Consistency Is Critical for the Effective Use of Baited Remote Video
Abstract
:1. Introduction
2. Materials and Methods
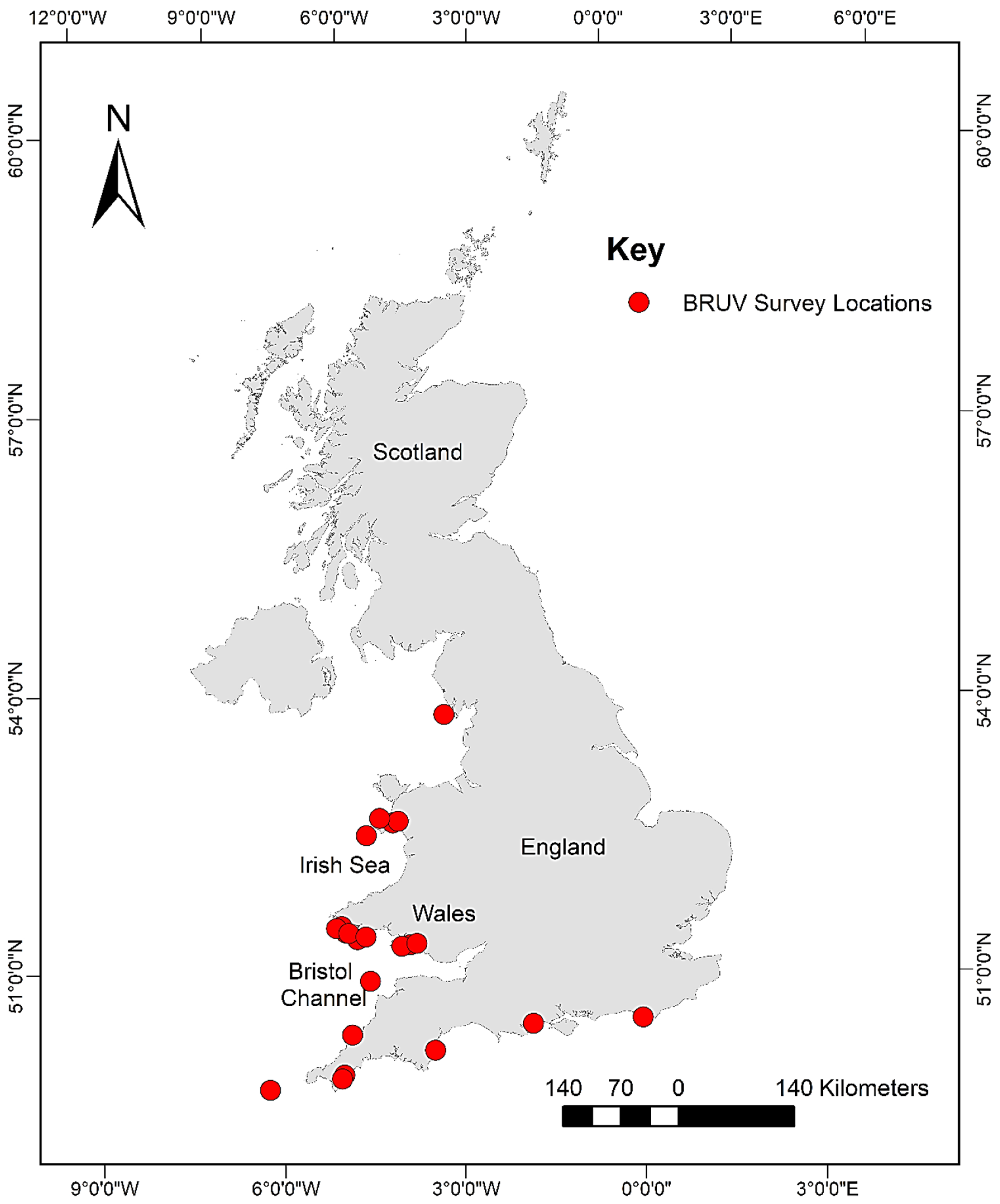
2.1. Database Compilation
2.2. Image Quality Criteria
2.3. Video Analysis
2.4. Data Analysis
3. Results
3.1. General Observations
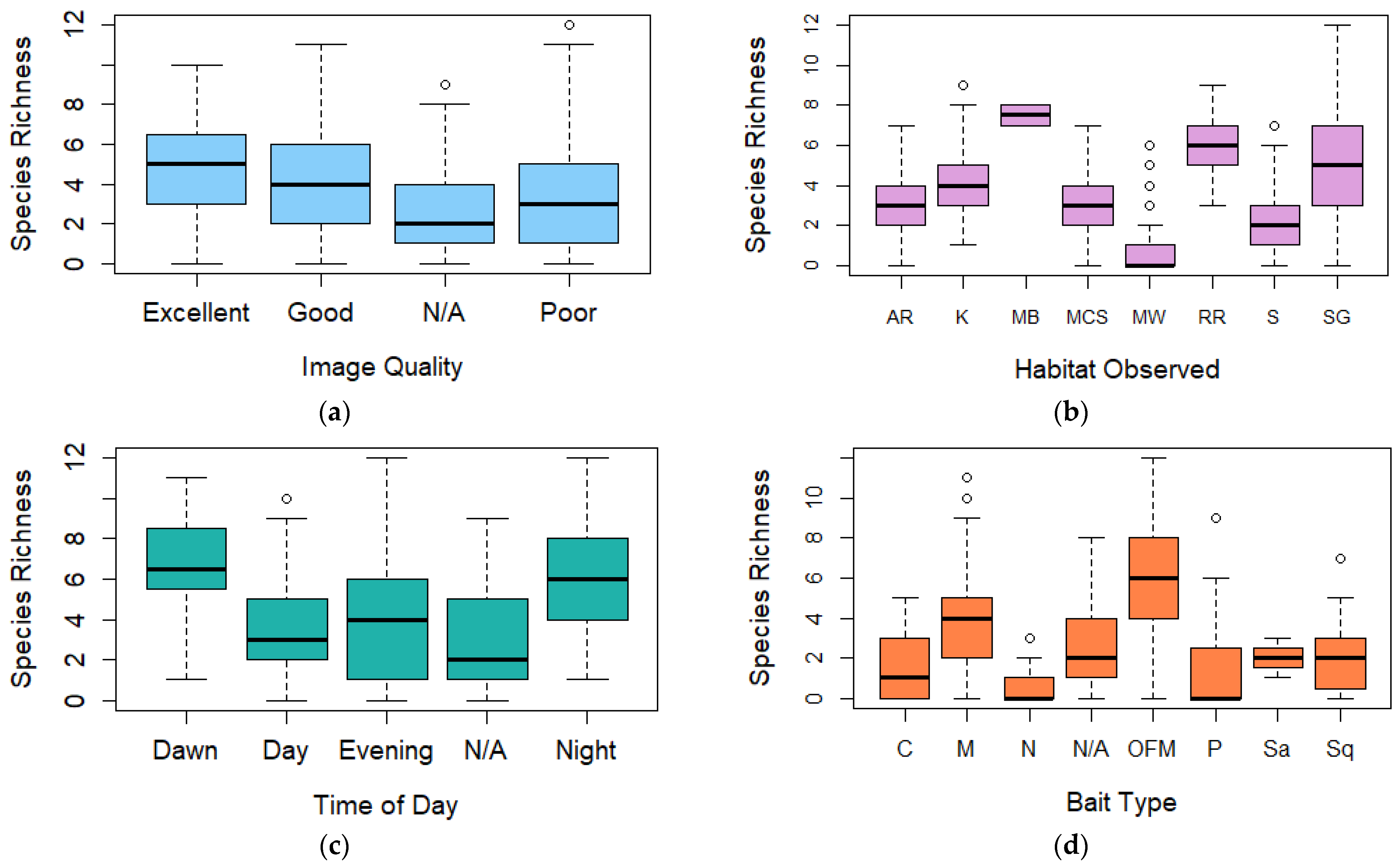
3.2. Species Richness
3.3. Relative Abundance
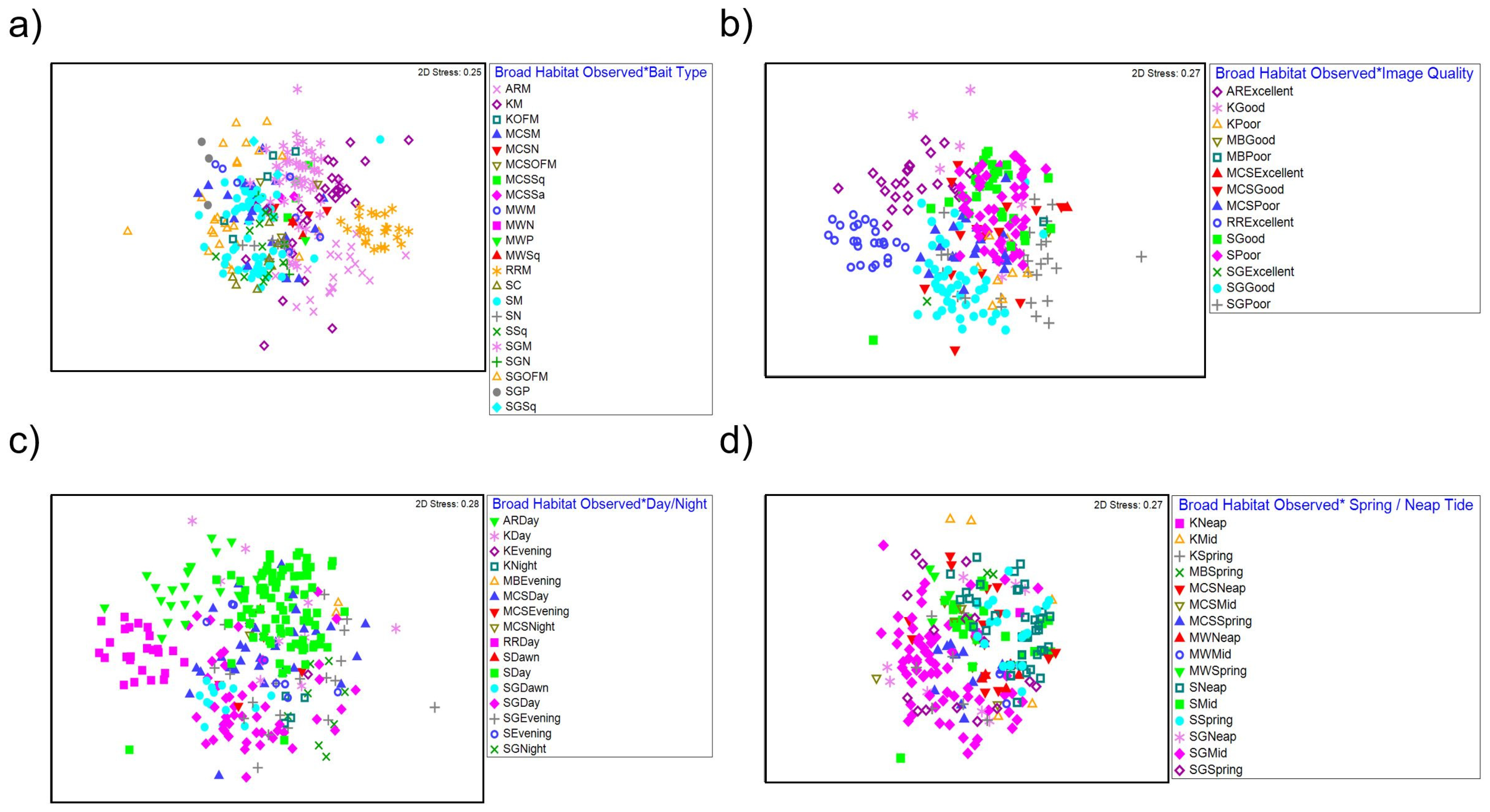
3.4. Faunal Assemblage Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitmarsh, S.K.; Fairweather, P.G.; Huveneers, C. What is Big BRUVver up to? Methods and uses of baited underwater video. Rev. Fish Biol. Fish. 2017, 27, 53–73. [Google Scholar] [CrossRef]
- Lowry, M.; Folpp, H.; Gregson, M.; Mckenzie, R. A comparison of methods for estimating fish assemblages associated with estuarine artificial reefs. Braz. J. Oceanogr. 2011, 59, 119–131. [Google Scholar] [CrossRef]
- Mallet, D.; Pelletier, D. Underwater video techniques for observing coastal marine biodiversity: A review of sixty years of publications (1952–2012). Fish. Res. 2014, 154, 44–62. [Google Scholar] [CrossRef]
- Cappo, M.; Harvey, E.S.; Shortis, M. Counting and Measuring Fish with Baited Video Techniques—An Overview. In Proceedings of the Australian Society for Fish Biology Workshop Proceedings, Hobart, Australia, 28–29 August 2006; pp. 101–114. [Google Scholar] [CrossRef]
- Wraith, J.; Lynch, T.; Minchinton, T.E.; Broad, A.; Davis, A.R. Bait type affects fish assemblages and feeding guilds observed at baited remote underwater video stations. Mar. Ecol. Prog. Ser. 2013, 477, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Ellis, D.M.; DeMartini, E.E. Evaluation of a video camera technique for indexing abundances of juvenile pink snapper, Pristipomoides filamentosus, and other Hawaiian insular shelf fishes. Fish. Bull. 1995, 93, 67–77. [Google Scholar]
- Martinez, I.; Jones, E.G.; Davie, S.L.; Neat, F.C.; Wigham, B.D.; Priede, I.G. Variability in behaviour of four fish species attracted to baited underwater cameras in the North Sea. Hydrobiologia 2011, 670, 23–34. [Google Scholar] [CrossRef]
