TIPS for Scaling up Research in Upper Limb Prosthetics
Abstract
:1. Introduction
2. Scaling Up
2.1. Translation
- -
- Focus more research on translational research stage 3
- -
- Consider home try-outs to be part of research proposals
- -
- Make use of the advantages of smart technology and telerehabilitation
- -
- Consider alternative study designs, such as small-N methodology
- -
- Scientific journals should accept and stimulate the submission of small-N papers
2.2. Innovation
- -
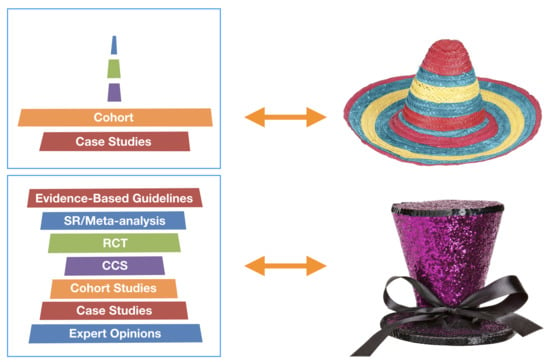
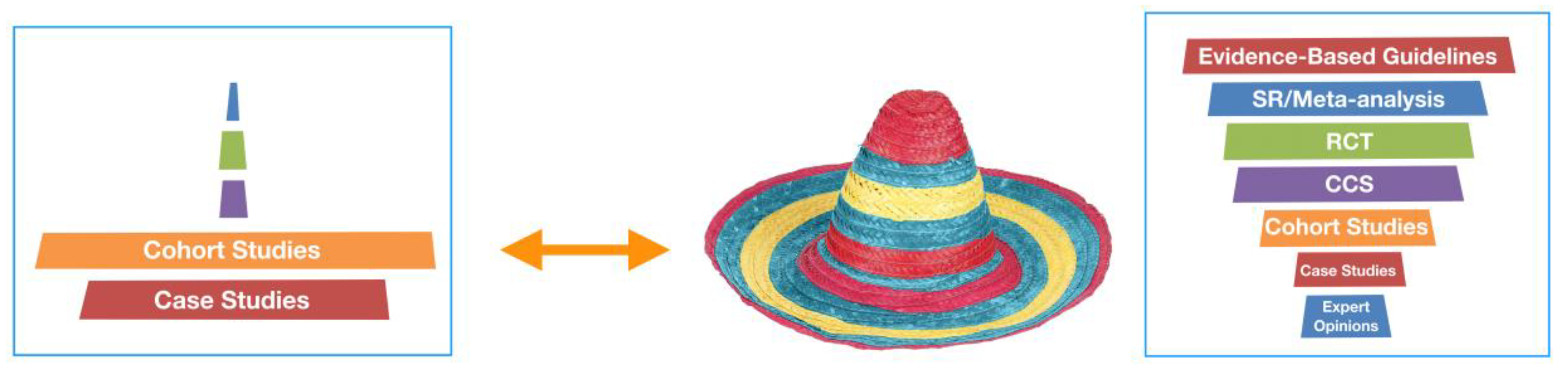
- Strive for a shape of a tall hat instead of a sombrero for the body of ULP research
- -
- Compose more evidence-based guidelines and systematic reviews
- -
- Develop standardized protocols, including a core set of questionnaires, to improve the quality of evidence-based guidelines, systematic reviews, and meta-analyses
- -
- Establish a dedicated database to host guidelines and systematic reviews on ULP topics
- -
- Rely much less on research on able-bodied persons
- -
- In all studies, always include at least some persons with ULD
- -
- Use established reporting guidelines when reporting study results
- -
- Base design innovations on theories
- -
- Scientific journals should encourage authors to submit theoretical papers
- -
- Provide good clinical practice education to all clinical staff
2.3. Patients
- -
- Include patient perspectives in each research project
- -
- Involve patients in each research project from the start of the project
- -
- Evaluations should be based on opinions of prosthetic users
- -
- Consider qualitative study designs to elucidate patient perspectives
- -
- Have an active attitude in your contacts with patient associations
2.4. Spreading of Research Findings: Implementation, Dissemination, and Collaboration
- -
- Implementation should be part of each research proposal
- -
- Take advantage of the feel-good content of ULP research by attracting media
- -
- Make it a habit to use social media and traditional media
- -
- Make a dissemination plan at the start of a project
- -
- (Inter)national collaboration and a team approach are essential
- -
- Establish shared databases and include each person with an upper limb defect in these databases
- -
- Publish in open access journals
- -
- Share data in public data repositories
- -
- Create a dedicated ULP data repository
- -
- Use conferences as a start of new beginnings
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Green, L.W.; Ottoson, J.M.; García, C.; Hiatt, R.A. Diffusion Theory and Knowledge Dissemination, Utilization, and Integration in Public Health. Annu. Rev. Public Health 2009, 30, 151–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization, Department of Reproductive Health and Research. Practical Guidelines for Scaling up Health Service Innovations; Report; WHO: Geneva, Switzerland, 2009; ISBN 978 92 4 159852 1. [Google Scholar]
- World Health Organization, Department of Reproductive Health and Research. Scaling up Health Service Delivery: From Pilot Innovations to Policies; Report; WHO: Geneva, Switzerland, 2007; ISBN 978 92 4 156351 2. [Google Scholar]
