The Effects of the UV-Blocker Oxybenzone (Benzophenone-3) on Planulae Swimming and Metamorphosis of the Scyphozoans Cassiopea xamachana and Cassiopea frondosa
Abstract
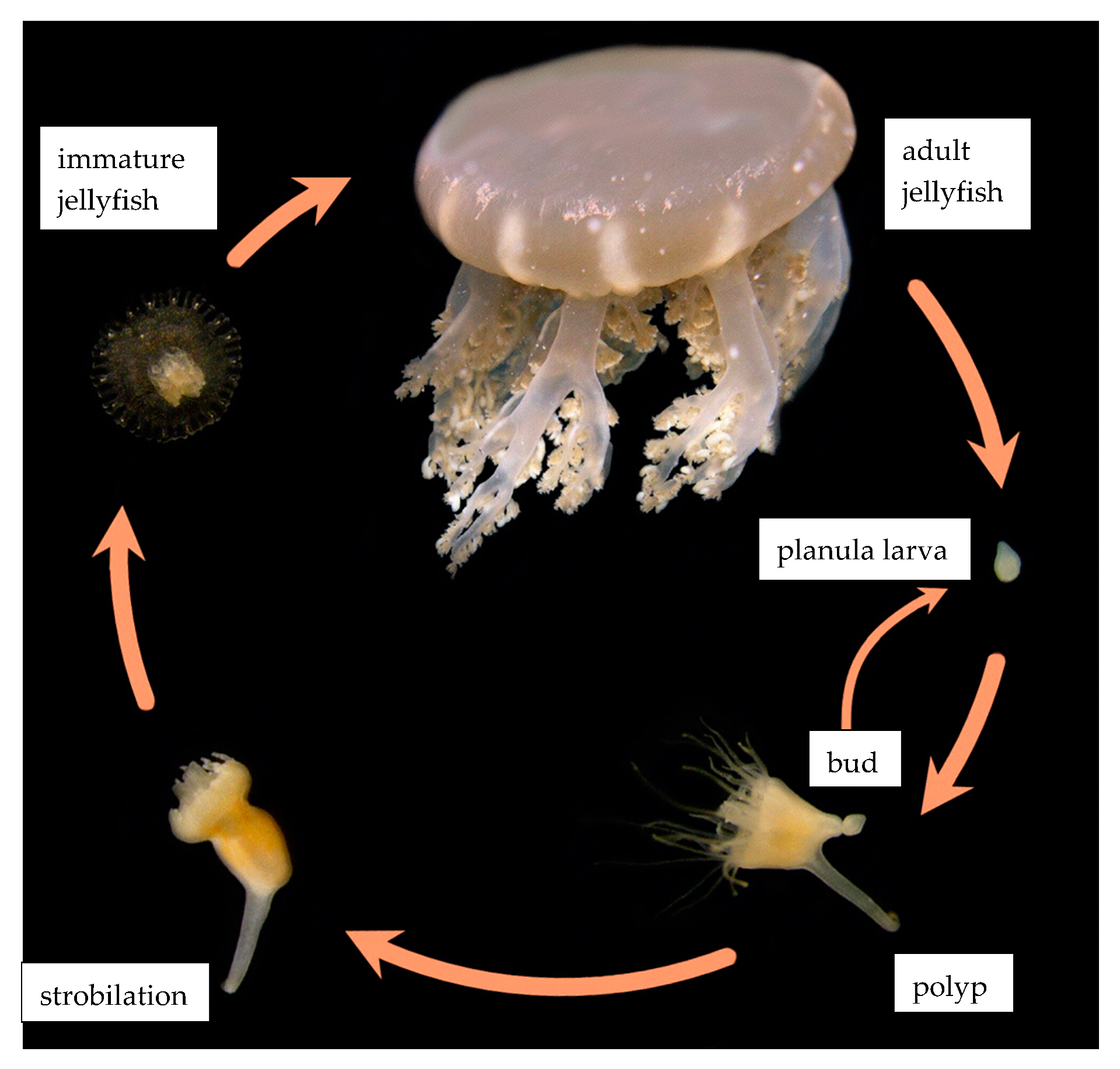
:1. Introduction
2. Materials and Methods
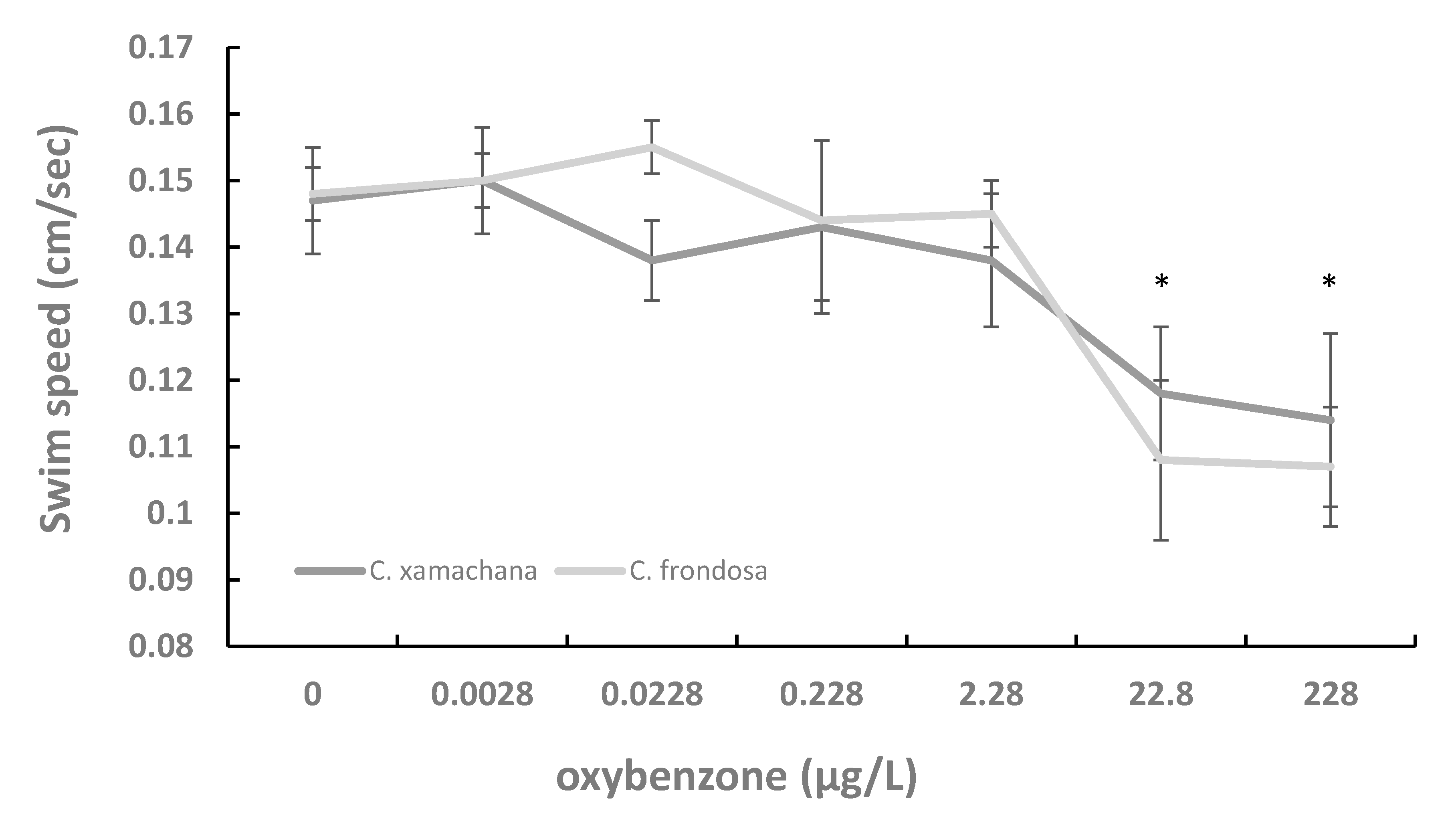
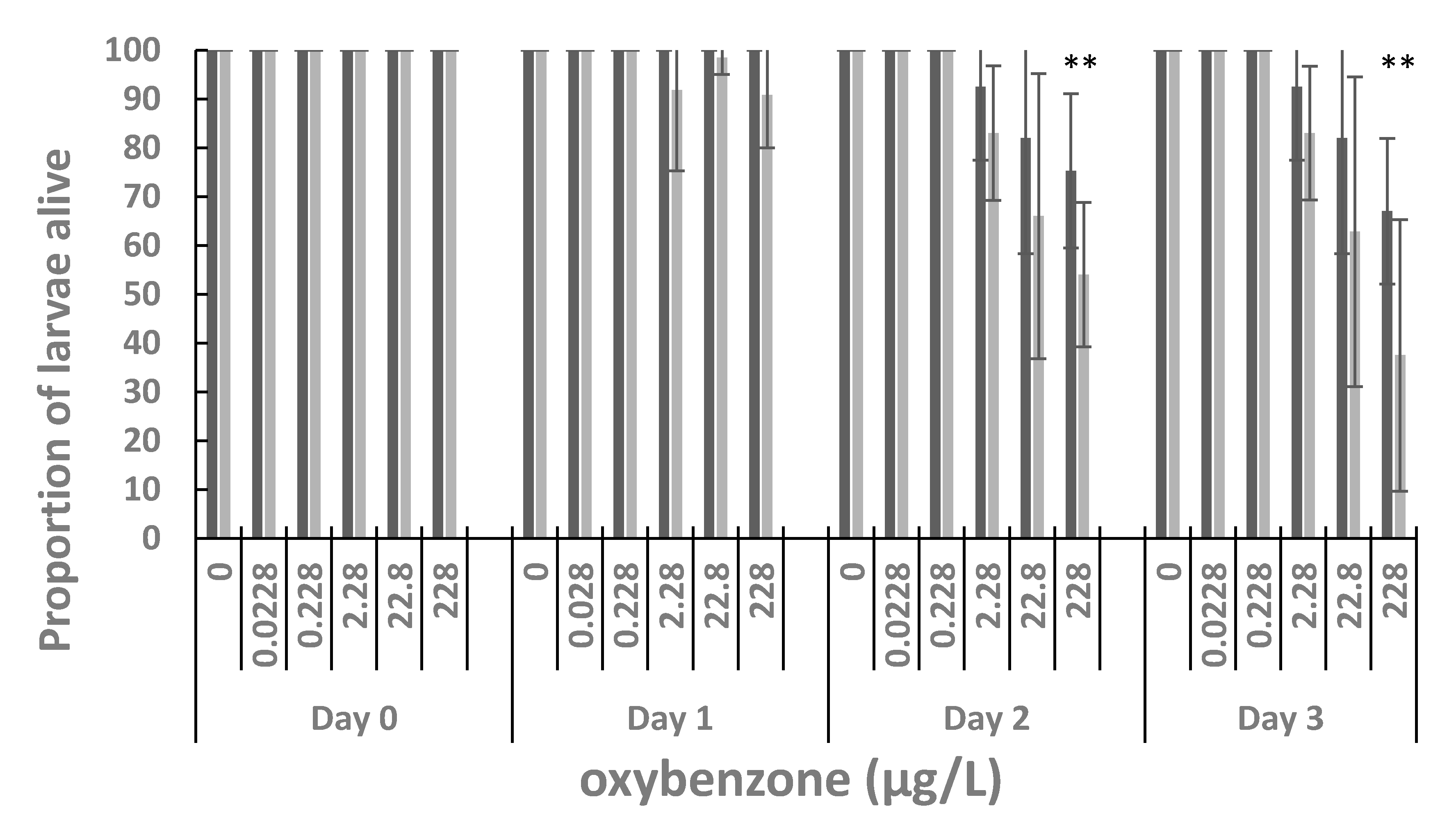
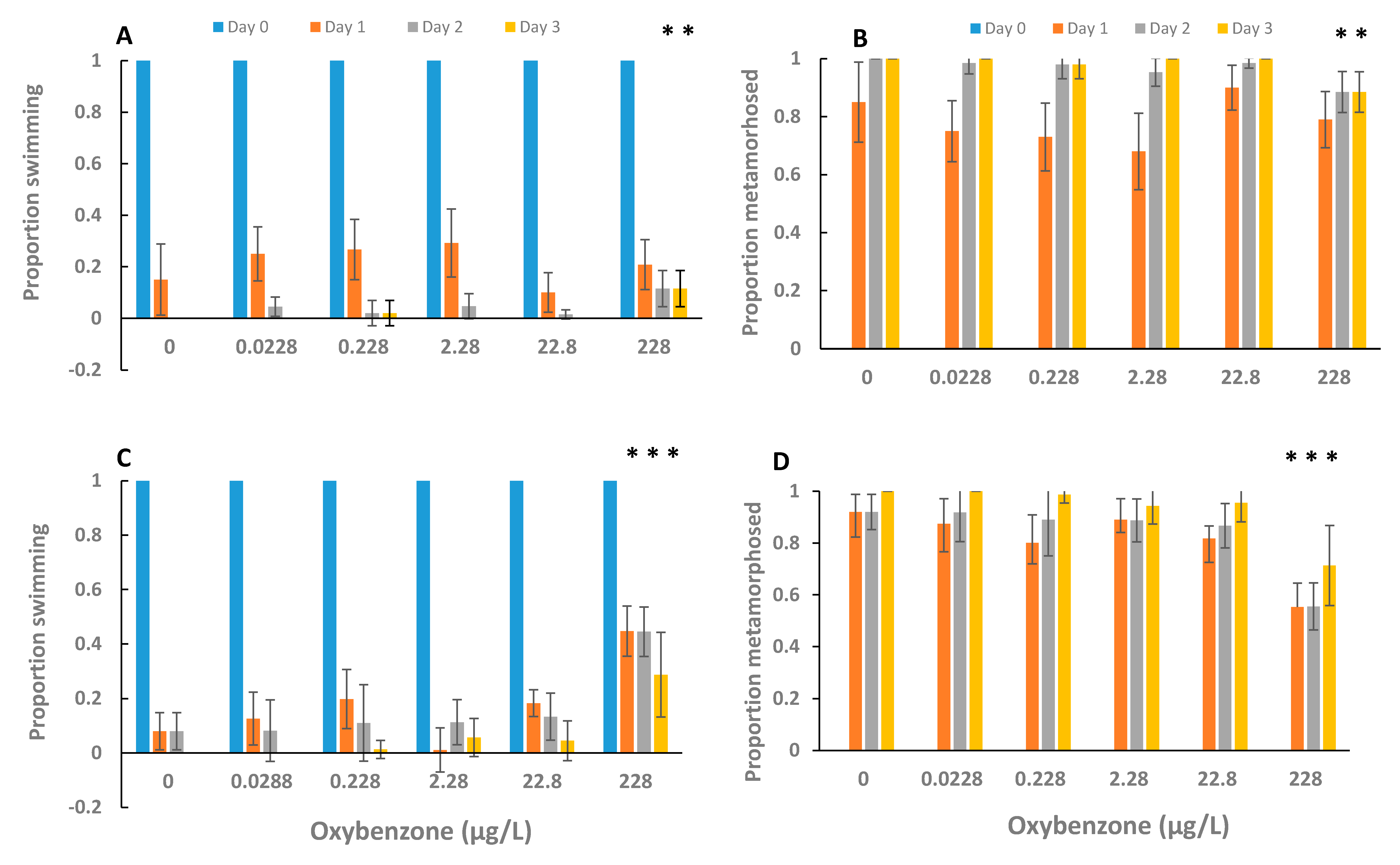
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shaath, N.A.; Shaath, M. Recent sunscreen market trends. In Sunscreens, Regulations and Commercial Development; Shaath, N.A., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2005; Volume 3, pp. 929–940. [Google Scholar]
- French, J.E. NTP technical report on the toxicity studies of 2-hydroxy-4-methoxybenzophenone (CAS No. 131-57-7) administered topically and in dosed feed to F344/N Rats and B6C3F1 mice. Toxic. Rep. Ser. 1992, 21, 1–14. [Google Scholar] [PubMed]
- Hany, J.; Nagel, R. Detection of sunscreen agents in human breast milk. Dtsch. Lebensm. Rundsch. 1995, 91, 341–345. [Google Scholar]
