Growth of Basil (Ocimum basilicum) in DRF, Raft, and Grow Pipes with Effluents of African Catfish (Clarias gariepinus) in Decoupled Aquaponics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydroponics Unit
2.1.1. Dynamic Root Floating Technique (DRF)
2.1.2. Floating Raft Culture (Raft)
2.1.3. Grow Pipes
2.2. Fish Production and Feeding
2.3. Plant Cultivation
2.4. Physicochemical Parameters
2.5. Mathematical and Statistical Analysis
3. Results
3.1. Fish Growth
3.2. Plant Growth
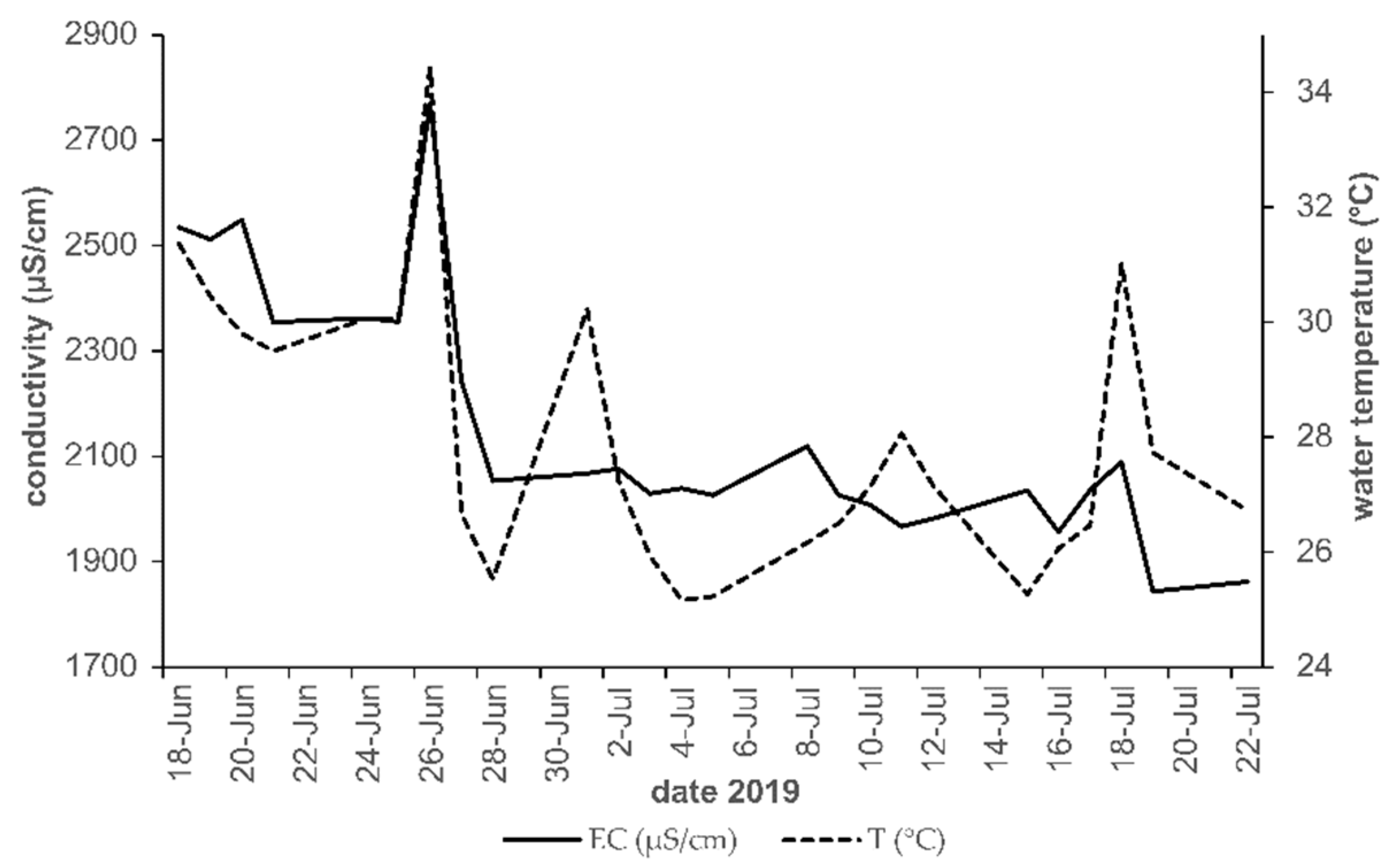
3.3. Physicochemical Parameters
4. Discussion
4.1. Fish Growth
4.2. Physio-Chemical Parameters
4.3. Plant Growth
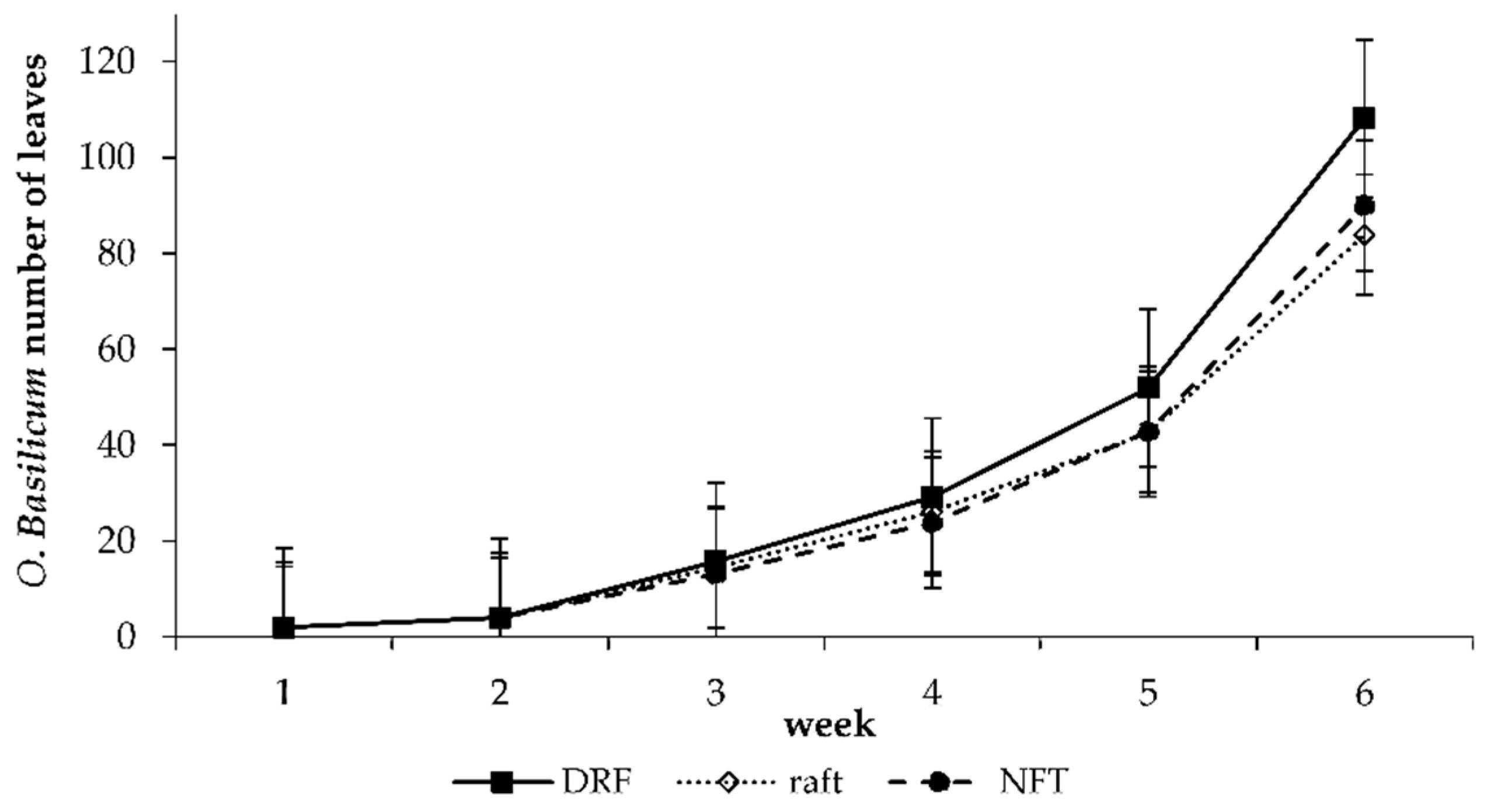
4.3.1. Leaf Development
4.3.2. Plant Biomass Development
4.3.3. Root Development
4.3.4. Plant Height Development
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palm, H.W.; Knaus, U.; Appelbaum, S.; Goddek, S.; Strauch, S.M.; Vermeulen, T.; Jijakli, M.H.; Kotzen, B. Towards commercial aquaponics: A review of systems, designs, scales and nomenclature. Aquac. Int. 2018, 26, 813–842. [Google Scholar] [CrossRef]
- Maucieri, C.; Nicoletto, C.; van Os, E.; Anseeuw, D.; Van Havermaet, R.; Junge, R. Chapter 4. Hydroponic Technologies. In Aquaponic Food Production Systems; Goddek, S., Joyce, A., Kotzen, B., Burnell, G., Eds.; Springer International Publishing: Basel, Switzerland, 2019; pp. 77–110. [Google Scholar]
- Love, D.C.; Fry, J.P.; Li, X.; Hill, E.S.; Genello, L.; Semmens, K.; Thompson, R.E. Commercial aquaponics production and profitability: Findings from an international survey. Aquaculture 2015, 435, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Engle, C.R. Economics of Aquaponics, SRAC-5006; Oklahoma State University: Stillwater, OK, USA, 2016; p. 4. [Google Scholar]
- Espinosa-Moya, A.; Álvarez-González, A.; Albertos-Alpuche, P.; Guzmán-Mendoza, R.; Martínez-Yáñez, R. Growth and development of herbaceous plants in aquaponic systems. Acta Univ. 2018, 28, 1–8. [Google Scholar] [CrossRef]
- Rakocy, J.E. Aquaponics: Integrating fish and plant culture. In Aquaculture Production Systems; Tidwell, J.H., Ed.; Wiley-Blackwell: Oxford, UK, 2012; pp. 344–386. [Google Scholar]
