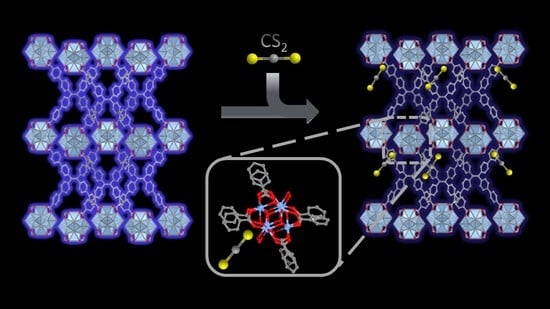
Fluorescent Detection of Carbon Disulfide by a Highly Emissive and Robust Isoreticular Series of Zr-Based Luminescent Metal Organic Frameworks (LMOFs)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
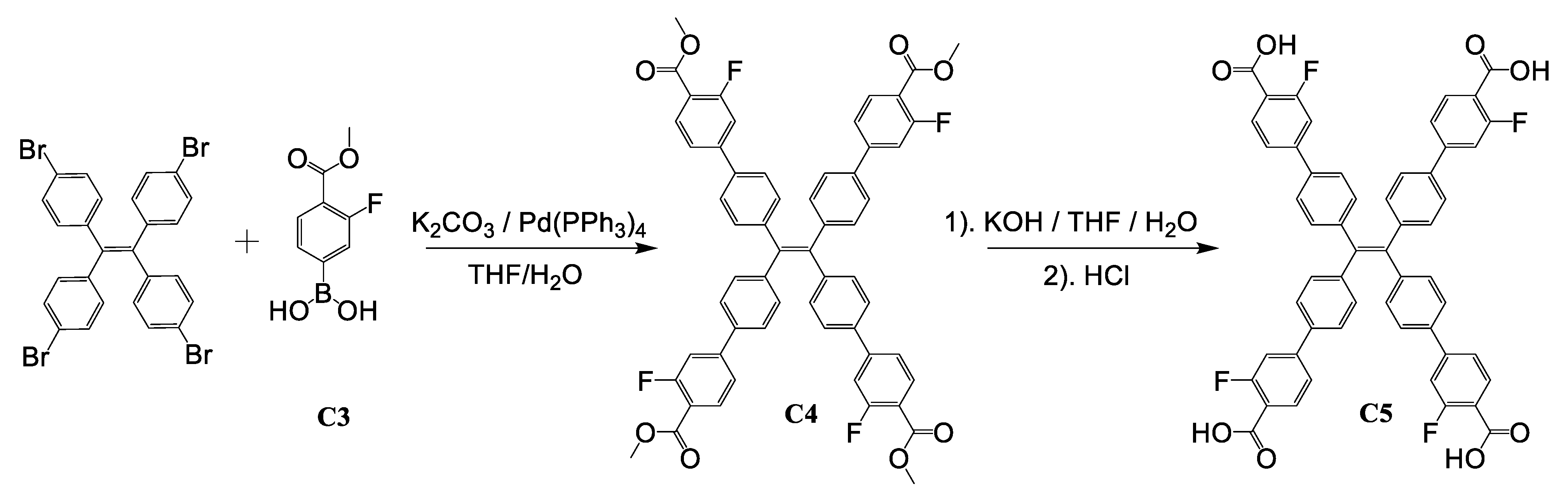
2.2. Synthesis of 1 and 2
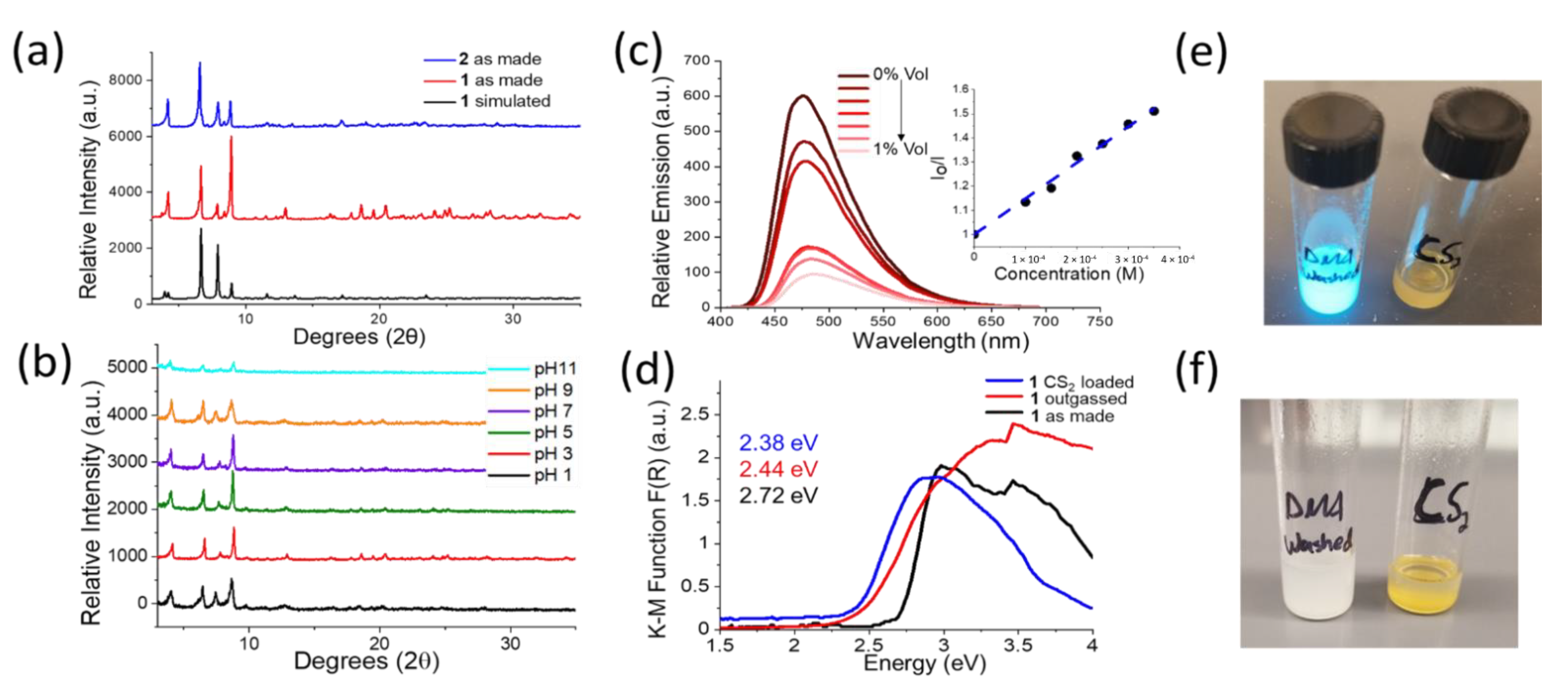
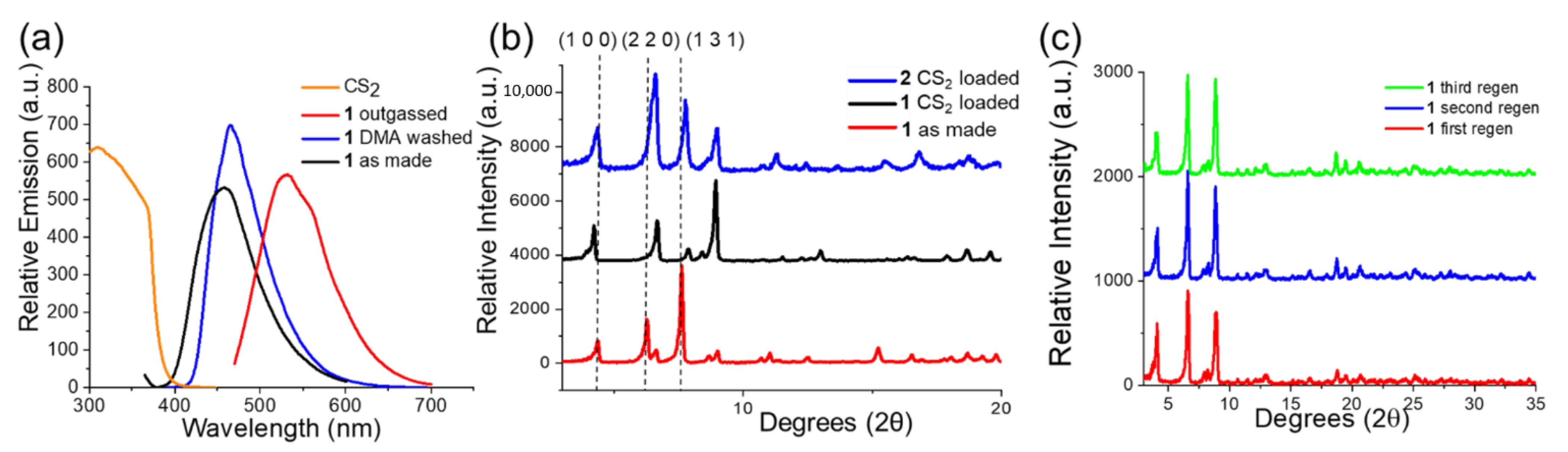
2.3. Powder X-ray Diffraction (PXRD)
2.4. Thermogravimetric Analysis (TGA)
2.5. Diffuse Reflectance Measurement(UV-VIS)
2.6. Photoluminescence Spectra (PL)
2.7. Internal Quantum Yield Measurements (QY)
2.8. Chemical Stability Experiments
2.9. Fluorescent Sensing Experiments
2.10. In-Situ Infrared Spectroscopy (FTIR)
2.11. Computational Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal-organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J. Microporous Metal-Organic Frameworks for Adsorptive Separation of C5-C6 Alkane Isomers. Acc. Chem. Res. 2019, 52, 1968–1978. [Google Scholar] [CrossRef]
- Adil, K.; Belmabkhout, Y.; Pillai, R.S.; Cadiau, A.; Bhatt, P.M.; Assen, A.H.; Maurin, G.; Eddaoudi, M. Gas/vapour separation using ultra-microporous metal-organic frameworks: Insights into the structure/separation relationship. Chem. Soc. Rev. 2017, 46, 3402–3430. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Chang, G.; Xing, H.; Krishna, R.; Ren, Q.; Chen, B. Potential of microporous metal–organic frameworks for separation of hydrocarbon mixtures. Energy Environ. Sci. 2016, 9, 3612–3641. [Google Scholar] [CrossRef]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal-organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal-organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, S.; Day, G.; Wang, X.; Yang, X.; Zhou, H.-C. Luminescent sensors based on metal-organic frameworks. Coord. Chem. Rev. 2018, 354, 28–45. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, D.-S.; Bu, X.; Feng, P. Metal–Organic Frameworks for Separation. Adv. Mater. 2018, 30, 1705189. [Google Scholar] [CrossRef]
