Growth of Basil (Ocimum basilicum) in Aeroponics, DRF, and Raft Systems with Effluents of African Catfish (Clarias gariepinus) in Decoupled Aquaponics (s.s.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydroponics Unit
2.1.1. Dynamic Root Floating Technique (DRF)
2.1.2. Floating Raft Culture (Raft)
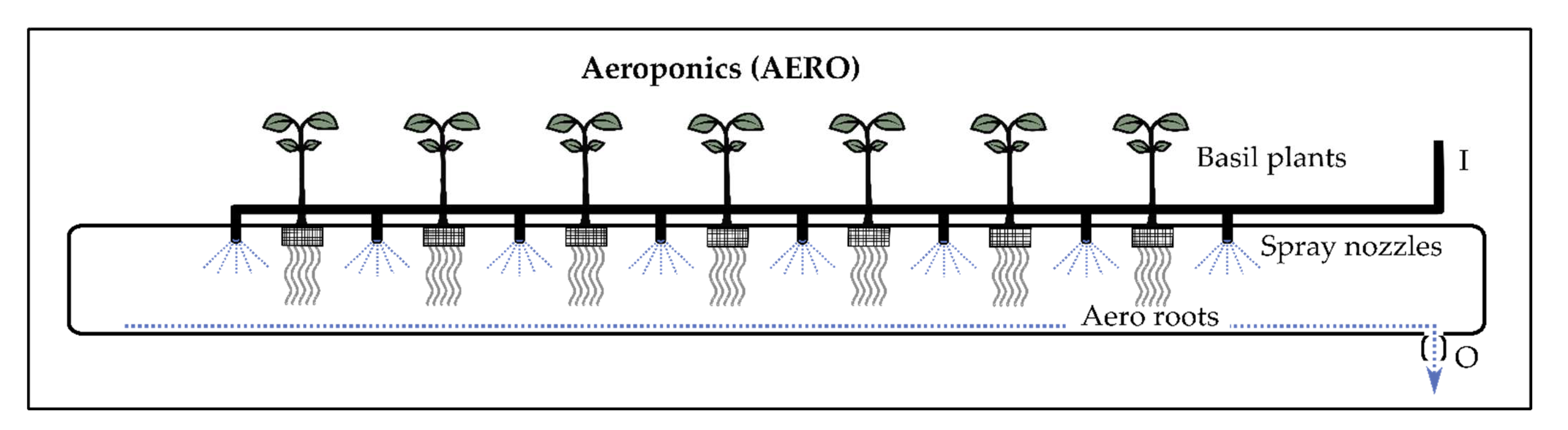
2.1.3. Aeroponics (AERO)
2.1.4. Fish Production and Feeding
2.2. Plant Cultivation
2.3. Physicochemical Parameters
2.4. Mathematical and Statistical Analysis
3. Results
3.1. Fish Growth
3.2. Plant Growth
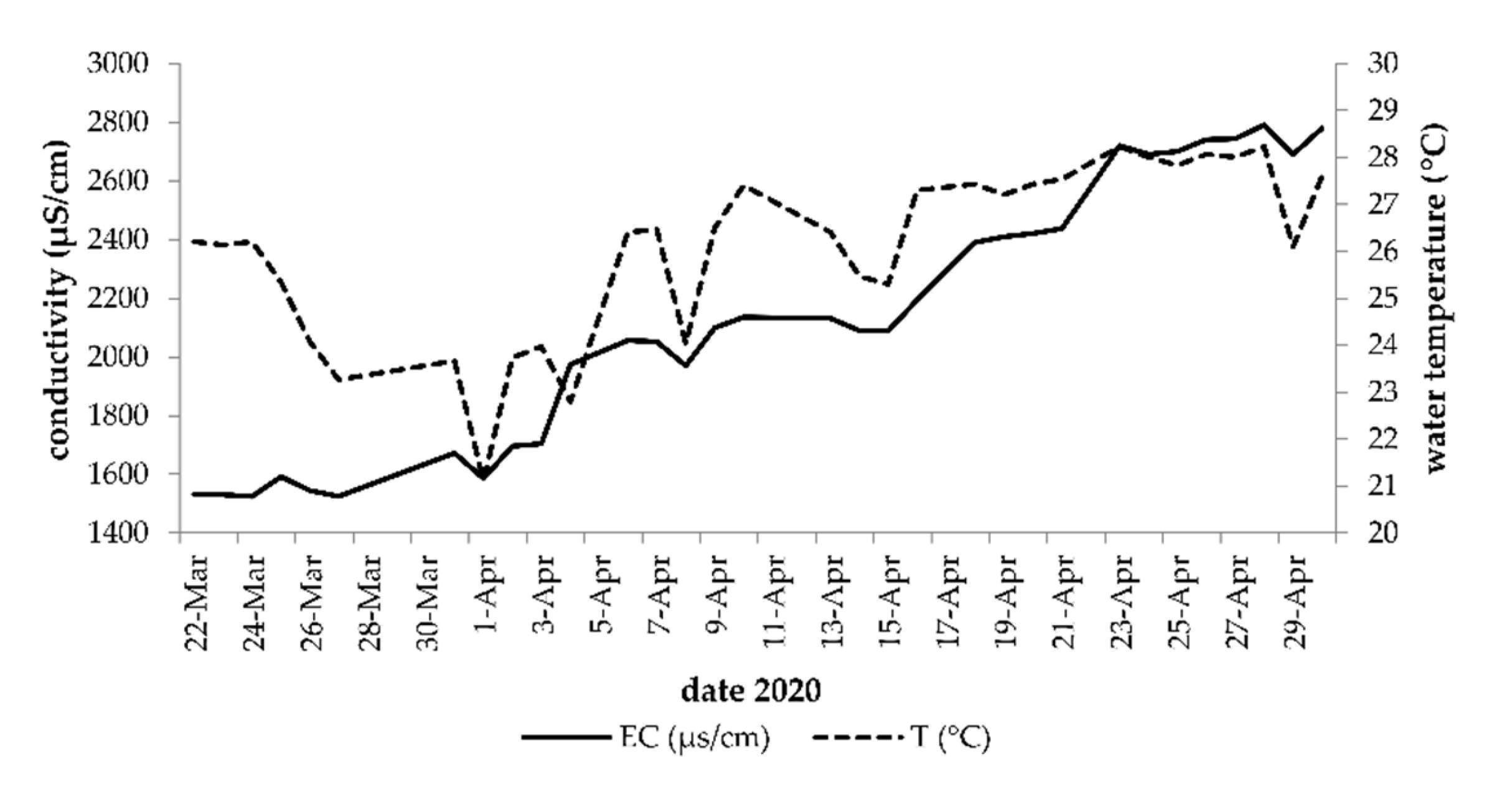
3.3. Physicochemical Parameters
4. Discussion
4.1. Fish Growth
4.2. Physicochemical Parameters
4.3. Plant Growth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palm, H.W.; Knaus, U.; Appelbaum, S.; Goddek, S.; Strauch, S.M.; Vermeulen, T.; Jijakli, M.H.; Kotzen, B. Towards commercial aquaponics: A review of systems, designs, scales and nomenclature. Aquac. Int. 2018, 26, 813–842. [Google Scholar] [CrossRef]
- Love, D.C.; Fry, J.P.; Li, X.; Hill, E.S.; Genello, L.; Semmens, K.; Thompson, R.E. Commercial aquaponics production and profitability: Findings from an international survey. Aquaculture 2015, 435, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Rakocy, J.E. Aquaponics: Integrating fish and plant culture. Aquac. Prod. Syst. 2012, 1, 343–386. [Google Scholar]
- Simon, J.E.; Quinn, J.; Murray, R.G. Basil: A source of essential oils. In Advances in New Crops, 1st ed.; Janick, J., Simon, J.E., Eds.; Timber Press: Portland, OR, USA, 1990; Volume 1, pp. 484–489. [Google Scholar]