- World Health Organization, Department of Reproductive Health and Research—ExpandNet. Nine Steps for Developing a Scaling-up Strategy; Report; WHO: Geneva, Switzerland, 2010; ISBN 978 92 4 150031 9. [Google Scholar]
- Zoellner, J.; Van Horn, L.; Gleason, P.; Boushey, C.J. What Is Translational Research? Concepts and Applications in Nutrition and Dietetics. J. Acad. Nutr. Diet. 2015, 115, 1057–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dougherty, D.; Conway, P.H. The “3T’s” Road Map to Transform US Health Care. JAMA 2008, 299, 2319–2321. [Google Scholar] [CrossRef]
- Kuiken, T.A.; Miller, L.A.; Turner, K.; Hargrove, L.J. A Comparison of Pattern Recognition Control and Direct Control of a Multiple Degree-of-Freedom Transradial Prosthesis. IEEE J. Transl. Eng. Heal. Med. 2016, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Resnik, L.; Acluche, F.; Borgia, M.; Cancio, J.; Latlief, G.; Sasson, N. Function, quality of life, and community integration of DEKA Arm users after discharge from prosthetic training: Impact of home use experience. Prosthetics Orthot. Int. 2018, 42, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, N.; Sreenivasan, N.; Bifulco, P.; Cesarelli, M.; Savino, S.; Niola, V.; Esposito, D.; Hamilton, T.J.; Naik, G.R.; Gunawardana, U.; et al. Real-Time EMG Based Pattern Recognition Control for Hand Prostheses: A Review on Existing Methods, Challenges and Future Implementation. Sensors 2019, 19, 4596. [Google Scholar] [CrossRef] [Green Version]
- Simon, A.M.; Turner, K.L.; Miller, L.A.; Hargrove, L.J.; Kuiken, T.A. Pattern recognition and direct control home use of a multi-articulating hand prosthesis. In Proceedings of the 16th International Conference on Rehabilitation Robotics (ICORR), Toronto, ON, Canada, 24–28 June 2019; pp. 386–391. [Google Scholar]
- Graham, J.E.; Karmarkar, A.M.; Ottenbacher, K.J. Small Sample Research Designs for Evidence-Based Rehabilitation: Issues and Methods. Arch. Phys. Med. Rehabil. 2012, 93 (Suppl. 8), S111–S116. [Google Scholar] [CrossRef] [Green Version]
- Krasny-Pacini, A.; Evans, J. Single-case experimental designs to assess intervention effectiveness in rehabilitation: A practical guide. Ann. Phys. Rehabil. Med. 2018, 61, 164–179. [Google Scholar] [CrossRef]
- Sackett, D.L.; Rosenberg, W.M.C.; Gray, J.A.M.; Haynes, R.B.; Richardson, W.S. Evidence based medicine: What it is and what it isn’t. BMJ 1996, 312, 71–72. [Google Scholar] [CrossRef] [Green Version]
- Doherty, S. History of evidence-based medicine. Oranges, chloride of lime and leeches: Barriers to teaching old dogs new tricks. Emerg. Med. Austral. 2005, 17, 314–321. [Google Scholar] [CrossRef]
- Strauss, S.E. Evidence-Based Medicine: How to Practice and Teach EBM, 3rd ed.; Elsevier: Edinburgh, UK; Churchill Livingstone: New York, NY, USA, 2005; ISBN 0443074445. [Google Scholar]
- Grade Working Group. Available online: http://www.gradeworkinggroup.org/ (accessed on 14 October 2020).
- Equator Network. Available online: http://www.equator-network.org/ (accessed on 14 October 2020).