- Downs, C.A.; Kramarsky-Winter, E.; Segal, R.; Fauth, J.; Knutson, S.; Bronstein, O.; Ciner, F.R.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; et al. Toxic pathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the U.S. Virgin Islands. Arch. Environ. Contam. Toxicol. 2016, 70, 265–288. [Google Scholar]
- Schallreuter, K.U.; Wood, J.M.; Farwell, D.W.; Moore, J.; Edwards, H.G.M. Oxybenzone oxidation following solar irradiation of skin: Photoprotection versus antioxidant inactivation. J. Investig. Derm. 1996, 106, 583–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Wnag, P.; Law, J.C.; Zhao, Y.; Wei, Y.; Zhou, Y.; Zang, Y.; Shi, H.; Leung, K.S. Organic UV filter exposure and pubertal development: A prospective follow-up study of urban Chinese adolescents. Environ. Int. 2020, 143. [Google Scholar] [CrossRef] [PubMed]
- Frikeche, J. Research on the immunosuppressive activity of ingredients contained in sunscreens. Arch. Dermatol. Res. 2015, 307, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens cause coral bleaching by promoting viral infections. Environ. Health Perspect. 2008, 116, 337–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, T.; Tsui, M.M.P.; Tan, C.J.; Ma, C.Y.; Yui, S.K.F.; Wang, L.H.; Chen, T.H.; Fan, T.Y.; Lam, P.K.S.; Murphy, M.B. Toxicological effects of two organic ultraviolet filters and a related commercial sunscreen product in adult corals. Environ. Pollut. 2019, 245, 462–471. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Tsui, M.M.P.; Tan, C.J.; Ng, K.Y.; Guo, F.W.; Wang, L.H.; Fan, T.Y.; Lam, P.K.S.; Murphy, M.B. Comparative toxicities of four benzophenone ultraviolet filters to two life stages of two coral species. Sci. Total Environ. 2019, 651, 2391–2399. [Google Scholar] [CrossRef]
- Wijgerde, T.; van Ballegooijen, M.; Nijland, R.; van der Loos, L.; Kwadijk, C.; Osinga, R.; Murk, A.; Slijkerman, D. Adding insult to injury: Effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci. Total Environ. 2020, 733, 139130. [Google Scholar] [CrossRef]
- DiNardo, J.C.; Downs, C.A. Dermatological and environmental toxicological impact of the sunscreen ingredient oxybenzone/benzophenone-3. J. Cosmet. Derm. 2017. [CrossRef] [PubMed]
- Blüthgen, N.; Zucchi, S.; Fent, K. Effects of the UV filter benzophenone-3 (oxybenzone) at low concentrations in zebrafish (Danio rerio). Toxicol. Appl. Pharm. 