- Makri, O.; Kintzios, S. Ocimum sp.(basil): Botany, cultivation, pharmaceutical properties, and biotechnology. J. Herbs Spices Med. Plants 2008, 13, 123–150. [Google Scholar] [CrossRef]
- Beaman, A.R. Irradiance, Total Nitrogen, and Nitrate-N: Ammonium-N Ratio Requirements for Optimal Edible Biomass Production of Basil. Master’s Thesis, Iowa State University, Ames, IA, USA, 2008; p. 64. [Google Scholar]
- Knaus, U.; Pribbernow, M.; Xu, L.; Appelbaum, S.; Palm, H.W. Basil (Ocimum basilicum) cultivation in decoupled aquaponics with three hydro-components (grow pipes, raft, gravel) and African catfish (Clarias gariepinus) production in Northern Germany. Sustainability 2020, 12, 8745. [Google Scholar] [CrossRef]
- Dunwoody, R.K. Aquaponics and Hydroponics: The Effects of Nutrient Source and Hydroponic Subsystem Design on Sweet Basil Production. Master’s Thesis, University of Central Missouri, Warrensburg, MO, USA, 2013. [Google Scholar]
- Raimondi, G.; Orsini, F.; Maggio, A.; De Pascale, S.; Barbieri, G. Yield and quality of hydroponically grown sweet basil cultivars. In Proceedings of the I International Symposium on the Labiatae: Advances in Production, Biotechnology and Utilisation, Sanremo, Italy, 30 November 2006; pp. 357–360. [Google Scholar]
- Baßmann, B.; Harbach, H.; Weißbach, S.; Palm, H.W. Effect of plant density in coupled aquaponics on the welfare status of African catfish Clarias gariepinus. J. World Aquac. Soc. 2018, 51, 183–199. [Google Scholar] [CrossRef]
- FAO.org. Available online: http://www.fao.org/fishery/species/2982/en (accessed on 16 December 2020).
