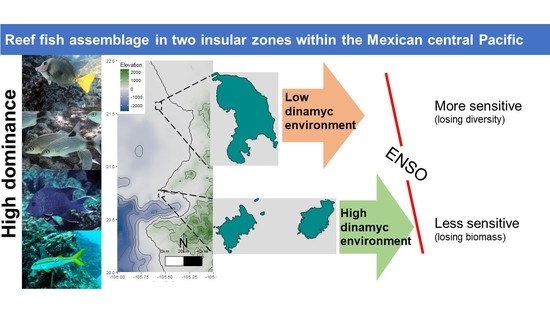
Reef Fish Assemblage in Two Insular Zones within the Mexican Central Pacific
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Fieldwork
2.3. Species-Abundance Analysis
2.4. Taxonomic Distinctness
2.5. Biomass Data Analysis
2.6. Influence of Environmental Variables
3. Results
3.1. Species-Abundance Data Analysis
- (1)
- The pattern of the sample coverage with respect to the q = 0, 1, and two indicators show an increasing tendency (Figure S1a) for the order of total species indicator (q < 0.5); i.e., the sample coverage was lower for MI, meaning that in this island there are more unrecorded species. At MI, the lowest SC value was recorded for the year 2010 (79%, Table 1, Figure S1a), which implies that the remaining 21% accounts for a total of 17 species that were not detected according to the asymptotic model; in contrast, 2015 presented the highest SC (95%), representing an absence of three species accounting for 5% of the SC. Meanwhile, II showed the highest annual SC value corresponding to 2014 (97%), reflecting a difference from the asymptotic model of fewer than one species. However, 2011 and 2017 presented the lowest SC values (86%) for this island, representing a total of 10 and seven species not observed, respectively, according to the asymptotic model (Table 1).
- (2)
- The comparison of each order of q = 1 and 2 showed that the numbers of both abundant and dominant species were the same as those from the asymptotic model in both islands (Table 1, Figure S1a,b). At MI, the year 2011 had the highest value of abundant species (17.4) and the year 2010 showed the highest value of dominant species (10.2). In contrast, the lowest values of both indicators of abundant and dominant species were recorded in 2015 with 13.2 and 6.9 species, respectively; these values suggest that for this year there was a total of 68 rare species (q = 0 − q = 1). At II, the same indicators showed that the year 2011 had the highest number of abundant (19.6) and dominant (14) species, while 2012 resulted in the lowest value of these indicators with 9.8 abundant and 4.4 dominant species, and a total of 50.2 rare species.
- (3)
- The non-asymptotic coverage-based rarefaction and extrapolation analysis (Table 1, Figure S1a,b) shows that although our data are insufficient to infer the true richness of the whole set, inference and significance testing can be extended to an SC cut-off value for both islands of SCmax ≥ 99%. At this confidence level, at MI the year 2011 reached the highest richness (86.27 species), followed by 2016 (77.05), and the lowest was found in 2015 (69). In contrast, at II the highest richness value was obtained in the year 2013 (69.54), followed by 2011 (66.95) and the lowest values were observed in 2014 (40.06).
- (4)
- Under the coverage value of 99%, the evenness profile and Pielou’s measure (Table 1, Figure S1a,b) were similar in the three q = 1, 2, 3 levels (p-value = 0.05); nevertheless, at II, the confidence intervals of the evenness profile show a wider range among the years, which implies more year-on-year variation at this island (Figure S1b).
3.2. Taxonomic Distinctness
3.3. Year-to-Year Biomass Variation
3.3.1. Marietas Islands
3.3.2. Isabel Island
3.4. Influence of Environmental Variables on the Community Indicators
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs High diversity of trees and corals is maintained. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moberg, F.; Folke, C. Ecological goods and services of coral reef ecosystems. Ecol. Econ. 1999, 29, 215–233. [Google Scholar] [CrossRef]
- Wild, C.; Hoegh-Guldberg, O.; Naumann, M.S.; Colombo-Pallota, M.F.; Ateweberham, M.; Fitt, W.K.; Iglesias-Prieto, R.; Palmer, C.; Bythel, J.C.; Ortiz, J.-C.; et al. Climate change impedes scleractinian corals as primary reef ecosystem engineers. Mar. Freshw. Res. 2011, 62, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Germain, N.; Hartmann, H.J.; Fernández-Rivera Melo, F.J.; Reyes-Bonilla, H. Ornamental reef fish fisheries: New indicators of sustainability and human development at a coastal community level. Ocean Coast. Manag. 2015, 104, 136–149. [Google Scholar] [CrossRef]
- Bruno, J.F.; Côté, I.M.; Toth, L.T. Climate Change, coral loss, and the curious case of the parrotfish paradigm: Why don’t marine protected areas improve reef resilience? Ann. Rev. Mar. Sci. 2019, 11, 307–334. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Bonilla, H.; Alvarez-Filip, L. Long-term changes in taxonomic distinctness and trophic structure of reef fishes at Cabo Pulmo reef, Gulf of California. In Proceedings of the 11th International Coral Reef Symposium, Fort Lauderdale, FL, USA, 7–11 July 2008; pp. 790–794. [Google Scholar]