- Zhai, Q.-G.; Bu, X.; Zhao, X.; Li, D.-S.; Feng, P. Pore Space Partition in Metal–Organic Frameworks. Acc. Chem. Res. 2017, 50, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J. Luminescent metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef]
- Lustig, W.P.; Li, J. Luminescent metal–organic frameworks and coordination polymers as alternative phosphors for energy efficient lighting devices. Coord. Chem. Rev. 2018, 373, 116–147. [Google Scholar] [CrossRef]
- Zhou, X.-S.; Fan, R.-Q.; Du, X.; Hao, S.-E.; Fang, R.; Wang, P.; Xing, K.; Wang, A.N.; Yang, Y.-L.; Liu, Z.-G. Key effect of robust π⋯π stacking on AIE performance for supramolecular indium(III)–organic assemblies and application in PMMA-doped hybrid material. Inorg. Chem. Commun. 2018, 90, 39–44. [Google Scholar] [CrossRef]
- Nagarkar, S.S.; Desai, A.V.; Ghosh, S.K. A fluorescent metal-organic framework for highly selective detection of nitro explosives in the aqueous phase. Chem. Commun. 2014, 50, 8915–8918. [Google Scholar] [CrossRef]
- Mollick, S.; Mandal, T.N.; Jana, A.; Fajal, S.; Desai, A.V.; Ghosh, S.K. Ultrastable Luminescent Hybrid Bromide Perovskite@MOF Nanocomposites for the Degradation of Organic Pollutants in Water. ACS Appl. Nano Mater. 2019, 2, 1333–1340. [Google Scholar] [CrossRef]
- Zhai, Z.-W.; Yang, S.-H.; Cao, M.; Li, L.-K.; Du, C.-X.; Zang, S.-Q. Rational Design of Three Two-Fold Interpenetrated Metal–Organic Frameworks: Luminescent Zn/Cd-Metal–Organic Frameworks for Detection of 2,4,6-Trinitrophenol and Nitrofurazone in the Aqueous Phase. Cryst. Growth Des. 2018, 18, 7173–7182. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Huang, H.-L.; Wang, J.-Y.; Li, H.-Y.; Zang, S.-Q. A Flexible Fluorescent SCC-MOF for Switchable Molecule Identification and Temperature Display. Chem. Mater. 2018, 30, 2160–2167. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal-organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [Green Version]
- Gargurevich, I.A. Hydrogen Sulfide Combustion: Relevant Issues under Claus Furnace Conditions. Ind. Eng. Chem. Res. 2005, 44, 7706–7729. [Google Scholar] [CrossRef]