- Knaus, U.; Wenzel, L.C.; Appelbaum, S.; Palm, H.W. Aquaponics (s.l.) Production of spearmint (Mentha spicata) with African catfish (Clarias gariepinus) in Northern Germany. Sustainability 2020, 12, 8717. [Google Scholar] [CrossRef]
- Knaus, U.; Palm, H.W. Effects of fish biology on ebb and flow aquaponical cultured herbs in northern Germany (Mecklenburg Western Pomerania). Aquaculture 2017, 466, 51–63. [Google Scholar] [CrossRef]
- Palm, H.W.; Bissa, K.; Knaus, U. Significant factors affecting the economic sustainability of closed aquaponic systems; Part II: Fish and plant growth. AACL Bioflux 2014, 7, 162–175. [Google Scholar]
- Pantanella, E. Nutrition and Quality of Aquaponic Systems. Ph.D. Thesis, Universita degli Studi della Tuscia, Viterbo, Italy, 2012. [Google Scholar]
- Walters, K.J.; Currey, C.J. Hydroponic greenhouse basil production: Comparing systems and cultivars. HortTechnology 2015, 25, 645–650. [Google Scholar] [CrossRef] [Green Version]
- Pasch, J.; Ratajczak, B.; Appelbaum, S.; Palm, H.W.; Knaus, U. Growth of basil (Ocimum basilicum) in DRF, raft, and grow pipes with effluents of African catfish (Clarias gariepinus) in decoupled aquaponics. AgriEngineering 2021, 3, 92–109. [Google Scholar] [CrossRef]
- Pasch, J. Einfluss Einer Wurzel-Belüftung auf das Wachstum der Marokkanischen Minze (Mentha spicata L.) bei Drei Berschiedenen Hydroponik-Subsystemen unter Aquaponischer Produktion. Master´s Thesis, University of Rostock, Rostock, Germany, 2018. [Google Scholar]
- Silva, L.; Gasca-Leyva, E.; Escalante, E.; Fitzsimmons, K.M.; Lozano, D.V. Evaluation of biomass yield and water treatment in two aquaponic systems using the dynamic root floating technique (DRF). Sustainability 2015, 7, 15384–15399. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.; Valdés-Lozano, D.; Escalante, E.; Gasca-Leyva, E. Dynamic root floating technique: An option to reduce electric power consumption in aquaponic systems. J. Clean. Prod. 2018, 183, 132–142. [Google Scholar] [CrossRef]
- Kao, T.C.; Hsiang, T.; Changhua, R.O.C. The Dynamic Root Floating Hydroponic Technique: Year-Round Production of Vegetables in Roc on Taiwan; ASPAC Food & Fertilizer Technology Centerz: Changhua, Taiwan, 1991. [Google Scholar]
- Buckseth, T.; Sharma, A.K.; Pandey, K.K.; Singh, B.P.; Muthuraj, R. Methods of pre-basic seed potato production with special reference to aeroponics—A review. Sci. Hortic. 2016, 204, 79–87. [Google Scholar] [CrossRef]
- Barak, P.; Smith, J.D.; Krueger, A.R.; Peterson, L.A. Measurement of short-term nutrient uptake rates in cranberry by aeroponics. Plant. Cell Environ. 1996, 19, 237–242. [Google Scholar] [CrossRef]
- Mbiyu, M.W.; Muthoni, J.; Kabira, J.; Elmar, G.; Muchira, C.; Pwaipwai, P.; Pwaipwai, J.; Otieno, S.; Onditi, J. Use of aeroponics technique for potato (Solanum tuberosum) minitubers production in Kenya. J. Hortic. For. 2012, 4, 172–177. [Google Scholar]