- Chalchat, J.C.; Özcan, M.M. Comparative essential oil composition of flowers, leaves and stems of basil (Ocimum basilicum L.) used as herb. Food Chem. 2008, 110, 501–503. [Google Scholar] [CrossRef]
- Ahmed, E.A.; Hassan, E.A.; Tobgy, K.M.; Ramadan, E.M. Evaluation of rhizobacteria of some medicinal plants for plant growth promotion and biological control. Ann. Agric. Sci. 2014, 59, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, P.M.; Kwee, E.M.; Niemeyer, E.D. Potassium rate alters the antioxidant capacity and phenolic concentration of basil (Ocimum basilicum L.) leaves. Food Chem. 2010, 123, 1235–1241. [Google Scholar] [CrossRef]
- Al-Maskari, M.Y.; Hanif, M.A.; Al-Maskri, A.Y.; Al-Adawi, S. Basil: A natural source of antioxidants and neutraceuticals. In Natural Products and Their Active Compounds on Disease Prevention; Mohamed Essa, M., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2012; pp. 463–471. [Google Scholar]
- Succop, C.E.; Newman, S.E. Organic fertilization of fresh market sweet basil in a greenhouse. HortTechnoloy 2004, 14, 235–239. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.; Monroe, A.; Day, M.R. Growth, yield, plant quality and nutrition of basil (Ocimum basilicum L.) under soilless agricultural systems. Ann. Agric. Sci. 2016, 61, 181–186. [Google Scholar] [CrossRef]
- Ferrarezi, R.S.; Bailey, D.S. Basil performance evaluation in aquaponics. HortTechnology 2019, 29, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Knaus, U.; Pribbernow, M.; Xu, L.; Appelbaum, S.; Palm, H.W. Basil (Ocimum basilicum) Cultivation in Decoupled Aquaponics with Three Hydro-Components (Grow Pipes, Raft, Gravel) and African Catfish (Clarias gariepinus) Production in Northern Germany. Sustainability 2020, 12, 8745. [Google Scholar] [CrossRef]
- Rakocy, J.E.; Bailey, D.S.; Shultz, R.C.; Thoman, E.S. Update on tilapia and vegetable production in the UVI aquaponic system. In New Dimensions on Farmed Tilapia, Proceedings of the 6th International Symposium on Tilapia in Aquaculture Philippine International Convention, Manila, Philippines, 12–16 September 2004; Bolivar, R., Fitzsimmons, K.M., Mair, G.C., Eds.; FAO: Rome, Italy, 2004; pp. 12–16. [Google Scholar]
- Palm, H.W.; Bissa, K.; Knaus, U. Significant factors affecting the economic sustainability of closed aquaponic systems. Part II: Fish and plant growth. Aquac. Aquar. Conserv. Legis. 2014, 7, 162–175. [Google Scholar]