- Meng, X.; de Jong, W.; Pal, R.; Verkooijen, A.H.M. In bed and downstream hot gas desulphurization during solid fuel gasification: A review. Fuel Process. Technol. 2010, 91, 964–981. [Google Scholar] [CrossRef]
- Glarborg, P.; Halaburt, B.; Marshall, P.; Guillory, A.; Troe, J.; Thellefsen, M.; Christensen, K. Oxidation of reduced sulfur species: Carbon disulfide. J. Phys. Chem. A 2014, 118, 6798–6809. [Google Scholar] [CrossRef] [Green Version]
- Anstey, M.; Sun, A.; Paap, S.M.; Foltz, G.; Jaeger, C.; Hoette, T.; Ochs, M.E. Inherently Safer Technology Gaps Analysis Study; United States. Department of Energy: Washington, DC, USA, 2012. [Google Scholar]
- Wayne, D.; Monnery, W.Y.S.; Behie, L.A. Modelling the modified claus process reaction furnace and the implications on plant design and recovery. Can. J. Chem. Eng. 1993, 71, 711–724. [Google Scholar]
- McGuirk, C.M.; Siegelman, R.L.; Drisdell, W.S.; Runcevski, T.; Milner, P.J.; Oktawiec, J.; Wan, L.F.; Su, G.M.; Jiang, H.Z.H.; Reed, D.A.; et al. Cooperative adsorption of carbon disulfide in diamine-appended metal-organic frameworks. Nat. Commun. 2018, 9, 5133. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.-F.; Tan, B.; Feng, M.-L.; Lan, A.-J.; Huang, X.-Y. A magnesium MOF as a sensitive fluorescence sensor for CS2 and nitroaromatic compounds. J. Mater. Chem. A 2014, 2, 6426–6431. [Google Scholar] [CrossRef]
- Dong, Y. Tetranuclear cluster-based Pb(II)-MOF: Synthesis, crystal structure and luminescence sensing for CS2. J. Mol. Struct. 2018, 1160, 46–49. [Google Scholar] [CrossRef]
- Wu, Z.-F.; Tan, B.; Feng, M.-L.; Du, C.-F.; Huang, X.-Y. A magnesium-carboxylate framework showing luminescent sensing for CS2 and nitroaromatic compounds. J. Solid State Chem. 2015, 223, 59–64. [Google Scholar] [CrossRef]
- Wu, Z.-F.; Tan, B.; Velasco, E.; Wang, H.; Shen, N.-N.; Gao, Y.-J.; Zhang, X.; Zhu, K.; Zhang, G.-Y.; Liu, Y.-Y.; et al. Fluorescent In based MOFs showing “turn on” luminescence towards thiols and acting as a ratiometric fluorescence thermometer. J. Mater. Chem. C 2019, 7, 3049–3055. [Google Scholar] [CrossRef]