- Priede, I.G.; Ragley, P.M.; Smith, K.L. Seasonal change in activity of abyssal demersal scavenging grenadiers Coryphaenoides (Nematonums) armatus in the eastern North Pacific Ocean. Limnol. Oceanogr. 1994, 39, 279–285. [Google Scholar] [CrossRef]
- Jones, R.E.; Griffin, R.A.; Rees, S.C.; Unsworth, R.K. Improving visual biodiversity assessments of motile fauna in turbid aquatic environments. Limnol. Oceanogr. Methods 2019, 17, 544–554. [Google Scholar] [CrossRef] [Green Version]
- Davies, J.; Baxter, J.; Bradley, M.; Connor, D.; Khan, J.; Murray, E.; Sanderson, W.; Turnbull, C.; Vincent, M. Marine Monitoring Handbook; Joint Nature Conservation Committee: Peterborough, UK, 2001; ISBN 1 86107 5243.
- Borland, H.; Schlacher, T.; Gilby, B.; Connolly, R.; Yabsley, N.; Olds, A. Habitat type and beach exposure shape fish assemblages in the surf zones of ocean beaches. Mar. Ecol. Prog. Ser. 2017, 570, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Schultz, A.L.; Malcolm, H.A.; Ferrari, R.; Smith, S.D. Wave energy drives biotic patterns beyond the surf zone: Factors influencing abundance and occurrence of mobile fauna adjacent to subtropical beaches. Reg. Stud. Mar. Sci. 2019, 25, 100467. [Google Scholar] [CrossRef]
- Vargas-Fonseca, E.; Olds, A.D.; Gilby, B.L.; Connolly, R.M.; Schoeman, D.S.; Huijbers, C.M.; Hyndes, G.A.; Schlacher, T.A. Combined effects of urbanization and connectivity on iconic coastal fishes. Divers. Distrib. 2016, 22, 1328–1341. [Google Scholar] [CrossRef] [Green Version]
- Haggitt, T.; Freeman, D.; Lily, C. Baited Remote Underwater Video Guidelines; eCoast Ltd.: Raglan, New Zealand, 2014; p. 82. [Google Scholar]
- Langlois, T.; Goetze, J.; Bond, T.; Monk, J.; Abesamis, R.A.; Asher, J.; Barrett, N.; Bernard, A.T.F.; Bouchet, P.J.; Birt, M.J.; et al. A field and video annotation guide for baited remote underwater stereo-video surveys of demersal fish assemblages. Methods Ecol. Evol. 2020, 11, 1401–1409. [Google Scholar] [CrossRef]