- Knaus, U. Wachstum Verschiedener Fisch—und Pflanzenarten und Deren Auswirkungen auf Die Chemisch-Physikalischen Parameter in geschlossenen Warmwasser-Aquaponiksystemen. Ph.D. Thesis, University of Rostock, Rostock, Germany, 2016. [Google Scholar]
- Destatis E2. Betriebe mit Erzeugung in Aquakultur sowie erzeugter Menge im Jahr 2019 nach Art der Bewirtschaftung. Afrikanischer Raubwels. In Land und Forstwirtschaft, Fischerei. Erzeugung in Aquakulturbetrieben; Fachserie 3, Reihe 4.6; Statistisches Bundesamt (Destatis): Wiesbaden, Germany, 2019; p. 13. [Google Scholar]
- Fatollahi, M.; Kasumyan, A.O. The study of sensory bases of the feeding behavior of the African catfish Clarias gariepinus (Clariidae, Siluriformes). J. Ichthyol. 2006, 46, 161–172. [Google Scholar] [CrossRef]
- Oellermann, L.K. A Comparison of the Aquaculture Potential of Clarias gariepinus (Burchell, 1822) and Its Hybrid with Heterobranchus longifilis Valenciennes, 1840 in Southern Africa. Ph.D. Thesis, Rhodes University, Makhanda, South Africa, 1995. [Google Scholar]
- Huisman, E.A.; Richter, C.J.J. Reproduction, growth, health control and aquacultural potential of the African catfish, Clarias gariepinus (Burchell 1822). Aquaculture 1987, 63, 1–14. [Google Scholar] [CrossRef]
- Baßmann, B.; Brenner, M.; Palm, H.W. Stress and welfare of African catfish (Clarias gariepinus Burchell, 1822) in a coupled aquaponic system. Water 2017, 9, 504. [Google Scholar] [CrossRef] [Green Version]
- Endut, A.; Lananan, F.; Abdul Hamid, S.H.; Jusoh, A.; Wan Nik, W.N. Balancing of nutrient uptake by water spinach (Ipomoea aquatica) and mustard green (Brassica juncea) with nutrient production by African catfish (Clarias gariepinus) in scaling aquaponic recirculation system. Desalination Water Treat. 2016, 57, 29531–29540. [Google Scholar] [CrossRef]
- Lennard, W.A.; Leonard, B.V. A comparison of three different hydroponic sub-systems (gravel bed, floating and nutrient film technique) in an aquaponic test system. Aquac. Int. 2006, 14, 539–550. [Google Scholar] [CrossRef]
- Zweig, R.D. An integrated fish culture hydroponic vegetable production system. Aquac. Mag. 1986, 12, 34–40. [Google Scholar]
