Spin-Crossover 2-D Hofmann Frameworks Incorporating an Amide-Functionalized Ligand: N-(pyridin-4-yl)benzamide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of N-(pyridin-4-yl)benzamide (benpy)
2.2. Synthesis of [FeII(benpy)2Pd(CN)4]·2H2O (Pd(benpy))
2.3. Synthesis of [FeII(benpy)2Pt(CN)4]·2H2O (Pt(benpy))
2.4. Thermogravimetric Analysis (TGA)
2.5. Variable-Temperature, Single-Crystal X-ray Diffraction Analysis (VT-SCXRD)
2.6. Variable-Temperature, Powder X-ray Diffraction Analysis (VT-PXRD)
2.7. Variable-Temperature Magnetic Susceptibility
2.8. Differential Scanning Calorimetry (DSC)
2.9. Calculation of the Magnetic Susceptibility
2.10. Variable-Temperature Raman Spectroscopy
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Spin-Crossover Properties
3.2.1. Variable-Temperature Magnetic Susceptibility
3.2.2. Differential Scanning Calorimetry
3.2.3. Magnetic Susceptibility Calculation
3.3. Variable-Temperature Structural Analysis
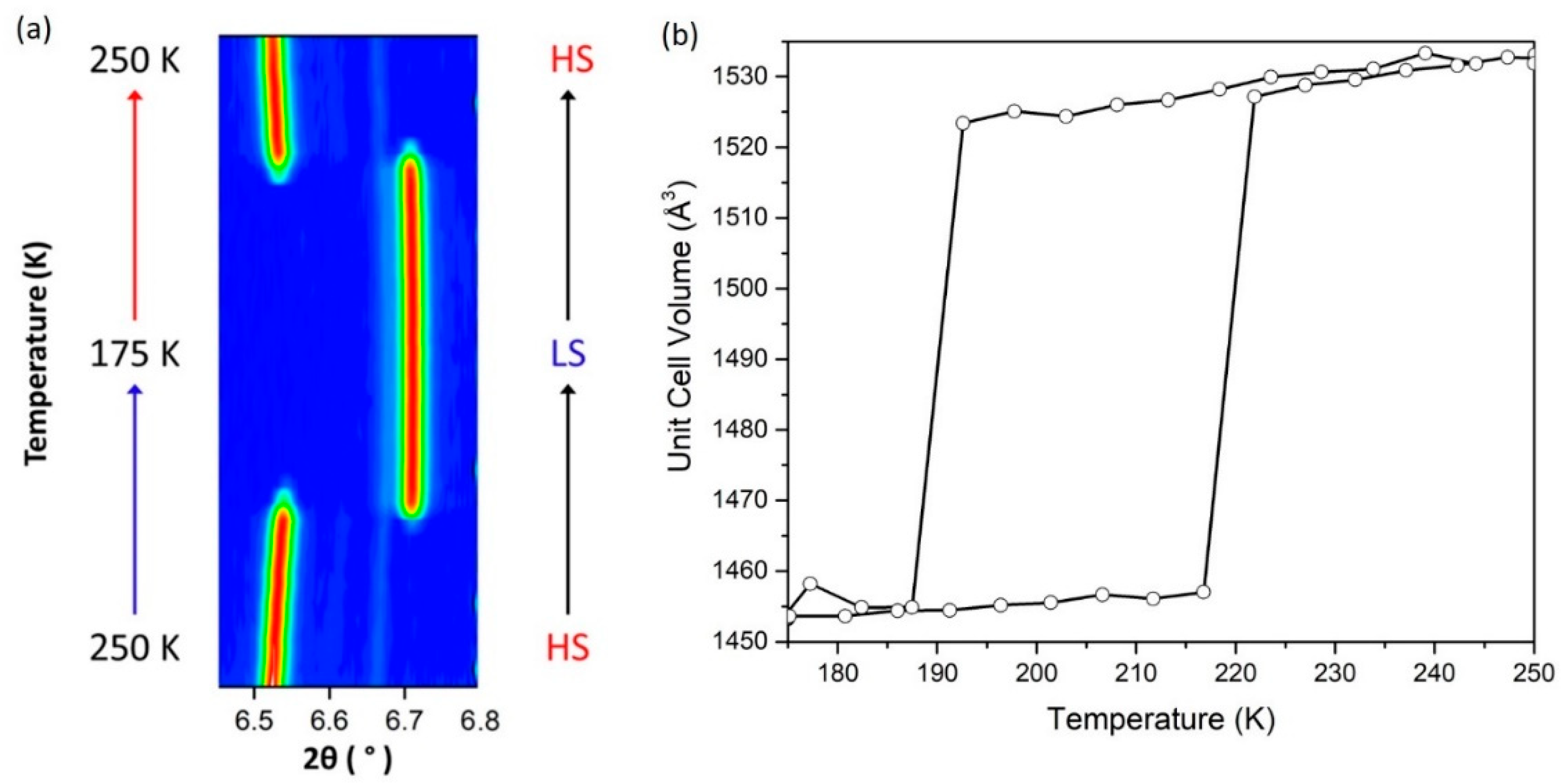
3.3.1. Variable-Temperature Powder X-ray Diffraction
3.3.2. Variable-Temperature Raman Spectroscopy
3.3.3. Variable-Temperature, Single-Crystal X-ray Diffraction
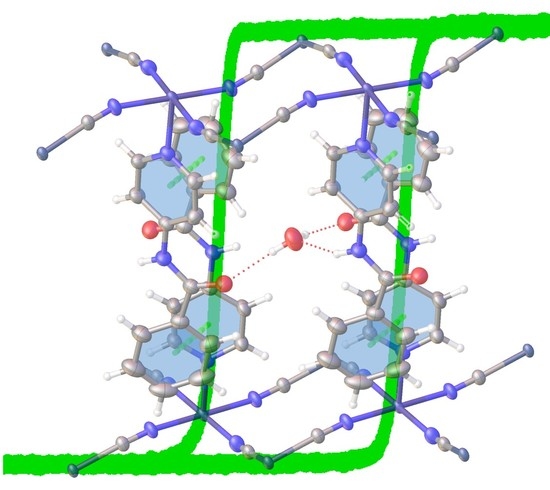
Overall Structural Description
Variable-Temperature Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kahn, O.; Kröber, J.; Jay, C. Spin Transition Molecular Materials for displays and data recording. Adv. Mater. 1992, 4, 718–728. [Google Scholar] [CrossRef]
- Létard, J.-F.; Guionneau, P.; Goux-Capes, L. Towards Spin Crossover Applications. Top. Curr. Chem. 2004, 235, 221–249. [Google Scholar]
- Gütlich, P.; Goodwin, H.A. Spin Crossover—An Overall Perspective. Top. Curr. Chem. 2004, 233, 1–47. [Google Scholar]
- Gütlich, P.; Gaspar, A.B.; Garcia, Y. Spin state switching in iron coordination compounds. Beilstein J. Org. Chem. 2013, 9, 342–391. [Google Scholar] [CrossRef] [Green Version]
- Halcrow, M.A. Spin-Crossover Materials: Properties and Applications; John Wiley & Sons: London, UK, 2013. [Google Scholar]
- Kumar, K.S.; Ruben, M. Emerging trends in spin crossover (SCO) based functional materials and devices. Coord. Chem. Rev. 2017, 346, 176–205. [Google Scholar] [CrossRef]
- Gütlich, P.; Hauser, A.; Spiering, H. Thermal and optical switching of Iron(II) Complexes. Angew. Chem. Int. Ed. 1994, 33, 2024. [Google Scholar] [CrossRef]
- Real, J.A.; Gaspar, A.B.; Niel, V.; Muñoz, M.C. Communication between iron(II) building blocks in cooperative spin transi-tion phenomena. Coord. Chem. Rev. 2003, 236, 121–141. [Google Scholar] [CrossRef]
- Real, J.A.; Gaspar, A.B.; Muñoz, M.C. Thermal, pressure and light switchable spin-crossover materials. Dalton Trans. 2005, 2062–2079. [Google Scholar] [CrossRef]