- Salachas, G.; Savvas, D.; Argyropoulou, K.; Tarantillis, P.A.; Kapotis, G. Yield and nutritional quality of aeroponically cultivated basil as affected by the available root-zone volume. Emir. J. Food Agric. 2015, 27, 911–918. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Li, X.; Tang, B.; Gu, M. Growth responses and root characteristics of lettuce grown in aeroponics, hydroponics, and substrate culture. Horticulturae 2018, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- NASA. Environmental and Agricultural Resources, progressive plant growing has business blooming. In NASA Spinoff; NASA: New York, NY, USA, 2006; pp. 64–77. [Google Scholar]
- Clawson, J.M.; Hoehn, A.; Stodieck, L.S.; Todd, P.; Stoner, R.J. Re-Examining Aeroponics for Spaceflight Plant Growth; SAE Technical Paper 2000-01-2507; SAE: Toulouse, France, 2000; p. 9. [Google Scholar]
- Farran, I.; Mingo-Castel, A.M. Potato minituber production using aeroponics: Effect of plant density and harvesting intervals. Am. J. Potato Res. 2006, 83, 47–53. [Google Scholar] [CrossRef]
- Tabatabaei, S.J. Effects of cultivation systems on the growth, and essential oil content and composition of valerian. J. Herbs Spices Med. Plants 2008, 14, 54–67. [Google Scholar] [CrossRef]
- Phan-Huy, S.A. Hydroponic products in Switzerland. Agrar Forsch. 1994, 1, 367–370. [Google Scholar]
- Microsoft® Corporation. Microsoft Excel®, Microsoft® Corporation: Redmond, WA, USA, 2010.
- IBM Deutschland GmbH. IBM SPSS Statistics for Windows, Version 20.0; IBM Deutschland GmbH: Ehningen, Germany, 2011.
- Zimmermann, J. Vergleich des Wachstums von Marokkanischer Minze (Mentha spicata) in Drei Verschiedenen Hydroponik Subsystemen unter Aquaponischer Produktion. Master´s Thesis, University of Rostock, Rostock, Germany, 2017. [Google Scholar]
- Martins, C.I.; Aanyu, M.; Schrama, J.W.; Verreth, J.A. Size distribution in African catfish (Clarias gariepinus) affects feeding behaviour but not growth. Aquaculture 2005, 250, 300–307. [Google Scholar] [CrossRef]
- Hepher, B. Nutrition of Pond Fishes; Cambridge University Press: Cambridge, UK, 1988; p. 388. [Google Scholar]
- Degani, G.; Ben-Zvi, Y.; Levanon, D. The relationship between body size and growth of African catfish (Clarias gariepinus) (Burchell, 1922) fed practical diet. Indian J. Fish. 1988, 35, 207–210. [Google Scholar]
- Anene, A. Condition factor of four Cichlid species of a man-made lake in Imo State, Southeastern Nigeria. Turk. J. Fish. Aquat. Sci. 2005, 5, 43–47. [Google Scholar]
- Saha, S.; Monroe, A.; Day, M.R. Growth, yield, plant quality and nutrition of basil (Ocimum basilicum L.) under soilless agricultural systems. Ann. Agric. Sci. 2016, 61, 181–186. [Google Scholar] [CrossRef]
- Somerville, C.; Cohen, M.; Pantanella, E.; Stankus, A.; Lovatelli, A. Small-Scale Aquaponic Food Production: Integrated Fish and Plant Farming; FAO Fisheries and Aquaculture Technical Paper No. 589; FAO: Rome, Italy, 2014; p. 262. [Google Scholar]
- Saaid, M.F.; Sanuddin, A.; Ali, M.; Yassin, M.S.A.I.M. Automated pH controller system for hydroponic cultivation. In Proceedings of the 2015 IEEE Symposium on Computer Applications & Industrial Electronics (ISCAIE), Langkawi, Malaysia, 12–14 April 2015; IEEE: New York, NY, USA, 2015; pp. 186–190. [Google Scholar]