- Somerville, C.; Cohen, M.; Pantanella, E.; Stankus, A.; Lovatelli, A. Small-Scale Aquaponic Food Production: Integrated Fish and Plant Farming; FAO Fisheries and Aquaculture Technical Paper No. 589; FAO: Rome, Italy, 2014; p. 262. [Google Scholar]
- Cooper, A. The ABC of NFT. Nutrient Film Technique; Grower Books: London, UK, 1979; p. 181. [Google Scholar]
- Wattanapreechanon, E.; Sukprasert, P. Development of soilless culture for crop production in Thailand. Kasetsart J. 2012, 33, 475–485. [Google Scholar]
- Remy, M.; Singh, B.K.; Taylor, R. Evaluación de Dos Técnicas Hidropónicas Adaptadas Para las Condiciones del Trópico húmedo. Bachelor’s Thesis, Universidad EARTH, San José, Costa Rica, 2005. [Google Scholar]
- Kao, T.C.; Hsiang, T.; Changhua, R.O.C. The Dynamic Root Floating Hydroponic Technique: Year-Round Production of Vegetables in Roc on Taiwan; ASPAC Food & Fertilizer Technology Center: Taipei, Taiwan, 1991. [Google Scholar]
- Silva, L.; Gasca-Leyva, E.; Escalante, E.; Fitzsimmons, K.M.; Lozano, D.V. Evaluation of biomass yield and water treatment in two aquaponic systems using the dynamic root floating technique (DRF). Sustainability 2015, 7, 15384–15399. [Google Scholar] [CrossRef] [Green Version]
- Pantanella, E. Aquaponics Production, Practices and Opportunities. In Sustainable Aquaculture, Applied Environmental Science and Engineering for a Sustainable Future; Hai, F.I., Visvanathan, C., Boopathy, R., Eds.; Springer: Cham, Switzerland, 2018; pp. 191–248. [Google Scholar]
- Silva, L.; Valdés-Lozano, D.; Escalante, E.; Gasca-Leyva, E. Dynamic root floating technique: An option to reduce electric power consumption in aquaponic systems. J. Clean. Prod. 2018, 183, 132–142. [Google Scholar] [CrossRef]
- Bione, M.A.A.; Paz, V.P.S.; da Silva, F.; Sartoratto, A.; Soares, T.M. Production of hydroponic basil essential oil with conventional nutrient solution in brackish waters and organic nutrient solution. In Proceedings of the II Inovagri International Meeting, Fortaleza, Brazil, 13–16 April 2014; pp. 438–448. [Google Scholar]
- Putievsky, E.; Galambosi, B. Production systems of sweet basil. In Basil: The Genus Ocimum; Hiltunen, R., Holm, Y., Eds.; Harwood Academic Publishers: Amsterdam, The Netherlands, 1999; pp. 39–65. [Google Scholar]