- Wilkinson, M.D.; Dumontier, M.; Aalbersberg, I.J.; Appleton, G.; Axton, M.; Baak, A.; Blomberg, N.; Boiten, J.-W.; da Silva Santos, L.B.; Bourne, P.E.; et al. Comment: The FAIR Guiding Principles for scientific data management and stewardship. Sci. Data 2016, 3, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unsworth, R.; Peters, J.; McCloskey, R.; Hinder, S. Optimising stereo baited underwater video for sampling fish and invertebrates in temperate coastal habitats. Estuar. Coast. Shelf Sci. 2014, 150, 281–287. [Google Scholar] [CrossRef]
- Dorman, S.R.; Harvey, E.S.; Newman, S.J. Bait Effects in Sampling Coral Reef Fish Assemblages with Stereo-BRUVs. PLoS ONE 2012, 7, e41538. [Google Scholar] [CrossRef] [Green Version]
- Hannah, R.W.; Blume, M.T.O. The Influence of Bait and Stereo Video on the Performance of a Video Lander as a Survey Tool for Marine Demersal Reef Fishes in Oregon Waters. Mar. Coast. Fish. 2014, 6, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Harvey, E.S.; Cappo, M.; Butler, J.J.; Hall, N.; Kendrick, G.A. Bait attraction affects the performance of remote underwater video stations in assessment of demersal fish community structure. Mar. Ecol. Prog. Ser. 2007, 350, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.E.; Griffin, R.A.; Januchowski-Hartley, S.R.; Unsworth, R.K. The influence of bait on remote underwater video observations in shallow-water coastal environments associated with the North-Eastern Atlantic. PeerJ 2020, 8, e9744. [Google Scholar] [CrossRef]
- Bassett, D.; Montgomery, J. Investigating nocturnal fish populations in situ using baited underwater video: With special reference to their olfactory capabilities. J. Exp. Mar. Biol. Ecol. 2011, 409, 194–199. [Google Scholar] [CrossRef]
- Birt, M.J.; Harvey, E.S.; Langlois, T.J. Within and between day variability in temperate reef fish assemblages: Learned response to baited video. J. Exp. Mar. Biol. Ecol. 2012, 416–417, 92–100. [Google Scholar] [CrossRef]
- Taylor, M.D.; Baker, J.; Suthers, I.M. Tidal currents, sampling effort and baited remote underwater video (BRUV) surveys: Are we drawing the right conclusions? Fish. Res. 2013, 140, 96–104. [Google Scholar] [CrossRef]