- Wei, Z.; Gu, Z.Y.; Arvapally, R.K.; Chen, Y.P.; McDougald, R.N., Jr.; Ivy, J.F.; Yakovenko, A.A.; Feng, D.; Omary, M.A.; Zhou, H.C. Rigidifying fluorescent linkers by metal-organic framework formation for fluorescence blue shift and quantum yield enhancement. J. Am. Chem. Soc. 2014, 136, 8269–8276. [Google Scholar] [CrossRef] [PubMed]
- Lustig, W.P.; Wang, F.; Teat, S.J.; Hu, Z.; Gong, Q.; Li, J. Chromophore-Based Luminescent Metal-Organic Frameworks as Lighting Phosphors. Inorg. Chem. 2016, 55, 7250–7256. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for \textit{ab initio} total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Thonhauser, T.; Cooper, V.R.; Li, S.; Puzder, A.; Hyldgaard, P.; Langreth, D.C. Van der Waals density functional: Self-consistent potential and the nature of the van der Waals bond. Phys. Rev. B 2007, 76, 125112. [Google Scholar] [CrossRef] [Green Version]
- Langreth, D.C.; Lundqvist, B.I.; Chakarova-Käck, S.D.; Cooper, V.R.; Dion, M.; Hyldgaard, P.; Kelkkanen, A.; Kleis, J.; Lingzhu, K.; Shen, L.; et al. A density functional for sparse matter. J. Phys. Condens. Matter 2009, 21, 084203. [Google Scholar] [CrossRef] [Green Version]
- Kristian, B.; Valentino, R.C.; Kyuho, L.; Elsebeth, S.; Thonhauser, T.; Per, H.; Bengt, I.L. van der Waals forces in density functional theory: A review of the vdW-DF method. Rep. Prog. Phys. 2015, 78, 066501. [Google Scholar]
- Chen, C.-X.; Wei, Z.-W.; Cao, C.-C.; Yin, S.-Y.; Qiu, Q.-F.; Zhu, N.-X.; Xiong, Y.-Y.; Jiang, J.-J.; Pan, M.; Su, C.-Y. All Roads Lead to Rome: Tuning the Luminescence of a Breathing Catenated Zr-MOF by Programmable Multiplexing Pathways. Chem. Mater. 2019, 31, 5550–5557. [Google Scholar] [CrossRef]
- Hu, Z.; Huang, G.; Lustig, W.P.; Wang, F.; Wang, H.; Teat, S.J.; Banerjee, D.; Zhang, D.; Li, J. Achieving exceptionally high luminescence quantum efficiency by immobilizing an AIE molecular chromophore into a metal-organic framework. Chem. Commun. 2015, 51, 3045–3048. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, W.; Teat, S.J.; Xu, F.; Wang, H.; Wang, X.; An, L.; Li, J. Chromophore-immobilized luminescent metal-organic frameworks as potential lighting phosphors and chemical sensors. Chem. Commun. (Camb.) 2016, 52, 10249–10252. [Google Scholar] [CrossRef]