- IBM. IBM SPSS Statistics for Windows, Version 23.0; IBM: New York, NY, USA, 2019. [Google Scholar]
- Microsoft®. Microsoft Excel®; Microsoft: Redmond, WA, USA, 2010. [Google Scholar]
- Palm, H.W.; Knaus, U.; Wasenitz, B.; Bischoff, A.A.; Strauch, S.M. Proportional up scaling of African catfish (Clarias gariepinus Burchell, 1822) commercial recirculating aquaculture systems disproportionally affects nutrient dynamics. Aquaculture 2018, 491, 155–168. [Google Scholar] [CrossRef]
- Pantanella, E. Nutrition and Quality of Aquaponic Systems. Ph.D. Thesis, Universita degli Studi della Tuscia, Viterbo, Italy, 2012; pp. 55–77. [Google Scholar]
- Zimmermann, J. Vergleich des Wachstums von Marokkanischer Minze (Mentha spicata) in drei verschiedenen Hydroponik Subsystemen unter aquaponischer Produktion. Master′s Thesis, University of Rostock, Rostock, Germany, 2017. [Google Scholar]
- Pasch, J. Einfluss einer Wurzel-Belüftung auf das Wachstum der Marokkanischen Minze (Mentha spicata L.) bei drei verschiedenen Hydroponik-Subsystemen unter aquaponischer Produktion. Master′s Thesis, University of Rostock, Rostock, Germany, 2018. [Google Scholar]
- Beaman, A.R.; Gladon, R.J.; Schrader, J.A. Sweet basil requires an irradiance of 500 μ mol· m−2·s−1 for greatest edible biomass production. HortScience 2009, 44, 64–67. [Google Scholar] [CrossRef] [Green Version]
- Walters, K.J.; Currey, C.J. Hydroponic greenhouse basil production: Comparing systems and cultivars. HortTechnology 2015, 25, 645–650. [Google Scholar] [CrossRef] [Green Version]
- Wetzel, R.G. Limnology: Lake and River Ecosystems, 3rd ed.; Academic Press: San Diego, CA, USA, 2001; pp. 151–167. [Google Scholar]
- Morard, P.; Lacoste, L.; Silvestre, J. Effect of oxygen deficiency on uptake of water and mineral nutrients by tomato plants in soilless culture. J. Plant Nutr. 2000, 23, 1063–1078. [Google Scholar] [CrossRef]
- Rakocy, J.E.; Masser, M.P.; Losordo, T.M. Recirculating aquaculture tank production systems: Aquaponics—integrating fish and plant culture. SRAC Publ. South. Reg. Aquac. Cent. 2006, 16, 454. [Google Scholar]
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O.P. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil Water Conserv. 2018, 17, 364–371. [Google Scholar] [CrossRef]