- Cochrane. Available online: https://consumers.cochrane.org/levels-evidence (accessed on 14 October 2020).
- Joanna Briggs Institute. Available online: https://joannabriggs.org/ (accessed on 14 October 2020).
- CEBM. Available online: https://www.cebm.net/ (accessed on 14 October 2020).
- Dijkers, M.P.; Murphy, S.L.; Krellman, J. Evidence-Based Practice for Rehabilitation Professionals: Concepts and Controversies. Arch. Phys. Med. Rehabil. 2012, 93 (Suppl. 8), S164–S176. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Catalan, M.; Esander, N.; Kristoffersen, M.B.; Håkansson, B.; Brånemark, R. Treatment of phantom limb pain (PLP) based on augmented reality and gaming controlled by myoelectric pattern recognition: A case study of a chronic PLP patient. Front. Neurosci. 2014, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Catalan, M.; A Guðmundsdóttir, R.; Kristoffersen, M.B.; Zepeda-Echavarria, A.; Caine-Winterberger, K.; Kulbacka-Ortiz, K.; Widehammar, C.; Eriksson, K.; Stockselius, A.; Ragnö, C.; et al. Phantom motor execution facilitated by machine learning and augmented reality as treatment for phantom limb pain: A single group, clinical trial in patients with chronic intractable phantom limb pain. Lancet 2016, 388, 2885–2894. [Google Scholar] [CrossRef]
- Lendaro, E.; Hermansson, L.; Burger, H.; Van Der Sluis, C.K.; E McGuire, B.; Pilch, M.; Bunketorp-Käll, L.; Kulbacka-Ortiz, K.; Rignér, I.; Stockselius, A.; et al. Phantom motor execution as a treatment for phantom limb pain: Protocol of an international, double-blind, randomised controlled clinical trial. BMJ Open 2018, 8, e021039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VA/DoD Clinical Practice Guidelines. The Management of Upper Extremity Amputation Rehabilitation (UEAR). 2014. Available online: https://www.healthquality.va.gov/guidelines/rehab/uear/index.asp (accessed on 14 October 2020).
- Meurs, M.; Maathuis, C.G.B.; Lucas, C.; Hadders-Algra, M.; Van Der Sluis, C.K. Prescription of the First Prosthesis and Later use in Children with Congenital Unilateral Upper Limb Deficiency: A Systematic Review. Prosthetics Orthot. Int. 2006, 30, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Ritchie, S.; Wiggins, S.; Sanford, A. Perceptions of cosmesis and function in adults with upper limb prostheses: A systematic literature review. Prosthetics Orthot. Int. 2011, 35, 332–341. [Google Scholar] [CrossRef]
- Romkema, S.; Bongers, R.M.; Van Der Sluis, C.K. Influence of mirror therapy and motor imagery on intermanual transfer effects in upper-limb prosthesis training of healthy participants: A randomized pre-posttest study. PLoS ONE 2018, 13, e0204839. [Google Scholar] [CrossRef] [Green Version]
- Bouwsema, H.; Van Der Sluis, C.K.; Bongers, R.M. Effect of Feedback during Virtual Training of Grip Force Control with a Myoelectric Prosthesis. PLoS ONE 2014, 9, e98301. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, L.; Van Der Sluis, C.K.; Van Dijk, H.W.; Bongers, R.M. Learning an EMG Controlled Game: Task-Specific Adaptations and Transfer. PLoS ONE 2016, 11, e0160817. [Google Scholar] [CrossRef]
- Terlaak, B.; Bouwsema, H.; Van Der Sluis, C.K.; Bongers, R.M. Virtual Training of the Myosignal. PLoS ONE 2015, 10, e0137161. [Google Scholar] [CrossRef]
- Romkema, S.; Bongers, R.M.; Van Der Sluis, C.K. Influence of Inter-Training Intervals on Intermanual Transfer Effects in Upper-Limb Prosthesis Training: A Randomized Pre-Posttest Study. PLoS ONE 2015, 10, e0128747. [Google Scholar] [CrossRef] [PubMed]
- Batzianoulis, I.; Krausz, N.E.; Simon, A.M.; Hargrove, L.; Billard, A. Decoding the grasping intention from electromyography during reaching motions. J. Neuroeng. Rehabil. 2018, 15, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Boer, E.; Romkema, S.; Cutti, A.G.; Brouwers, M.A.; Bongers, R.M.; Van Der Sluis, C.K. Intermanual Transfer Effects in Below-Elbow Myoelectric Prosthesis Users. Arch. Phys. Med. Rehabil. 2016, 97, 1924–1930. [Google Scholar] [CrossRef] [PubMed]