- Gong, Q.; Hu, Z.; Deibert, B.J.; Emge, T.J.; Teat, S.J.; Banerjee, D.; Mussman, B.; Rudd, N.D.; Li, J. Solution processable MOF yellow phosphor with exceptionally high quantum efficiency. J. Am. Chem. Soc. 2014, 136, 16724–16727. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Kalenak, A.P.; Wong-Foy, A.G.; Matzger, A.J. Rapid Guest Exchange and Ultra-Low Surface Tension Solvents Optimize Metal-Organic Framework Activation. Angew. Chem. Int. Ed. Engl. 2017, 56, 14618–14621. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Tan, K.; Lustig, W.P.; Wang, H.; Zhao, Y.; Zheng, C.; Banerjee, D.; Emge, T.J.; Chabal, Y.J.; Li, J. Effective sensing of RDX via instant and selective detection of ketone vapors. Chem. Sci. 2014, 5, 4873–4877. [Google Scholar] [CrossRef]
- Pramanik, S.; Hu, Z.; Zhang, X.; Zheng, C.; Kelly, S.; Li, J. A Systematic Study of Fluorescence-Based Detection of Nitroexplosives and Other Aromatics in the Vapor Phase by Microporous Metal–Organic Frameworks. Chem. A Eur. J. 2013, 19, 15964–15971. [Google Scholar] [CrossRef]
- Hu, Z.; Lustig, W.P.; Zhang, J.; Zheng, C.; Wang, H.; Teat, S.J.; Gong, Q.; Rudd, N.D.; Li, J. Effective Detection of Mycotoxins by a Highly Luminescent Metal–Organic Framework. J. Am. Chem. Soc. 2015, 137, 16209–16215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pramanik, S.; Zheng, C.; Zhang, X.; Emge, T.J.; Li, J. New Microporous Metal−Organic Framework Demonstrating Unique Selectivity for Detection of High Explosives and Aromatic Compounds. J. Am. Chem. Soc. 2011, 133, 4153–4155. [Google Scholar] [CrossRef] [PubMed]
- Lustig, W.P.; Shen, Z.; Teat, S.J.; Javed, N.; Velasco, E.; O’Carroll, D.M.; Li, J. Rational design of a high-efficiency, multivariate metal–organic framework phosphor for white LED bulbs. Chem. Sci. 2020, 11, 1814–1824. [Google Scholar] [CrossRef] [Green Version]