- McLean, D.L.; Langlois, T.J.; Newman, S.J.; Holmes, T.H.; Birt, M.J.; Bornt, K.R.; Bond, T.; Collins, D.L.; Evans, S.N.; Travers, M.J.; et al. Distribution, abundance, diversity and habitat associations of fishes across a bioregion experiencing rapid coastal development. Estuar. Coast. Shelf Sci. 2016, 178, 36–47. [Google Scholar] [CrossRef]
- Grimmel, H.M.; Bullock, R.W.; Dedman, S.L.; Guttridge, T.L.; Bond, M.E. Assessment of faunal communities and habitat use within a shallow water system using non-invasive BRUVs methodology. Aquac. Fish. 2020, 5, 224–233. [Google Scholar] [CrossRef]
- Costello, M.J.; Basher, Z.; Mcleod, L.; Asaad, I.; Claus, S.; Vandepitte, L.; Yasuhara, M.; Gislason, H.; Edwards, M.; Appeltans, W.; et al. The GEO Handbook on Biodiversity Observation Networks; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef] [Green Version]
- Turrell, W. Improving the implementation of marine monitoring in the northeast Atlantic. Mar. Pollut. Bull. 2018, 128, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Murphy, H.M.; Jenkins, G.P. Observational methods used in marine spatial monitoring of fishes and associated habitats: A review. Mar. Freshw. Res. 2010, 61, 236–252. [Google Scholar] [CrossRef]
- Zuur, A.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. Primer V6: User Manual—Tutorial; Plymouth Marine Laboratory: Plymouth, UK, 2007. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA); Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 1–15. [Google Scholar] [CrossRef]
- Sherman, C.S.; Heupel, M.R.; Johnson, M.; Kaimuddin, M.; Qamar, L.M.S.; Chin, A.; Simpfendorfer, C.A. Repeatability of baited remote underwater video station (BRUVS) results within and between seasons. PLoS ONE 2020, 15, e0244154. [Google Scholar] [CrossRef]
- Bernard, A.T.F.; Götz, A. Bait increases the precision in count data from remote underwater video for most subtidal reef fish in the warm-temperate Agulhas bioregion. Mar. Ecol. Prog. Ser. 2012, 471, 235–252. [Google Scholar] [CrossRef] [Green Version]