- Collet, E.; Guionneau, P. Structural analysis of spin-crossover materials: From molecules to materials. C. R. Chim. 2018, 21, 1133–1151. [Google Scholar] [CrossRef]
- Spiering, H. Elastic Interaction in Spin-Crossover Compounds. Top. Curr. Chem. 2006, 171–195. [Google Scholar] [CrossRef]
- Nishino, M.; Boukheddaden, K.; Konishi, Y.; Miyashita, S. Simple Two-Dimensional Model for the Elastic Origin of Coopera-tivity among Spin States of Spin-Crossover Complexes. Phys. Rev. Lett. 2007, 98, 247203. [Google Scholar] [CrossRef] [Green Version]
- Nishino, M.; Boukheddaden, K.; Miyashita, S. Molecular dynamics study of thermal expansion and compression in spin-crossover solids using a microscopic model of elastic interactions. Phys. Rev. B 2009, 79, 012409. [Google Scholar] [CrossRef]
- Kitazawa, T.; Gomi, Y.; Takahashi, M.; Takeda, M.; Enomoto, M.; Miyazaki, A. Spin-crossover behaviour of the coordination polymer FeII(C5H5N)2NiII(CN)4. J. Mater. Chem. 1996, 6, 119. [Google Scholar] [CrossRef]
- Niel, V.; Martinez-Agudo, J.M.; Munoz, M.C.; Gaspar, A.B.; Real, J.A. Cooperative spin crossover behavior in cya-nide-bridged Fe(II)−M(II) bimetallic 3D Hofmann-like networks (M= Ni, Pd, and Pt). Inorg. Chem. 2001, 40, 3838–3839. [Google Scholar] [CrossRef]
- Muñoz, M.C.; Real, J.A. Thermo-, piezo-, photo- and chemo-switchable spin crossover iron(II)-metallocyanate based coordination polymers. Coord. Chem. Rev. 2011, 255, 2068–2093. [Google Scholar] [CrossRef]
- Ni, Z.-P.; Liu, J.-L.; Hoque, N.; Liu, W.; Li, J.-Y.; Chen, Y.-C.; Tong, M.-L. Recent advances in guest effects on spin-crossover behavior in Hofmann-type metal-organic frameworks. Coord. Chem. Rev. 2017, 335, 28–43. [Google Scholar] [CrossRef]
- Ohtani, R.; Hayami, S. Guest-Dependent Spin-Transition Behavior of Porous Coordination Polymers. Chem. Eur. J. 2017, 23, 2236–2248. [Google Scholar] [CrossRef] [PubMed]
- Ohba, M.; Yoneda, K.; Agustí, G.; Muñoz, M.C.; Gaspar, A.B.; Real, J.A.; Yamasaki, M.; Ando, H.; Nakao, Y.; Sakaki, S.; et al. Bidirectional Chemo-Switching of Spin State in a Microporous Framework. Angew. Chem. Int. Ed. 2009, 48, 4767–4771. [Google Scholar] [CrossRef] [PubMed]
- Southon, P.D.; Liu, L.; Fellows, E.A.; Price, D.J.; Halder, G.J.; Chapman, K.W.; Moubaraki, B.; Murray, K.S.; Létard, J.-F.; Kepert, C.J. Dynamic Interplay between Spin-Crossover and Host−Guest Function in a Nanoporous Metal−Organic Framework Material. J. Am. Chem. Soc. 2009, 131, 10998–11009. [Google Scholar] [CrossRef] [PubMed]