- Pang, J.; Yuan, S.; Qin, J.; Liu, C.; Lollar, C.; Wu, M.; Yuan, D.; Zhou, H.-C.; Hong, M. Control the Structure of Zr-Tetracarboxylate Frameworks through Steric Tuning. J. Am. Chem. Soc. 2017, 139, 16939–16945. [Google Scholar] [CrossRef]
- Grissom, T.G.; Driscoll, D.M.; Troya, D.; Sapienza, N.S.; Usov, P.M.; Morris, A.J.; Morris, J.R. Molecular-Level Insight into CO2 Adsorption on the Zirconium-Based Metal–Organic Framework, UiO-66: A Combined Spectroscopic and Computational Approach. J. Phys. Chem. C 2019, 123, 13731–13738. [Google Scholar] [CrossRef]
- Tan, K.; Jensen, S.; Feng, L.; Wang, H.; Yuan, S.; Ferreri, M.; Klesko, J.P.; Rahman, R.; Cure, J.; Li, J.; et al. Reactivity of Atomic Layer Deposition Precursors with OH/H2O-Containing Metal Organic Framework Materials. Chem. Mater. 2019, 31, 2286–2295. [Google Scholar] [CrossRef]
- Mivehvar, F.; Lauzin, C.; McKellar, A.R.W.; Moazzen-Ahmadi, N. Infrared spectra of rare gas–carbon disulfide complexes: He–CS2, Ne–CS2, and Ar–CS2. J. Mol. Spectrosc. 2012, 281, 24–27. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velasco, E.; Osumi, Y.; Teat, S.J.; Jensen, S.; Tan, K.; Thonhauser, T.; Li, J. Fluorescent Detection of Carbon Disulfide by a Highly Emissive and Robust Isoreticular Series of Zr-Based Luminescent Metal Organic Frameworks (LMOFs). Chemistry 2021, 3, 327-337. https://0-doi-org.brum.beds.ac.uk/10.3390/chemistry3010024
Velasco E, Osumi Y, Teat SJ, Jensen S, Tan K, Thonhauser T, Li J. Fluorescent Detection of Carbon Disulfide by a Highly Emissive and Robust Isoreticular Series of Zr-Based Luminescent Metal Organic Frameworks (LMOFs). Chemistry. 2021; 3(1):327-337. https://0-doi-org.brum.beds.ac.uk/10.3390/chemistry3010024
Chicago/Turabian StyleVelasco, Ever, Yuki Osumi, Simon J. Teat, Stephanie Jensen, Kui Tan, Timo Thonhauser, and Jing Li. 2021. "Fluorescent Detection of Carbon Disulfide by a Highly Emissive and Robust Isoreticular Series of Zr-Based Luminescent Metal Organic Frameworks (LMOFs)" Chemistry 3, no. 1: 327-337. https://0-doi-org.brum.beds.ac.uk/10.3390/chemistry3010024