- Cyr, C.; Sainte-Marie, B. Catch of Japanese crab traps in relation to bait quantity and shielding. Fish. Res. 1995, 24, 129–139. [Google Scholar] [CrossRef]
- Miller, R.J. How Many Traps Should a Crab Fisherman Fish? N. Am. J. Fish. Manag. 1983, 3, 1–8. [Google Scholar] [CrossRef]
- Hardinge, J.; Harvey, E.S.; Saunders, B.J.; Newman, S.J. A little bait goes a long way: The influence of bait quantity on a temperate fish assemblage sampled using stereo-BRUVs. J. Exp. Mar. Biol. Ecol. 2013, 449, 250–260. [Google Scholar] [CrossRef]
- Pattiaratchi, C.; Collins, M. Sediment transport under waves and tidal currents: A case study from the northern Bristol Channel, U.K. Mar. Geol. 1984, 56, 27–40. [Google Scholar] [CrossRef]
- Heathershaw, A.; Langhorne, D. Observations of near-bed velocity profiles and seabed roughness in tidal currents flowing over sandy gravels. Estuar. Coast. Shelf Sci. 1988, 26, 459–482. [Google Scholar] [CrossRef]
- O’Byrne, M.; Schoefs, F.; Pakrashi, V.; Ghosh, B. An underwater lighting and turbidity image repository for analysing the performance of image-based non-destructive techniques. Struct. Infrastruct. Eng. 2017, 14, 104–123. [Google Scholar] [CrossRef] [Green Version]
- Whitmarsh, S.K.; Huveneers, C.; Fairweather, P.G. What are we missing? Advantages of more than one viewpoint to estimate fish assemblages using baited video. R. Soc. Open Sci. 2018, 5, 171993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, M.A.; Francis, M.P.; Hartill, B.W.; Parkinson, D.M. Diurnal and Tidal Variation in the Abundance of the Fish Fauna of a Temperate Tidal Mudflat. Estuar. Coast. Shelf Sci. 2002, 54, 793–807. [Google Scholar] [CrossRef]
- Hampel, H.; Cattrijsse, A.; Vincx, M. Tidal, diel and semi-lunar changes in the faunal assemblage of an intertidal salt marsh creek. Estuar. Coast. Shelf Sci. 2003, 56, 795–805. [Google Scholar] [CrossRef]
- Reis-Filho, J.A.; Barros, F.; Nunes, J.; Sampaio, C.L.S.; De Souza, G.B.G. Moon and tide effects on fish capture in a tropical tidal flat. J. Mar. Biol. Assoc. UK 2011, 91, 735–743. [Google Scholar] [CrossRef]