- Klein, Y.M.; Sciortino, N.F.; Ragon, F.; Housecroft, C.E.; Kepert, C.J.; Neville, S.M. Spin crossover intermediate plateau stabilization in a flexible 2-D Hofmann-type coordination polymer. Chem. Commun. 2014, 50, 3838–3840. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Peng, Y.-Y.; Wu, S.-G.; Chen, Y.-C.; Hoque, N.; Ni, Z.-P.; Chen, X.-M.; Tong, M. Guest-Switchable Multi-Step Spin Transitions in an Amine-Functionalized Metal-Organic Framework. Angew. Chem. Int. Ed. 2017, 56, 14982–14986. [Google Scholar] [CrossRef]
- Seredyuk, M.; Gaspar, A.B.; Ksenofontov, V.; Verdaguer, M.; Villain, F.; Gütlich, P. Thermal- and Light-Induced Spin Crossover in Novel 2D Fe(II) Metalorganic Frameworks {Fe(4-PhPy)2[MII(CN)x]y}∙sH2O: Spectroscopic, Structural, and Mag-netic Study. Inorg. Chem. 2009, 48, 6130–6141. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, L.; Su, Y.-J.; Chen, Y.-C.; Tucek, J.; Zboril, R.; Ni, Z.-P.; Tong, M.-L. Hysteretic Spin Crossover in Two-Dimensional (2D) Hofmann-Type Coordination Polymers. Inorg. Chem. 2015, 54, 8711–8716. [Google Scholar] [CrossRef] [PubMed]
- Noveron, J.C.; Lah, M.S.; Del Sesto, R.E.; Arif, A.M.; Miller, J.S.; Stang, P.J. Engineering the Structure and Magnetic Properties of Crystalline Solids via the Metal-Directed Self-Assembly of a Versatile Molecular Building Unit. J. Am. Chem. Soc. 2002, 124, 6613–6625. [Google Scholar] [CrossRef] [PubMed]
- Valverde-Muñoz, F.J.; Bartual-Murgui, C.; Piñeiro-López, L.; Muñoz, M.C.; Real, J.A. Influence of host-guest and host-host interactions on the spin-crossover of 3D Hofmann-type clathrates {FeII(pina)[MI(CN)2]2}∙xMeOH (MI = Ag, Au). Inorg. Chem. 2019, 58, 10038–10046. [Google Scholar] [CrossRef]
- Lan, W.; Valverde-Muñoz, F.J.; Dou, Y.; Hao, Y.; Muñoz, M.C.; Zhou, Z.; Liu, H.; Liu, Q.; Real, J.A.; Zhang, D. A thermal- and light-induced switchable one-dimensional rare loo-like spin crossover coordination polymer. Dalton Trans. 2019, 48, 17014. [Google Scholar] [CrossRef]
- Hao, X.; Dou, T.; Cao, L.; Yang, L.; Liu, H.; Li, D.; Zhang, D.; Zhou, Z. Tuning of crystallization method and ligand confor-mation to give a mononuclear compound or two-dimensional SCO coordination polymer based on a new semi-rigid V-shaped bis-pyridyl bis-amide ligand. Acta Cryst. 2020, C76, 412–418. [Google Scholar]
- Mondal, D.J.; Roy, S.; Yadav, J.; Zeller, M.; Konar, S. Solvent-induced reversible spin-crossover in a 3D Hofmann-type coor-dination polymer and unusual enhancement of the lattice cooperativity at the desolvated state. Inorg. Chem. 2020, 59, 13024–13028. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Xie, Z.; Thoonen, S.; Hua, C.; Kepert, C.J.; Price, J.R.; Neville, S.M. A new spin crossover FeII coordination environment in a two-fold interpenetrated 3-D Hofmann-type framework material. Chem. Commun. 2021, 57, 85–88. [Google Scholar] [CrossRef]