2012, 263, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Downs, C.A.; Kramarsky-Winter, E.; Fauth, J.E.; Segal, R.; Bronstein, O.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; Pennington, P.; Kushmaro, A.; et al. Toxicological Effects of the Sunscreen UV Filter, Benzophenone-2, on Planulae and In Vitro Cells of the Coral. Stylophora Pist. Ecotoxicol. 2014, 23, 175. [Google Scholar] [CrossRef] [PubMed]
- Raffa, R.B.; Pergolizzi, J.V.; Taylor, R.; Kitzen, J.M. Sunscreen bans: Coral reefs and skin cancer. J. Clin. Pharm. Ther. 2019, 44, 134–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banning Personal Care Products. In Proceedings of the Hawaii State Legislature, SB 260, Honolulu, HI, USA, January 2017.
- Hofmann, D.K.; Fitt, W.K.; Fleck, J. Checkpoints in the life-cycle of Cassiopea spp.: Control of metagenesis and metamorphosis in a tropical jellyfish. Int. J. Dev. Biol. 1996, 40, 331–338. [Google Scholar] [PubMed]
- Tsui, M.M.P.; Lam, J.C.; Ng, T.Y.; Murphy, M.B.; Lam, P.K.S. Occurrence, distribution and fate of organic UV filters in coral communities. Environ. Sci. Technol. 2017, 51, 4182–4190. [Google Scholar] [CrossRef] [PubMed]