- Fulton, C.J.; Bellwood, D.R.; Wainwright, P.C. Wave energy and swimming performance shape coral reef fish assemblages. Proc. R. Soc. B Biol. Sci. 2005, 272, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Dominici-Arosemena, A.; Wolff, M. Reef fish community structure in the Tropical Eastern Pacific (Panamá): Living on a relatively stable rocky reef environment. Helgol. Mar. Res. 2006, 60, 287–305. [Google Scholar] [CrossRef]
- Robertson, D.R.; Cramer, K.L. Shore fishes and biogeographic subdivisions of the Tropical Eastern Pacific. Mar. Ecol. Prog. Ser. 2009, 380, 1–17. [Google Scholar] [CrossRef]
- Salomón-Aguilar, C.A.; Villavicencio-Garayzar, C.J.; Reyes-Bonilla, H. Zonas y temporadas de reproducción y crianza de tiburones en el Golfo de California: Estrategia para su conservación y manejo pesquero. Cien. Mar. 2009, 35, 369–388. [Google Scholar] [CrossRef] [Green Version]
- Villegas-Sánchez, C.A.; Abitia-Cárdenas, L.A.; Gutiérrez-Sánchez, F.J.; Galván-Magaña, F. Rocky-reef fish assemblages at San José Island, Mexico. Rev. Mex. Biodivers. 2009, 80, 169–179. [Google Scholar]
- Alvarado, J.J.; Beita-Jiménez, A.; Mena, S.; Fernández, J.; Cortés, J.; Sánchez-Noguera, C.; Jiménez, C.; Guzmán-Mora, A.G. Cuando la conservación no puede seguir el ritmo del desarrollo: Estado de salud de los ecosistemas coralinos del Pacífico Norte de Costa Rica. Rev. Biol. Trop. 2018, 66, S280–S308. [Google Scholar] [CrossRef] [Green Version]
- Crowder, L.B.; Lyman, S.J.; Figueira, W.F.; Priddy, J. Source-sink population dynamics and the problems of siting marine reserves. Bull. Mar. Sci. 2000, 66, 799–820. [Google Scholar]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Bostford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; et al. Historical overfishing and the recent collapse of coastal ecosystems. Science 2001, 293, 629–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.S. Marine protected area strategies: Issues, divergence and the search for middle ground. Rev. Fish Biol. Fish. 2002, 11, 197–216. [Google Scholar] [CrossRef]
- Martínez-Árroyo, A.; Manzanilla-Naim, S.; Zavala Hidalgo, J. Vulnerability to climate change of marine and coastal fisheries in México. Atmósfera 2011, 24, 103–123. [Google Scholar]
- Roberts, C.M.; McClean, C.J.; Veron, J.E.; Hawkins, J.P.; Allen, G.R.; McAllister, D.E.; Mittermeier, C.G.; Schueler, F.W.; Spalding, M.; Wells, F.; et al. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 2002, 295, 1280–1284. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Troncoso, A.P.; Carpizo-Ituarte, E.; Pettay, D.T.; Warner, M.E.; Cupul-Magaña, A.L. The effects of an abnormal decrease in temperature on the Eastern Pacific reef-building coral Pocillopora verrucosa. Mar. Biol. 2014, 161, 131–139. [Google Scholar] [CrossRef]
- Reyes-Bonilla, H. Coral reefs of the Pacific coast of México. In Latin American Coral Reefs; Cortés, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 331–349. [Google Scholar] [CrossRef]
- Kramer, P.; Kramer, P.; Arias-Gonzalez, E.; McField, M. Status of coral reefs of northern Central America: Mexico, Belize, Guatemala, Honduras, Nicaragua and El Salvador. In Status of Coral Reefs of the World: 2000; Wilkinson, C., Ed.; Australian Institute of Marine Science: Townsville, Australia, 2000; pp. 287–313. [Google Scholar]
- Collins, C.A.; Garfield, N.; Mascarenhas, A.S.; Spearman, M.G.; Rago, T.A. Ocean Current Measurements Across the Entrance to the Gulf of California. J. Geophys. Res. 1997, 102, 20927–20936. [Google Scholar] [CrossRef]
- Escalante, F.; Valdez-Holguín, J.E.; Álvarez-Borrego, S.; Lara-Lara, J.R. Temporal and spatial variation of sea surface temperature, chlorophyll a, and primary productivity in the Gulf of California. Cienc. Mar. 2013, 39, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Welch, D. What should protected areas managers do in the face of climate change? In The George Wright Forum; George Wright Society: Michigan, MI, USA, 2005; Volume 22, pp. 75–93. [Google Scholar]
- Aburto-Oropeza, O.; Erisman, B.; Galland, G.R.; Mascareñas-Osorio, I.; Sala, E.; Ezcurra, E. Large recovery of fish biomass in a no-take marine reserve. PLoS ONE 2011, 6, e23601. [Google Scholar] [CrossRef] [Green Version]