- Morano, G.; Amalfitano, C.; Sellitto, M.; Cuciniello, A.; Maiello, R.; Caruso, G. Effects of nutritive solution electrical conductivity and plant density on growth, yield and quality of sweet basil grown in gullies by subirrigation. Adv. Hortic. Sci. 2017, 31, 25–30. [Google Scholar]
- Bittsanszky, A.; Uzinger, N.; Gyulai, G.; Mathis, A.; Junge, R.; Villarroel, M.; Kotzen, B.; Komives, T. Nutrient supply of plants in aquaponic systems. Ecocycles 2016, 2, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Trejo-Téllez, L.I.; Gómez-Merino, F.C. Nutrient solutions for hydroponic systems. Hydroponics A Stand. Methodol. Plant Biol. Res. 2012, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Sharafzadeh, S.; Alizadeh, O. Nutrient supply and fertilization of basil. Adv. Environ. Biol. 2011, 5, 956–960. [Google Scholar]
- López-Millán, A.F.; Grusak, M.A.; Abadía, A.; Abadía, J. Iron deficiency in plants: An insight from proteomic approaches. Front. Plant Sci. 2013, 4, 254. [Google Scholar] [CrossRef] [Green Version]
- Kiferle, C.; Maggini, R.; Pardossi, A. Influence of nitrogen nutrition on growth and accumulation of rosmarinic acid in sweet basil (Ocimum basilicum L.) grown in hydroponic culture. Aust. J. Crop Sci. 2013, 7, 321–327. [Google Scholar]
- Elansary, H.O.; Yessoufou, K.; Shokralla, S.; Mahmoud, E.A.; Skalicka-Woźniak, K. Enhancing mint and basil oil composition and antibacterial activity using seaweed extracts. Ind. Crop. Prod. 2016, 92, 50–56. [Google Scholar] [CrossRef]
- Eck, M.; Sare, A.R.; Massart, S.; Schmautz, Z.; Junge, R.; Smits, T.H.M.; Jijakli, M.H. Exploring Bacterial Communities in Aquaponic Systems. Water 2019, 11, 260. [Google Scholar] [CrossRef] [Green Version]
- Janpen, C.; Kanthawang, N.; Inkham, C.; Tsan, F.Y.; Sommano, S.R. Physiological responses of hydroponically-grown Japanese mint under nutrient deficiency. PeerJ 2019, 7, e7751. [Google Scholar] [CrossRef]
- Pribbernow, M.; University of Rostock, Rostock, Germany; Knaus, U.; University of Rostock, Rostock, Germany; Xu, L.; University of Rostock, Rostock, Germany; Appelbaum, S.; Ben-Gurion University of the Negev, Midreshet Ben-Gurion, Israel; Palm, H.W.; University of Rostock, Rostock, Germany. Personal communication, 2020.
