphenol extraction from Hypericum triquetrifolium Turra using Box–Behnken experimental design and kinetics. Int. J. Ind. Chem. 2015, 6, 85–92. [Google Scholar] [CrossRef]
- Manousaki, A.; Jancheva, M.; Grigorakis, S.; Makris, D.P. Extraction of antioxidant phenolics from agri-food waste biomass using a newly designed glycerol-based natural low-transition temperature mixture: A comparison with conventional eco-friendly solvents. Recycling 2016, 1, 194–204. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Chiou, A.; Andrikopoulos, N.K. An investigation on factors affecting recovery of antioxidant phenolics and anthocyanins from red grape (Vitis vinifera L.) pomace employing water/ethanol-based solutions. Am. J. Food Technol. 2008, 3, 164–173. [Google Scholar] [CrossRef]
- Shi, H.; Niki, E. Stoichiometric and kinetic studies on Ginkgo biloba extract and related antioxidants. Lipids 1998, 33, 365. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P.; Passalidi, V.; Kallithraka, S.; Mourtzinos, I. Optimization of polyphenol extraction from red grape pomace using aqueous glycerol/tartaric acid mixtures and response surface methodology. Prep. Biochem. Biotechnol. 2016, 46, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.-G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Peng, X.; Yao, X.-H.; Wei, Z.-F.; Luo, M.; Wang, W.; Zhao, C.-J.; Fu, Y.-J.; Zu, Y.-G. Deep eutectic solvent-based microwave-assisted extraction of genistin, genistein and apigenin from pigeon pea roots. Sep. Purif. Technol. 2015, 150, 63–72. [Google Scholar] [CrossRef]
- Karageorgou, I.; Grigorakis, S.; Lalas, S.; Makris, D.P. Enhanced extraction of antioxidant polyphenols from Moringa oleifera Lam. leaves using a biomolecule-based low-transition temperature mixture. Eur. Food Res. Technol. 2017, 243, 1839–1848. [Google Scholar] [CrossRef]
- Patsea, M.; Stefou, I.; Grigorakis, S.; Makris, D.P. Screening of natural sodium acetate-based low-transition temperature mixtures (LTTMs) for enhanced extraction of antioxidants and pigments from red vinification solid wastes. Environ. Process. 2017, 4, 123–135. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, J.-Z.; Wang, L.-T.; Kang, Y.-F.; Meng, Y.; Jiao, J.; Fu, Y.-J. Sustainable deep eutectic solvents preparation and their efficiency in extraction and enrichment of main bioactive flavonoids from sea buckthorn leaves. J. Clean. Prod. 2018, 184, 826–835. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Grigorakis, S.; Lalas, S.; Makris, D.P. Highly efficient extraction of antioxidant polyphenols from Olea europaea leaves using an eco-friendly glycerol/glycine deep eutectic solvent. Waste Biomass Valor. 2018, 9, 1985–1992. [Google Scholar] [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J. Chromatogr. A 2013, 1285, 22–30. [Google Scholar] [CrossRef]
- Pinelo, M.; Sineiro, J.; Núñez, M.J. Mass transfer during continuous solid–liquid extraction of antioxidants from grape byproducts. J. Food Eng. 2006, 77, 57–63. [Google Scholar] [CrossRef]
- Rakotondramasy-Rabesiaka, L.; Havet, J.-L.; Porte, C.; Fauduet, H. Estimation of effective diffusion and transfer rate during the protopine extraction process from Fumaria officinalis L. Sep. Purif. Technol. 2010, 76, 126–131. [Google Scholar] [CrossRef]
- Shewale, S.; Rathod, V.K. Extraction of total phenolic content from Azadirachta indica or (neem) leaves: Kinetics study. Prep. Biochem. Biotechnol. 2018, 48, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Jancheva, M.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Optimised extraction of antioxidant polyphenols from Satureja thymbra using newly designed glycerol-based natural low-transition temperature mixtures (LTTMs). J. Appl. Res. Med. Aromat. Plants 2017, 6, 31–40. [Google Scholar] [CrossRef]
- Dedousi, M.; Mamoudaki, V.; Grigorakis, S.; Makris, D.P. Ultrasound-assisted extraction of polyphenolic antioxidants from olive (Olea europaea) leaves using a novel glycerol/sodium-potassium tartrate low-transition temperature mixture (LTTM). Environments 2017, 4, 31. [Google Scholar] [CrossRef]
- Slim, Z.; Jancheva, M.; Grigorakis, S.; Makris, D.P. Polyphenol extraction from Origanum dictamnus using low-transition temperature mixtures composed of glycerol and organic salts: Effect of organic anion carbon chain length. Chem. Eng. Commun. 2018, 205, 1494–1506. [Google Scholar] [CrossRef]