- Fleck, J.; Fitt, W.K. Degrading Mangrove Leaves of Rhizophora mangle Linne Provide a Natural Cue for Settlement and Metamorphosis of the Upside Down Jellyfish Cassiopea xamachana Bigelow. J. Exp. Mar. Biol. Ecol. 1999, 234, 83–94. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitt, W.K.; Hofmann, D.K. The Effects of the UV-Blocker Oxybenzone (Benzophenone-3) on Planulae Swimming and Metamorphosis of the Scyphozoans Cassiopea xamachana and Cassiopea frondosa. Oceans 2020, 1, 174-180. https://0-doi-org.brum.beds.ac.uk/10.3390/oceans1040013
Fitt WK, Hofmann DK. The Effects of the UV-Blocker Oxybenzone (Benzophenone-3) on Planulae Swimming and Metamorphosis of the Scyphozoans Cassiopea xamachana and Cassiopea frondosa. Oceans. 2020; 1(4):174-180. https://0-doi-org.brum.beds.ac.uk/10.3390/oceans1040013
Chicago/Turabian StyleFitt, William K., and Dietrich K. Hofmann. 2020. "The Effects of the UV-Blocker Oxybenzone (Benzophenone-3) on Planulae Swimming and Metamorphosis of the Scyphozoans Cassiopea xamachana and Cassiopea frondosa" Oceans 1, no. 4: 174-180. https://0-doi-org.brum.beds.ac.uk/10.3390/oceans1040013