- Wortman, S.E. Crop physiological response to nutrient solution electrical conductivity and pH in an ebb-and-flow hydroponic system. Sci. Hortic. 2015, 194, 34–42. [Google Scholar] [CrossRef]
- Rakocy, J.E.; Masser, M.P.; Losordo, T.M. Recirculating Aquaculture Tank Production Systems: Aquaponics—Integrating Fish and Plant Culture; SRAC Publication No. 454; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2006; 16p. [Google Scholar]
- Morano, G.; Amalfitano, C.; Sellitto, M.; Cuciniello, A.; Maiello, R.; Caruso, G. Effects of nutritive solution electrical conductivity and plant density on growth, yield and quality of sweet basil grown in gullies by subirrigation. Adv. Hortic. Sci. 2017, 31, 25–30. [Google Scholar]
- Trejo-Téllez, L.I.; Gómez-Merino, F.C. Nutrient solutions for hydroponic systems. In Hydroponics: A Standard Methodology for Plant Biological Researches, 1st ed.; Asao, T., Ed.; InTech: Rijeka, Croatia, 2012; Volume 1, pp. 1–22. [Google Scholar]
- Bittsanszky, A.; Uzinger, N.; Gyulai, G.; Mathis, A.; Junge, R.; Villarroel, M.; Kotzen, B.; Komives, T. Nutrient supply of plants in aquaponic systems. Ecocycles 2016, 2, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Tyson, R.V.; Treadwell, D.D.; Simonne, E.H. Opportunities and challenges to sustainability in aquaponic systems. HortTechnology 2011, 21, 6–13. [Google Scholar] [CrossRef]
- Frerichs, C.; Daum, D.; Koch, R. Ammonium toxicity-one cause for growth and quality impairments on organic fertilized basil? J. Kult. 2017, 69, 101–112. [Google Scholar]
- López-Millán, A.F.; Grusak, M.A.; Abadía, A.; Abadía, J. Iron deficiency in plants: An insight from proteomic approaches. Front. Plant Sci. 2013, 4, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafsi, C.; Debez, A.; Abdelly, C. Potassium deficiency in plants: Effects and signaling cascades. Acta Physiol. Plant. 2014, 36, 1055–1070. [Google Scholar] [CrossRef]
- Lucena, J.J. Synthetic iron chelates to correct iron deficiency in plants. In Iron Nutrition in Plants and Rhizospheric Microorganisms; Barton, L.L., Abadia, J., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 103–128. [Google Scholar]
- Teliban, G.C.; Stoleru, V.; Burducea, M.; Lobiuc, A.; Munteanu, N.; Popa, L.D.; Caruso, G. Biochemical, physiological and yield characteristics of red basil as affected by cultivar and fertilization. Agriculture 2020, 10, 48. [Google Scholar] [CrossRef] [Green Version]
- Sifola, M.I.; Barbieri, G. Growth, yield and essential oil content of three cultivars of basil grown under different levels of nitrogen in the field. Sci. Hortic. 2006, 108, 408–413. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K.; Shokralla, S.; Mahmoud, E.A.; Skalicka-Woźniak, K. Enhancing mint and basil oil composition and antibacterial activity using seaweed extracts. Ind. Crop. Prod. 2016, 92, 50–56. [Google Scholar] [CrossRef]