- Vetal, M.D.; Lade, V.G.; Rathod, V.K. Extraction of ursolic acid from Ocimum sanctum by ultrasound: Process intensification and kinetic studies. Chem. Eng. Process. 2013, 69, 24–30. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Prieto, M.; Vazquez, J.; Barreiro, M.F.; Barros, L.; Ferreira, I.C. Recovery of bioactive compounds from Arbutus unedo L. fruits: Comparative optimization study of maceration/microwave/ultrasound extraction techniques. Food Res. Int. 2018, 109, 455–471. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Bhushan, B.; Rosales, A.; Turienzo, L.R.; Cortina, J. Valorisation potential of Cabernet grape pomace for the recovery of polyphenols: Process intensification, optimisation and study of kinetics. Food Bioprod. Process. 2018, 109, 74–85. [Google Scholar] [CrossRef]
- Karageorgou, I.; Grigorakis, S.; Lalas, S.; Mourtzinos, I.; Makris, D.P. Incorporation of 2-hydroxypropyl β-cyclodextrin in a biomolecule-based low-transition temperature mixture (LTTM) boosts efficiency of polyphenol extraction from Moringa oleifera Lam leaves. J. Appl. Res. Med. Aromat. Plants 2018, 9, 62–69. [Google Scholar] [CrossRef]
- Seikova, I.; Simeonov, E.; Ivanova, E. Protein leaching from tomato seed––Experimental kinetics and prediction of effective diffusivity. J. Food Eng. 2004, 61, 165–171. [Google Scholar] [CrossRef]
- Peleg, M.; Normand, M.D.; Corradini, M.G. The Arrhenius equation revisited. Crit. Rev. Food Sci. Nutr. 2012, 52, 830–851. [Google Scholar] [CrossRef] [PubMed]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction optimisation using water/glycerol for the efficient recovery of polyphenolic antioxidants from two Artemisia species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Trasanidou, D.; Apostolakis, A.; Makris, D.P. Development of a green process for the preparation of antioxidant and pigment-enriched extracts from winery solid wastes using response surface methodology and kinetics. Chem. Eng. Commun. 2016, 203, 1317–1325. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]




| Independent Variables | Code Units | Coded Variable Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Concentration of DES in aqueous mixtures, CDES (%, w/v) | X1 | 55 | 70 | 85 |
| Liquid-to-solid ratio, RL/S (mL g−1) | X2 | 20 | 40 | 60 |
| Speed of stirring, SS (rpm) | X3 | 200 | 500 | 800 |
| Design Point | Independent Variables | Response | |||
|---|---|---|---|---|---|
| CDES (X1) | RL/S (X2) | SS (X3) | Yield in Total Polyphenols, YTP (mg Gallic Acid Equivalents (GAE) g−1 Dry Mass (dm)) | ||
| Measured | Predicted | ||||
| 1 | −1 | −1 | 0 | 64.57 | 66.11 |
| 2 | −1 | 1 | 0 | 86.98 | 83.88 |
| 3 | 1 | −1 | 0 | 92.00 | 95.10 |
| 4 | 1 | 1 | 0 | 119.47 | 117.93 |
| 5 | 0 | −1 | −1 | 85.54 | 82.67 |
| 6 | 0 | −1 | 1 | 86.97 | 85.20 |
| 7 | 0 | 1 | −1 | 103.83 | 105.60 |
| 8 | 0 | 1 | 1 | 100.01 | 102.88 |
| 9 | −1 | 0 | −1 | 81.37 | 82.70 |
| 10 | 1 | 0 | −1 | 105.09 | 104.86 |
| 11 | −1 | 0 | 1 | 73.02 | 73.25 |
| 12 | 1 | 0 | 1 | 115.45 | 114.12 |
| 13 | 0 | 0 | 0 | 102.08 | 102.49 |
| 14 | 0 | 0 | 0 | 105.00 | 102.49 |
| 15 | 0 | 0 | 0 | 100.39 | 102.49 |
| Temperature, T (°C) | Kinetic Parameters | |||
|---|---|---|---|---|
| Second-Order Extraction Rate, k (×10−3) (g mg−1 min−1) | Initial Extraction Rate, h (mg g−1 min−1) | YTP(s) (mg GAE g−1) | Half Time, t0.5 (min) | |
| No pretreatment | ||||
| 40 | 2.40 | 30.80 | 112.41 | 3.65 |
| 50 | 1.88 | 26.38 | 118.44 | 4.49 |
| 60 | 1.17 | 17.77 | 123.13 | 6.93 |
| 70 | 1.00 | 15.27 | 128.91 | 8.44 |
| 80 | 0.92 | 16.19 | 131.17 | 8.10 |
| Ultrasound-assisted pretreatment | ||||
| 40 | 13.19 | 79.25 | 96.78 | 0.98 |
| 50 | 4.10 | 32.51 | 108.37 | 2.74 |
| 60 | 1.78 | 16.55 | 115.86 | 5.83 |
| 70 | 1.69 | 20.11 | 128.49 | 5.43 |
| 80 | 1.66 | 29.96 | 153.51 | 4.48 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakka, A.; Karageorgou, I.; Kaltsa, O.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D. Polyphenol Extraction from Humulus lupulus (Hop) Using a Neoteric Glycerol/L-Alanine Deep Eutectic Solvent: Optimisation, Kinetics and the Effect of Ultrasound-Assisted Pretreatment. AgriEngineering 2019, 1, 403-417. https://0-doi-org.brum.beds.ac.uk/10.3390/agriengineering1030030
Lakka A, Karageorgou I, Kaltsa O, Batra G, Bozinou E, Lalas S, Makris D. Polyphenol Extraction from Humulus lupulus (Hop) Using a Neoteric Glycerol/L-Alanine Deep Eutectic Solvent: Optimisation, Kinetics and the Effect of Ultrasound-Assisted Pretreatment. AgriEngineering. 2019; 1(3):403-417. https://0-doi-org.brum.beds.ac.uk/10.3390/agriengineering1030030
Chicago/Turabian StyleLakka, Achillia, Ioanna Karageorgou, Olga Kaltsa, Georgia Batra, Eleni Bozinou, Stavros Lalas, and Dimitris Makris. 2019. "Polyphenol Extraction from Humulus lupulus (Hop) Using a Neoteric Glycerol/L-Alanine Deep Eutectic Solvent: Optimisation, Kinetics and the Effect of Ultrasound-Assisted Pretreatment" AgriEngineering 1, no. 3: 403-417. https://0-doi-org.brum.beds.ac.uk/10.3390/agriengineering1030030