- Cowieson, N.P.; Arago, D.; Clift, M.; Ericsson, D.J.; Gee, C.; Harrop, S.J.; Mudie, N.; Panjikar, S.; Price, J.R.; Ribol-di-Tunnicliffe, A.; et al. MX1: A bending-magnet crystallography beamline serving both chemical and macromolecular crystallographic communities at the Australian Synchrotron. J. Synch. Rad. 2015, 22, 187–190. [Google Scholar] [CrossRef]
- McPhillips, T.M.; McPhillips, S.E.; Chiu, H.-J.; Cohen, A.E.; Deacon, A.M.; Ellis, P.J.; Garman, E.; Gonzalez, A.; Sauter, N.K.; Phizackerley, R.P. Blu-Ice and the Distributed Control System: Software for data acqui-sition and instrument control at macromolecular crystallography beamlines. J. Synch. Rad. 2002, 9, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Kabsch, W. XDS. Acta Cryst. D 2010, 66, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Wallwork, K.S.; Kennedy, B.J.; Wang, D.C. The High Resolution Powder Diffraction Beamline for the Australian Synchrotron. In Fourth Huntsville Gamma-Ray Burst Symposium; AIP Publishing: New York, NY, USA, 2007; Volume 879, pp. 879–882. [Google Scholar]
- Bergamaschi, A.; Cervellino, A.; DiNapoli, R.; Gozzo, F.; Henrich, B.; Johnson, I.; Kraft, P.; Mozzanica, A.; Schmitt, B.; Shi, X. The MYTHEN detector for X-ray powder diffraction experiments at the Swiss Light Source. J. Synchrotron Radiat. 2010, 17, 653–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jong, L.; Ruben, G.; Spear, K. PDViPeR; Synchrotron Light Source Australia Pty Ltd.: Melbourne, Australia, 2014. [Google Scholar]
- Coelho, A. TOPAS Version 4.1; Bruker AXS GmbH: Karlsruhe, Germany, 2007. [Google Scholar]
- Wajnflasz, J.; Pick, R. Transitions «Low spin»-«High spin» dans les complexes de Fe2+. J. Phys. 1971, 32, 90–91. [Google Scholar] [CrossRef]
- Pavlik, J.; Boča, R. Established Static Models of Spin Crossover. Eur. J. Inorg. Chem. 2013, 5–6, 697–709. [Google Scholar] [CrossRef]
- Ruzzi, G.; Cruddas, J.; McKenzie, R.H.; Powell, B.J. Equivalence of elastic and Ising models for spin crossover materials. arXiv 2020, arXiv:2008.08738. [Google Scholar]
- Ragon, F.; Yaksi, K.; Sciortino, N.F.; Chastanet, G.; Létard, J.-F.; D’Alessandro, D.M.; Kepert, C.J.; Neville, S.M. Thermal Spin Crossover Behaviour of Two-Dimensional Hofmann-Type Coordination Polymers Incorporating Photoactive Ligands. Aust. J. Chem. 2014, 67, 1563–1573. [Google Scholar] [CrossRef]
- Roubeau, O.; Castro, M.; Burriel, R.; Haasnoot, J.G.; Reedijk, J. Calorimetric Investigation of Triazole-Bridged Fe(II) Spin-Crossover One-Dimensional Materials: Measuring the Cooperativity. J. Phys. Chem. B 2011, 115, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Molnár, G.; Mikolasek, M.; Ridier, K.; Fahs, A.; Nicolazzi, W.; Bousseksou, A. Molecular Spin Crossover Materials: Review of the Lattice Dynamical Properties. Annalen Physik 2019, 531, 1900076. [Google Scholar] [CrossRef]