- Grabemann, I.; Uncles, R.; Krause, G.; Stephens, J. Behaviour of Turbidity Maxima in the Tamar (U.K.) and Weser (F.R.G.) Estuaries. Estuar. Coast. Shelf Sci. 1997, 45, 235–246. [Google Scholar] [CrossRef]
- Uncles, R.J. Physical properties and processes in the Bristol Channel and Severn Estuary. Mar. Pollut. Bull. 2010, 61, 5–20. [Google Scholar] [CrossRef]
- Allen, G.; Salomon, J.; Bassoullet, P.; Du Penhoat, Y.; de Grandpré, C. Effects of tides on mixing and suspended sediment transport in macrotidal estuaries. Sediment. Geol. 1980, 26, 69–90. [Google Scholar] [CrossRef]
- Gonzalez-Santamaria, R.; Zou, Q.-P.; Pan, S. Impacts of a Wave Farm on Waves, Currents and Coastal Morphology in South West England. Estuaries Coasts 2013, 38, 159–172. [Google Scholar] [CrossRef]
- Fetterplace, L. The Ecology of Temperate Soft Sediment Fishes: Implications for Fisheries Management and Marine Protected Area Design. Ph.D. Thesis, University of Wollongong, Wollongong, Australia, 2017. Available online: https://ro.uow.edu.au/theses1/375 (accessed on 23 February 2021).
- Schultz, A.L.; Malcolm, H.A.; Bucher, D.J.; Smith, S.D.A. Effects of Reef Proximity on the Structure of Fish Assemblages of Unconsolidated Substrata. PLoS ONE 2012, 7, e49437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
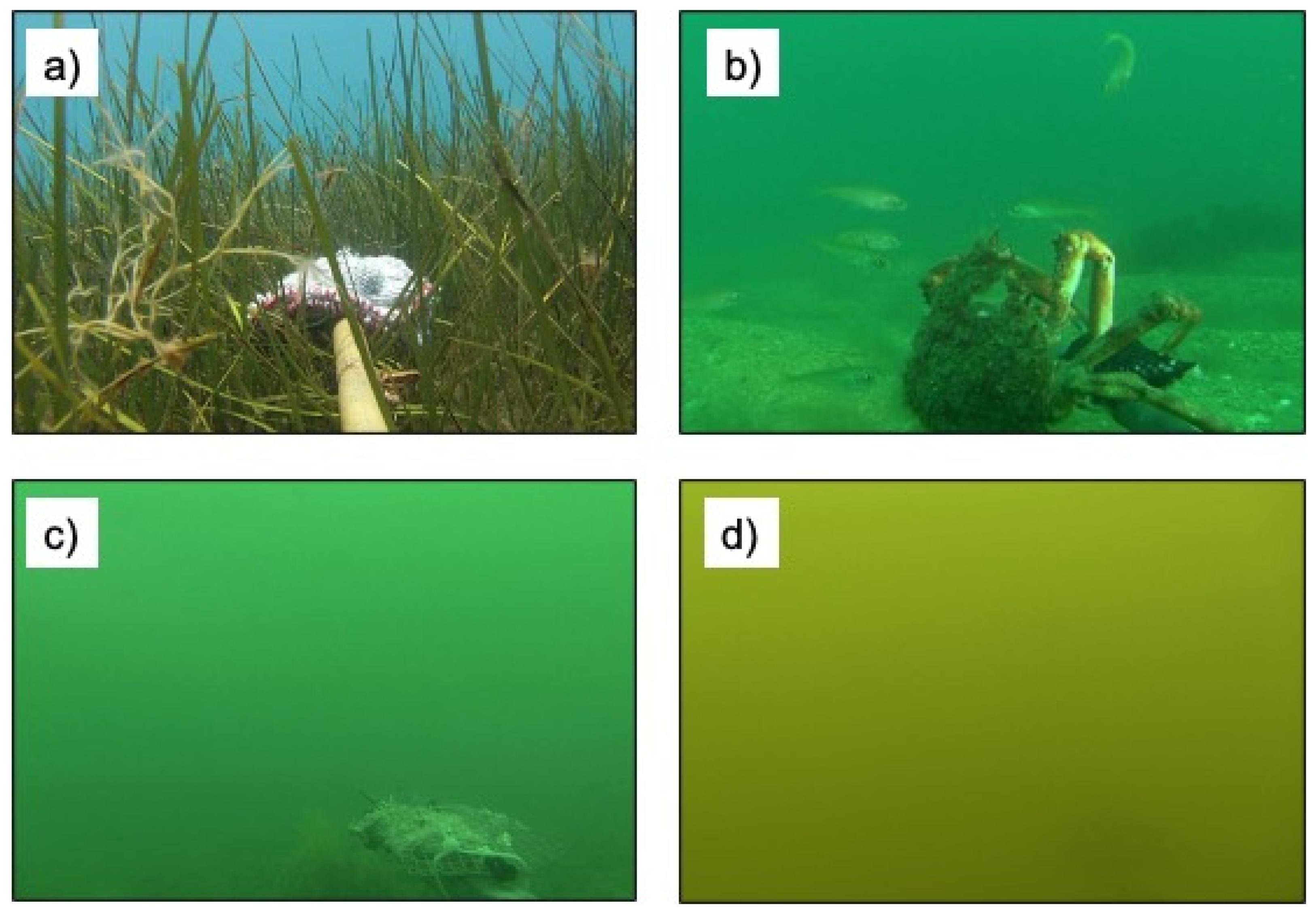



| Image Category | Image Code | Description |
|---|---|---|
| Excellent | 3 | Can clearly see the bait plus over 1 m into the distance. |
| Good | 2 | Can clearly see the bait and maximum 1 m into the distance. |
| Poor | 1 | Can see the bait only. |
| Unusable | 0 | Unable to see bait and fish not clearly visible for identification purposes. |
| Predictors | AIC | AICc | R2 |
|---|---|---|---|
| Base model: All 7 predictors | 346.05 | 352.45 | 35.68% |
| Bait Type | 328.64 | 329.34 | 26.09% |
| Bait Type + Tidal State | 330.62 | 331.57 | 26.11% |
| Bait Type + Tidal State + Image Quality | 331.43 | 332.99 | 30.76% |
| Broad Habitat + Bait Type + Tidal State | 333.06 | 334.97 | 31.30% |