- Ekren, S.; Sönmez, Ç.; Özçakal, E.; Kurttaş, Y.S.K.; Bayram, E.; Gürgülü, H. The effect of different irrigation water levels on yield and quality characteristics of purple basil (Ocimum basilicum L.). Agric. Water Manag. 2012, 109, 155–161. [Google Scholar] [CrossRef]
- Teixeira, J.S.; Ten Tusscher, K.H. The systems biology of lateral root formation: Connecting the dots. Mol. Plant 2019, 12, 784–803. [Google Scholar] [CrossRef] [PubMed]
- Valle-Echevarria, D.; Angel, R.; Kantar, M.B.; Branca, J.; Moore, S.; Frederiksen, M.K.; Hagen, L.; Hussain, T.; Baumler, D.J. Aeroponic cloning of Capsicum spp. Horticulturae 2019, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Sharma, U.; Barupal, M.; Shekhawat, N.S.; Kataria, V. Aeroponics for propagation of horticultural plants: An approach for vertical farming. Hortic. Int. J. 2018, 2, 443–444. [Google Scholar] [CrossRef] [Green Version]
- Mehandru, P.; Shekhawat, N.S.; Rai, M.K.; Kataria, V.; Gehlot, H.S. Evaluation of aeroponics for clonal propagation of Caralluma edulis, Leptadenia reticulata and Tylophora indica—Three threatened medicinal Asclepiads. Physiol. Mol. Biol. Plants 2014, 20, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.C.; Park, C.S.; Kim, S.Y.; Lee, Y.B. Growth and tuberization of hydroponically grown potatoes. Potato Res. 2012, 55, 69–81. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, R. Aeroponics: A Review on Modern Agriculture Technology. Indian Farmer 2019, 6, 286–292. [Google Scholar]





| Parameters | Weight Class 1 | Weight Class 2 | Weight Class 3 | P-I 1 | P-II 1 | P-III 1 |
|---|---|---|---|---|---|---|
| Fish initial length (cm) | 56.93 ± 1.38 a,2 | 48.10 ± 1.81 b | 17.33 ± 1.58 c | 0.001 | 0.001 | 0.001 |
| Fish final length (cm) | 57.43 ± 3.01 a | 53.26 ± 1.98 b | 27.81 ± 1.28 c | 0.001 | 0.001 | 0.001 |
| Fish initial weight (g) | 1266.68 ± 86.46 a | 838.46 ± 17.66 b | 42.78 ± 0.69 c | 0.023 | 0.003 | 0.001 |
| Fish final weight (g) | 1571.48 ± 44.06 a | 1147.13 ± 11.52 b | 133.24 ± 2.20 c | 0.005 | 0.001 | 0.001 |
| Tank initial mass (kg) | 148.00 ± 3.69 a | 100.71 ± 2.29 b | 5.13 ± 0.08 c | 0.001 | 0.001 | 0.001 |
| Tank final mass (kg) | 182.74 ± 1.71 a | 133.44 ± 3.21 b | 15.63 ± 0.30 c | 0.001 | 0.001 | 0.001 |
| Growth per tank (kg) | 34.74 ± 2.00 a | 32.73 ± 1.07 a | 10.50 ± 0.25 b | 0.226 | 0.001 | 0.001 |
| Growth per tank (%) | 23.50 ± 1.93 c | 32.73 ± 0.65 b | 204.56 ± 4.29 a | 0.020 | 0.001 | 0.001 |
| Feed conversion ratio (FCR) 3 | 1.27 ± 0.09 a | 1.17 ± 0.03 a | 0.84 ± 0.02 b | 0.132 | 0.001 | 0.001 |
| Specific growth rate (%/day) 3 | 0.45 ± 0.03 c | 0.60 ± 0.01 b | 2.37 ± 0.03 a | 0.001 | 0.001 | 0.001 |
| Condition factor (C) | 0.83 ± 0.10 a | 0.76 ± 0.07 a | 0.81 ± 0.15 a | 0.447 | 0.931 | 0.665 |
| Growth Parameters | DRF | Raft | AERO | P-I 1 | P-II 1 | P-III 1 |
|---|---|---|---|---|---|---|
| Total wet weight (g) | 46.80 ± 13.78 b,2 | 44.28 ± 13.19 b | 62.20 ± 17.01 a | 0.694 | 0.002 | 0.007 |
| Total height (cm) | 70.38 ± 10.40 a | 70.62 ± 10.46 a | 70.96 ± 6.81 a | 0.996 | 0.979 | 0.993 |
| Leaf mass wet weight (g) | 20.19 ± 6.57 b | 20.35 ± 7.14 b | 28.53 ± 8.74 a | 0.997 | 0.003 | 0.003 |