- Chadwell, A.; Kenney, L.; Thies, S.; Galpin, A.; Head, J. The Reality of Myoelectric Prostheses: Understanding What Makes These Devices Difficult for Some Users to Control. Front. Neurorobotics 2016, 10, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whyte, J. Contributions of Treatment Theory and Enablement Theory to Rehabilitation Research and Practice. Arch. Phys. Med. Rehabil. 2014, 95, S17–S23. [Google Scholar] [CrossRef] [PubMed]
- Whyte, J.; Dijkers, M.P.; Hart, T.; Zanca, J.M.; Packel, A.; Ferraro, M.; Tsaousides, T. Development of a Theory-Driven Rehabilitation Treatment Taxonomy: Conceptual Issues. Arch. Phys. Med. Rehabil. 2014, 95, S24–S32. [Google Scholar] [CrossRef] [PubMed]
- Bongers, R.M.; Kyberd, P.J.; Bouwsema, H.; Kenney, L.P.; Plettenburg, D.H.; Van Der Sluis, C.K. Bernstein’s Levels of Construction of Movements Applied to Upper Limb Prosthetics. JPO J. Prosthetics Orthot. 2012, 24, 67–76. [Google Scholar] [CrossRef]
- Van Dijk, L.; Van Der Sluis, C.; Bongers, R.M. Reductive and Emergent Views on Motor Learning in Rehabilitation Practice. J. Mot. Behav. 2016, 49, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Kelley, M.A.; Benz, H.; Engdahl, S.M.; Bridges, J.F.P. Identifying the benefits and risks of emerging integration methods for upper limb prosthetic devices in the United States: An environmental scan. Expert Rev. Med Devices 2019, 16, 631–641. [Google Scholar] [CrossRef]
- Coapt Engineering. Available online: https://www.coaptengineering.com/pattern-recognition.html (accessed on 14 October 2020).
- Ottobock Myoplus. Available online: https://www.ottobock-export.com/en/prosthetics/upper-limb/solution-overview/myo-plus-mustererkennung/ (accessed on 14 October 2020).
- Franzke, A.W.; Kristoffersen, M.B.; Bongers, R.M.; Murgia, A.; Pobatschnig, B.; Unglaube, F.; Van Der Sluis, C.K. Users’ and therapists’ perceptions of myoelectric multi-function upper limb prostheses with conventional and pattern recognition control. PLoS ONE 2019, 14, e0220899. [Google Scholar] [CrossRef] [Green Version]
- International Confederation of Amputee Associations. Available online: https://www.ic2a.eu/ (accessed on 14 October 2020).
- Benz, H.L.; Yao, J.; Rose, L.; Olgac, O.; Kreutz, K.; Saha, A.; Civillico, E.F. Upper Extremity Prosthesis User Perspectives on Unmet Needs and Innovative Technology. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 287–290. [Google Scholar]
- Wijdenes, P.; Brouwers, M.; Van Der Sluis, C.K. Prosthesis Prescription Protocol of the Arm (PPP-Arm): The implementation of a national prosthesis prescription protocol. Prosthetics Orthot. Int. 2018, 42, 56–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, E. The Culture Map, 1st ed.; Public Affairs: Philadelphia, PA, USA, 2014; p. 67. [Google Scholar]


| Topics of Interest: TIPS | Recommendations for Sustainable Scaling Up (WHO) [2] |
|---|---|
| Translation |
|
| Innovation |
|
| Patients |
|
| Spreading |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Sluis, C.K.; Bongers, R.M. TIPS for Scaling up Research in Upper Limb Prosthetics. Prosthesis 2020, 2, 340-351. https://0-doi-org.brum.beds.ac.uk/10.3390/prosthesis2040032
van der Sluis CK, Bongers RM. TIPS for Scaling up Research in Upper Limb Prosthetics. Prosthesis. 2020; 2(4):340-351. https://0-doi-org.brum.beds.ac.uk/10.3390/prosthesis2040032
Chicago/Turabian Stylevan der Sluis, Corry K., and Raoul M. Bongers. 2020. "TIPS for Scaling up Research in Upper Limb Prosthetics" Prosthesis 2, no. 4: 340-351. https://0-doi-org.brum.beds.ac.uk/10.3390/prosthesis2040032