| Bait Type + Tidal State + Spring/Neap | 334.23 | 335.79 | 26.68% |
| Habitat Observed + Bait Type + Tidal State + Duration | 334.31 | 336.62 | 32.40% |
| Habitat Observed + Bait Type + Tidal State + Spring/Neap + Duration | 334.69 | 336.60 | 28.92% |
| Habitat Observed + Bait Type + Tidal State + Image Quality | 335.44 | 338.20 | 33.66% |
| Habitat Observed + Bait Type + Tidal State + Spring/Neap | 336.82 | 339.58 | 31.65% |
| Habitat Observed + Bait Type + Tidal State + Image Quality + Duration | 336.82 | 340.07 | 34.56% |
| Habitat Observed + Bait Type + Tidal State + Time of Day | 338.79 | 342.04 | 31.69% |
| Duration + Tidal State | 339.68 | 339.87 | 11.92% |
| Habitat Observed + Bait Type + Tidal State + Time of Day + Duration | 340.11 | 343.89 | 32.69% |
| Habitat Observed + Bait Type + Tidal State + Time of Day +Image Quality | 341.06 | 345.43 | 34.21% |
| Habitat Observed + Bait Type + Tidal State + Time of Day + Image Quality + Duration | 342.51 | 347.50 | 35.01% |
| Predictors | AIC | AICc | R2 |
|---|---|---|---|
| Base model: All 7 predictors | 426.66 | 433.05 | 41.67% |
| Bait Type + Image Quality + Duration | 416.61 | 418.16 | 36.70% |
| Bait Type + Image Quality + Duration + Tidal State | 418.61 | 420.52 | 36.71% |
| Bait Type + Image Quality + Duration + Tidal State + Spring/Neap | 420.35 | 423.11 | 38.12% |
| Bait Type + Image Quality + Duration + Tidal State + Habitat Observed | 421.65 | 424.90 | 38.56% |
| Bait Type + Image Quality + Duration + Tidal State + Time of Day | 422.56 | 425.81 | 37.99% |
| Bait Type + Image Quality + Duration + Tidal State + Spring/Neap + Habitat Observed | 423.25 | 427.62 | 40.05% |
| Bait Type + Image Quality + Duration + Tidal State + Time of Day + Spring/Neap | 423.61 | 427.97 | 39.83% |
| Source | df | MS | Pseudo-F | P(Perm) | Unique Perms |
|---|---|---|---|---|---|
| Habitat Observed * Image Quality | |||||
| Habitat Observed | 6 | 19,518 | 9.969 | <0.001 | 9806 |
| Image Quality | 2 | 2361.7 | 1.2012 | 0.1965 | 9897 |
| Hab * Image | 5 | 5241.2 | 2.6656 | <0.001 | 9838 |