- Dou, H.; Niu, G.; Gu, M. Pre-harvest UV-B radiation and photosynthetic photon flux density interactively affect plant photosynthesis, growth, and secondary metabolites accumulation in basil (Ocimum basilicum) plants. Agronomy 2019, 9, 434. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, S.N.; Araujo, G.D.S.; Viana, A.E.S. Growth of sweet basil depending on nitrogen and potassium doses. Hortic. Bras. 2013, 31, 489–493. [Google Scholar] [CrossRef] [Green Version]
- Meng, Q.; Runkle, E.S. Far-red radiation interacts with relative and absolute blue and red photon flux densities to regulate growth, morphology, and pigmentation of lettuce and basil seedlings. Sci. Hortic. 2019, 255, 269–280. [Google Scholar] [CrossRef]
- Ramin, A.A. Effects of salinity and temperature on germination and seedling establishment of sweet basil (Ocimum basilicum L.). J. Herbs Spices Med. Plants 2006, 11, 81–90. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Kumar, S.; Sharma, R.S. Ocimum as a promising commercial crop. In The Ocimum Genome, Compendium of Plant Genomes; Shasany, A.K., Kole, C., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 1–7. [Google Scholar]
- Elhindi, K.M.; El-Din, A.S.; Elgorban, A.M. The impact of arbuscular mycorrhizal fungi in mitigating salt-induced adverse effects in sweet basil (Ocimum basilicum L.). Saudi J. Biol. Sci. 2017, 24, 170–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Parameters | Weight Class 1 | Weight Class 2 | Weight Class 3 | P-I 1 | P-II 1 | P-III 1 |
|---|---|---|---|---|---|---|
| Fish initial length (cm) | 55.76 ± 4.90 a,2 | 43.39 ± 2.99 b | 35.51 ± 2.18 c | 0.001 | 0.001 | 0.001 |
| Fish final length (cm) | 56.90 ± 2.54 a | 48.31 ± 2.57 b | 39.92 ± 2.96 b | 0.001 | 0.038 | 0.078 |
| Fish initial weight (g) | 1310.09 ± 29.00 a | 623.74 ± 14.90 b | 297.13 ± 6.52 c | 0.001 | 0.001 | 0.001 |
| Fish final weight (g) | 1395.30 ± 15.32 a | 702.22 ± 12.54 b | 377.81 ± 7.73 c | 0.001 | 0.001 | 0.001 |
| Tank initial mass (kg) | 152.83 ± 2.64 a | 81.27 ± 0.37 b | 37.63 ± 0.87 c | 0.001 | 0.001 | 0.001 |
| Tank final mass (kg) | 161.66 ± 1.80 a | 90.45 ± 2.55 b | 46.34 ± 0.44 c | 0.001 | 0.001 | 0.001 |
| Growth per tank (kg) | 8.83 ± 3.48 a | 9.18 ± 2.53 a | 8.71 ± 0.62 a | 0.984 | 0.998 | 0.973 |
| Growth per tank (%) | 5.80 ± 2.37 b | 11.29 ± 3.11 b | 23.19 ± 2.10 a | 0.087 | 0.003 | 0.001 |
| Feed conversion ratio (FCR) 3 | 2.40 ± 0.85 a | 1.43 ± 0.45 ab | 0.94 ± 0.07 b | 0.161 | 0.042 | 0.561 |
| Specific growth rate (%/day) 3 | 0.16 ± 0.06 b | 0.30 ± 0.08 b | 0.58 ± 0.05 a | 0.080 | 0.001 | 0.004 |
| Condition factor (C) | 0.70 ± 0.09 a | 0.69 ± 0.09 a | 0.67 ± 0.15 a | 0.977 | 0.977 | 0.977 |
| Growth Parameters | DRF | Raft | Grow Pipes | P-I 1 | P-II 1 | P-III 1 |
| Total wet weight (g) | 107.70 ± 34.03 a,2 | 82.02 ± 22.74 b | 77.86 ± 23.93 b | 0.006 | 0.002 | 0.755 |
| Total height 3 (cm) | 108.41 ± 13.45 a | 99.66 ± 10.80 a | 106.20 ± 18.94 a | 0.142 | 0.879 | 0.330 |
| Leaf mass wet weight (g) | 45.36 ± 13.53 a | 34.94 ± 9.44 b | 32.74 ± 9.84 b | 0.010 | 0.001 | 0.796 |