| Leaves (No.) | 102.25 ± 23.67 a | 101.33 ± 29.42 a | 119.45 ± 29.55 a | 0.994 | 0.131 | 0.100 |
| Shoot axis wet weight (g) | 12.48 ± 4.20 b | 12.22 ± 3.92 b | 17.93 ± 5.51 a | 0.996 | 0.004 | 0.002 |
| Shoot axis height (cm) | 40.81 ± 5.48 b | 42.22 ± 7.29 b | 52.04 ± 6.83 a | 0.772 | 0.001 | 0.001 |
| Root wet weight (g) | 14.13 ± 3.74 a | 11.71 ± 2.90 b | 15.72 ± 4.24 a | 0.031 | 0.364 | 0.002 |
| Root length (cm) | 29.58 ± 6.44 a | 28.41 ± 6.11 a | 18.80 ± 5.00 b | 0.800 | 0.001 | 0.001 |
| Leaf wet weight (mg) 3 | 696.83 ± 143.47 b | 704.62 ± 138.11 b | 789.33 ± 159.41 a | 0.927 | 0.031 | 0.036 |
| Leaf length (cm) 3 | 7.31 ± 0.69 b | 7.35 ± 0.72 b | 7.89 ± 0.74 a | 0.820 | 0.005 | 0.010 |
| Leaf width (cm) 3 | 4.81 ± 0.59 b | 4.79 ± 0.52 b | 5.14 ± 0.52 a | 0.928 | 0.017 | 0.020 |
| Leaf SPAD-Value 3 | 37.00 ± 2.86 a | 33.58 ± 3.15 b | 36.58 ± 4.08 a | 0.006 | 0.921 | 0.019 |
| Dry Weight Parameters | DRF | Raft | AERO | P-I 1 | P-II 1 | P-III 1 |
| Total dry weight (g) | 5.20 ± 1.63 b | 4.96 ± 1.78 b | 7.60 ± 2.21 a | 0.913 | 0.001 | 0.001 |
| Leaf mass dry weight (g) | 2.83 ± 0.90 b | 2.84 ± 1.04 b | 4.26 ± 1.23 a | 0.999 | 0.001 | 0.001 |
| Shoot axis dry weight (g) | 1.55 ± 0.50 b | 1.45 ± 0.55 b | 2.26 ± 0.75 a | 0.848 | 0.001 | 0.001 |
| Root dry weight (g) | 0.82 ± 0.36 b | 0.67 ± 0.27 b | 1.08 ± 0.38 a | 0.336 | 0.047 | 0.001 |
| Total Weight Parameters 4 | DRF | Raft | AERO | |||
| Total wet mass (g) | 935.90 | 929.90 | 1244.00 | - | - | - |
| Wet mass of leaves (g) | 403.80 | 427.40 | 570.60 | - | - | - |
| Total dry mass (g) | 103.95 | 104.10 | 151.99 | - | - | - |
| Dry mass of leaves (g) | 56.55 | 59.65 | 85.24 | - | - | - |
| Colorimeter Index 5 | DRF | Raft | AERO | P-I 1 | P-II 1 | P-III 1 |
| L | 42.96 ± 1.89 a | 44.36 ± 1.95 a | 44.24 ± 2.33 a | 0.084 | 0.134 | 0.983 |
| a | −13.46 ± 0.82 a | −14.11 ± 0.71 b | −13.60 ± 1.03 ab | 0.045 | 0.861 | 0.150 |
| b | 23.89 ± 2.04 b | 25.56 ± 1.59 a | 25.41 ± 2.01 a | 0.017 | 0.039 | 0.965 |
| Color Difference (∆Ex,y) | ∆EDRF,raft 6 | ∆Eraft,AERO 6 | ∆EDRF,AERO 6 | |||
| 2.59 | 0.15 | 1.98 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasch, J.; Appelbaum, S.; Palm, H.W.; Knaus, U. Growth of Basil (Ocimum basilicum) in Aeroponics, DRF, and Raft Systems with Effluents of African Catfish (Clarias gariepinus) in Decoupled Aquaponics (s.s.). AgriEngineering 2021, 3, 559-574. https://0-doi-org.brum.beds.ac.uk/10.3390/agriengineering3030036
Pasch J, Appelbaum S, Palm HW, Knaus U. Growth of Basil (Ocimum basilicum) in Aeroponics, DRF, and Raft Systems with Effluents of African Catfish (Clarias gariepinus) in Decoupled Aquaponics (s.s.). AgriEngineering. 2021; 3(3):559-574. https://0-doi-org.brum.beds.ac.uk/10.3390/agriengineering3030036
Chicago/Turabian StylePasch, Johannes, Samuel Appelbaum, Harry Wilhelm Palm, and Ulrich Knaus. 2021. "Growth of Basil (Ocimum basilicum) in Aeroponics, DRF, and Raft Systems with Effluents of African Catfish (Clarias gariepinus) in Decoupled Aquaponics (s.s.)" AgriEngineering 3, no. 3: 559-574. https://0-doi-org.brum.beds.ac.uk/10.3390/agriengineering3030036