| Residual | 270 | 1966.2 | |||
| Total | 283 | ||||
| Habitat Observed * Time of Day | |||||
| Habitat Observed | 6 | 25,071 | 12.65 | <0.001 | 9830 |
| Time of Day | 3 | 4899.5 | 2.4721 | <0.001 | 9858 |
| Hab * Time | 6 | 4212.4 | 2.1254 | <0.001 | 9816 |
| Residual | 317 | 1981.9 | |||
| Total | 332 | ||||
| Habitat Observed * Bait Type | |||||
| Habitat Observed | 7 | 25,553 | 14.634 | <0.001 | 9809 |
| Bait Type | 7 | 6663.6 | 3.8161 | <0.001 | 9801 |
| Hab * Bait | 8 | 6049.2 | 3.4643 | <0.001 | 9807 |
| Residual | 325 | 1746.2 | |||
| Total | 347 | ||||
| Habitat Observed * Tide (Spring/Neap) | |||||
| Habitat Observed | 5 | 14,915 | 7.5186 | <0.001 | 9847 |
| Tide | 2 | 6924.2 | 3.4906 | <0.001 | 9903 |
| Hab * Tide | 8 | 6849.6 | 3.4530 | <0.001 | 9820 |
| Residual | 267 | 1983.7 | |||
| Total | 282 | ||||
| Habitat Observed * Tidal State | |||||
| Habitat Observed | 4 | 10,272 | 5.2581 | <0.001 | 9885 |
| Tidal State | 1 | 2601.9 | 1.3319 | 0.2148 | 9921 |
| Hab * State | 3 | 1721.7 | 0.88133 | 0.6547 | 9896 |
| Residual | 119 | 1953.5 | |||
| Total | 127 | ||||
| Habitat Observed * Slack Tide | |||||
| Habitat Observed | 4 | 14,867 | 7.6298 | <0.001 | 9862 |
| Slack Tide | 1 | 2831.9 | 1.4533 | 0.1518 | 9928 |
| Hab*Slack | 3 | 2584.9 | 1.3266 | 0.1177 | 9915 |
| Residual | 119 | 1948.5 | |||
| Total | 127 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, R.E.; Griffin, R.A.; Herbert, R.J.H.; Unsworth, R.K.F. Consistency Is Critical for the Effective Use of Baited Remote Video. Oceans 2021, 2, 215-232. https://0-doi-org.brum.beds.ac.uk/10.3390/oceans2010013
Jones RE, Griffin RA, Herbert RJH, Unsworth RKF. Consistency Is Critical for the Effective Use of Baited Remote Video. Oceans. 2021; 2(1):215-232. https://0-doi-org.brum.beds.ac.uk/10.3390/oceans2010013
Chicago/Turabian StyleJones, Robyn E., Ross A. Griffin, Roger J. H. Herbert, and Richard K. F. Unsworth. 2021. "Consistency Is Critical for the Effective Use of Baited Remote Video" Oceans 2, no. 1: 215-232. https://0-doi-org.brum.beds.ac.uk/10.3390/oceans2010013