| Leaves (No.) | 108.05 ± 47.43 a | 83.81 ± 19.64 a | 89.90 ± 26.84 a | 0.152 | 0.152 | 0.152 |
| Shoot axis wet weight (g) | 27.33 ± 10.11 a | 18.42 ± 6.73 b | 20.01 ± 7.29 b | 0.002 | 0.022 | 0.424 |
| Shoot axis height (cm) | 54.09 ± 8.86 a | 46.78 ± 7.22 b | 55.75 ± 10.65 a | 0.010 | 0.520 | 0.001 |
| Root wet weight (g) | 35.00 ± 12.03 a | 28.65 ± 7.69 a b | 25.11 ± 8.40 b | 0.178 | 0.005 | 0.629 |
| Root length (cm) | 54.32 ± 8.53 a | 53.60 ± 10.00 a | 50.46 ± 11.77 a | 0.971 | 0.440 | 0.581 |
| Leaf wet weight 4 (g) | 1.84 ± 0.49 a | 1.83 ± 0.38 a | 1.53 ± 0.28 b | 0.995 | 0.039 | 0.049 |
| Leaf length 4 (cm) | 14.29 ± 1.62 a | 13.12 ± 1.72 b | 12.77 ± 1.25 b | 0.043 | 0.006 | 0.744 |
| Leaf width 4 (cm) | 9.18 ± 1.33 a | 8.50 ± 1.08 ab | 8.31 ± 1.06 b | 0.147 | 0.049 | 0.869 |
| Leaf SPAD-Value3 | 29.76 ± 2.61 a | 29.09 ± 2.67 a | 28.63 ± 2.23 a | 0.659 | 0.316 | 0.826 |
| Dry Weight Parameters | DRF | Raft | Grow Pipes | P-I 1 | P-II 1 | P-III 1 |
| Total dry weight (g) | 10.38 ± 3.44 a | 8.07 ± 1.92 b | 8.21 ± 2.35 a b | 0.034 | 0.064 | 0.996 |
| Leaf dry weight (g) | 4.96 ± 1.57 a | 3.74 ± 1.04 b | 3.75 ± 1.22 b | 0.009 | 0.009 | 0.999 |
| Shoot axis dry weight (g) | 3.03 ± 1.33 a | 1.90 ± 0.69 b | 2.04 ± 0.80 b | 0.019 | 0.033 | 0.833 |
| Root dry weight (g) | 2.39 ± 0.81 a | 2.44 ± 0.41 a | 2.43 ± 0.44 a | 0.994 | 0.997 | 0.999 |
| Total Weight Parameters 5 | DRF | Raft | Grow Pipes | |||
| Total wet weight (g) | 2261.68 | 1722.31 | 1635.04 | - | - | - |
| Total leaf wet weight (g) | 952.66 | 733.77 | 687.50 | - | - | - |
| Total dry weight (g) | 218.06 | 169.55 | 172.36 | - | - | - |
| Total leaf dry weight (g) | 104.23 | 78.45 | 78.65 | - | - | - |
| Light Parameters | DRF | Raft | Grow Pipes | P-I 1 | P-II 1 | P-III 1 |
|---|---|---|---|---|---|---|
| PPFD 2 (μm/m2s) | 155.83 ± 32.29 b,3 | 157.92 ± 32.14 b | 173.10 ± 22.40 a | 0.404 | 0.001 | 0.002 |
| Light (lx (×10)) | 985.00 ± 116.70 a | 1013.00 ± 93.40 a | 1024.00 ± 161.30 a | 0.063 | 0.063 | 0.063 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasch, J.; Ratajczak, B.; Appelbaum, S.; Palm, H.W.; Knaus, U. Growth of Basil (Ocimum basilicum) in DRF, Raft, and Grow Pipes with Effluents of African Catfish (Clarias gariepinus) in Decoupled Aquaponics. AgriEngineering 2021, 3, 92-109. https://0-doi-org.brum.beds.ac.uk/10.3390/agriengineering3010006
Pasch J, Ratajczak B, Appelbaum S, Palm HW, Knaus U. Growth of Basil (Ocimum basilicum) in DRF, Raft, and Grow Pipes with Effluents of African Catfish (Clarias gariepinus) in Decoupled Aquaponics. AgriEngineering. 2021; 3(1):92-109. https://0-doi-org.brum.beds.ac.uk/10.3390/agriengineering3010006
Chicago/Turabian StylePasch, Johannes, Benny Ratajczak, Samuel Appelbaum, Harry W. Palm, and Ulrich Knaus. 2021. "Growth of Basil (Ocimum basilicum) in DRF, Raft, and Grow Pipes with Effluents of African Catfish (Clarias gariepinus) in Decoupled Aquaponics" AgriEngineering 3, no. 1: 92-109. https://0-doi-org.brum.beds.ac.uk/10.3390/agriengineering3010006