- Kulmaczewski, R.; Olguín, J.; Kitchen, J.A.; Feltham, H.L.C.; Jameson, G.N.L.; Tallon, J.L.; Brooker, S. Remarkable Scan Rate Dependence for a Highly Constrained Dinuclear Iron(II) Spin Crossover Complex with a Wide Thermal Hysteresis Loop. J. Am. Chem. Soc. 2014, 136, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Paez-Espejo, M.; Sy, M.; Boukheddaden, K. Elastic Frustration Causing Two-Step and Multistep Transitions in Spin-Crossover Solids: Emergence of Complex Antiferroelastic Structures. J. Am. Chem. Soc. 2016, 138, 3202–3210. [Google Scholar] [CrossRef]
- Cruddas, J.; Powell, B.J. Structure-property relationships and the mechanism of multistep transitions in spin crossover mate-rials and frameworks. Inorg. Chem. Front. 2020, 7, 4424–4437. [Google Scholar] [CrossRef]
- Cruddas, J.; Powell, B.J. Spin-State Ice in Elastically Frustrated Spin-Crossover Materials. J. Am. Chem. Soc. 2019, 141, 19790–19799. [Google Scholar] [CrossRef] [PubMed]






| FeII(benpy)2Pd(CN)4·2H2O | FeII(benpy)2Pt(CN)4·2H2O | |||
|---|---|---|---|---|
| Spin state | LS | HS | LS | HS |
| Temperature (K) | 100 | 250 | 100 | 250 |
| Average Fe–N≡C | 1.9976 | 2.1411 | 1.926 | 2.125 |
| Average Fe–N(py) | 1.93775 | 2.2042 | 1.997 | 2.216 |
| Average Fe–N (Å) | 1.9577 | 2.1621 | 1.950 | 2.155 |
| O∙water (Å) | 2.767(2) | 2.846(2) | 2.730(2) | 2.805(2) |
| water∙N(H) (Å) | 2.852(4) | 2.904(2) | 2.895(2) | 2.915(3) |
| Σ (Fe) (°) | 13.20 | 8.160 | 12.82 | 12.40 |
| Ligand torsion (°) | 24.3 | 35.5 | 13.8 | 40.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, X.; Ahmed, M.; Xu, L.; Brennan, A.T.; Hua, C.; Zenere, K.A.; Xie, Z.; Kepert, C.J.; Powell, B.J.; Neville, S.M. Spin-Crossover 2-D Hofmann Frameworks Incorporating an Amide-Functionalized Ligand: N-(pyridin-4-yl)benzamide. Chemistry 2021, 3, 360-372. https://0-doi-org.brum.beds.ac.uk/10.3390/chemistry3010026
Ong X, Ahmed M, Xu L, Brennan AT, Hua C, Zenere KA, Xie Z, Kepert CJ, Powell BJ, Neville SM. Spin-Crossover 2-D Hofmann Frameworks Incorporating an Amide-Functionalized Ligand: N-(pyridin-4-yl)benzamide. Chemistry. 2021; 3(1):360-372. https://0-doi-org.brum.beds.ac.uk/10.3390/chemistry3010026
Chicago/Turabian StyleOng, Xandria, Manan Ahmed, Luonan Xu, Ashley T. Brennan, Carol Hua, Katrina A. Zenere, Zixi Xie, Cameron J. Kepert, Benjamin J. Powell, and Suzanne M. Neville. 2021. "Spin-Crossover 2-D Hofmann Frameworks Incorporating an Amide-Functionalized Ligand: N-(pyridin-4-yl)benzamide" Chemistry 3, no. 1: 360-372. https://0-doi-org.brum.beds.ac.uk/10.3390/chemistry3010026