- Villalobos, F.; Lira-Noriega, A.; Soberón, J.; Arita, H.T. Range-diversity plots for conservation assessments: Using richness and rarity in priority setting. Biol. Conserv. 2013, 158, 313–320. [Google Scholar] [CrossRef]
- Barneche, D.R.; Rezende, E.L.; Parravicini, V.; Maire, E.; Edgar, G.J.; Stuart-Smith, R.D.; Arias-González, J.E.; Ferreira, C.E.L.; Friedlander, A.M.; Green, A.L.; et al. Body size, reef area and temperature predict global reef-fish species richness across spatial scales. Glob. Ecol. Biogeogr. 2018, 28, 315–327. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. A taxonomic distinctness index and its statistical properties. J. Appl. Ecol. 1998, 35, 523–531. [Google Scholar] [CrossRef]
- Ellison, A.M. Partitioning diversity. Ecology 2010, 91, 1962–1963. [Google Scholar] [CrossRef]
- Chao, A.; Kubota, Y.; Zelený, D.; Chiu, C.; Li, C.; Kusumoto, B.; Yasuhara, M.; Thorn, S.; Wei, C.; Costello, M.J.; et al. Quantifying sample completeness and comparing diversities among assemblages. Ecol. Res. 2020, 35, 292–314. [Google Scholar] [CrossRef]
- DOF. Decreto Por el Que se Declara Parque Nacional a la Isla Isabel, Ubicada Frente a las Costas del Estado de Nayarit, Declarándose de Interés Público la Conservación y Aprovechamiento de sus Valores Naturales, Para Fines Recreativos, Culturales y de Investigacion. Diario Oficial de la Federación, Mexico. 8 December 1980. Available online: http://dof.gob.mx/nota_detalle.php?codigo=4862220&fecha=08/12/80#gsc.tab=0 (accessed on 4 June 2020).
- DOF. Decreto Por el Que se Declara área Natural Protegida, con la Categoría de Parque Nacional, la Región Conocida como Islas Marietas, de Jurisdicción Federal, Incluyendo la Zona Marina Que la Circunda, Localizada en la Bahía de Banderas, Frente a las Costas. Diario Oficial de la Federación, Mexico. 25 April 2005. Available online: http://dof.gob.mx/nota_detalle.php?codigo=2034060&fecha=25/04/2005#gsc.tab=0 (accessed on 4 June 2020).
- Ulloa, R.; Torre, J.; Bourillón, L.; Gondor, A.; Alcantar, N. Planeación Ecorregional para la Conservación Marina: Golfo de California y Costa Occidental de Baja California Sur; Final Report to the Nature Conservancy; Comunidad y Biodiversidad A.C.: Guaymas, México, 2006; p. 153. [Google Scholar]
- Lavín, M.F.; Marinone, S.G. An overview of the physical oceanography of the Gulf of California. In Nonlinear Processes in Geophysical Fluid Dynamics; Velasco Fuentes, O.U., Sheinbaum, J., Ochoa, J., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 173–204. [Google Scholar] [CrossRef] [Green Version]
- Chao, A.; Colwell, R.K.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods. Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER: Getting Started with V6; Prim-E Ltd.: Plymouth, UK, 2005. [Google Scholar]
- vegan: Community Ecology Package. Available online: http://CRAN.R-project.org/package=vegan (accessed on 28 November 2020).
- Boettiger, C.; Lang, D.T.; Wainwright, P.C. rfishbase: Exploring, manipulating and visualizing FishBase data from R. J. Fish. Biol. 2012, 81, 2030–2039. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase, Version (06/2021). Available online: www.fishbase.org (accessed on 15 June 2020).
- Hervé, M. Package ‘RVAideMemoire’. Testing and Plotting Procedures for Biostatistics; The R Project for Statistical Computing: Vienna, Austria, 2020; Available online: https://CRAN.R-project.org/package=RVAideMemoire (accessed on 28 June 2021).
- Simons, R.A.; Mendelssohn, R. ERDDAP-A Brokering Data Server for Gridded and Tabular Datasets. In AGU Fall Meeting Abstracts; American Geophysical Union: San Francisco, CA, USA, 2012; p. IN21B-1473. [Google Scholar]
- Plata, L.; Filonov, A. Marea interna en la parte noroeste de la Bahía de Banderas, México. Cienc. Mar. 2007, 33, 197–215. [Google Scholar]
- Godínez, V.M.; Beier, E.; Lavín, M.F.; Kurczyn, J.A. Circulation at the entrance of the Gulf of California from satellite altimeter and hydrographic observations. JGR Ocean. 2010, 115, C4. [Google Scholar] [CrossRef]
- Pantoja, D.A.; Marinone, S.G.; Parés-Sierra, A.; Gómez-Valdivia, F. Modelación numérica de la hidrografía y circulación estacional y de mesoescala en el Pacífico Central Mexicano. Cienc. Mar. 2012, 38, 363–379. [Google Scholar] [CrossRef]
- Alvarez-Borrego, S.; Lara-Lara, J.R. The Physical Environment and Primary Productivity of the Gulf of California. In The Gulf and Peninsular Province of the Californias; Dauphin, J.P., Simoneit, B.R.T., Eds.; American Association of Petroleum Geologists: Tulsa, OK, USA, 1991; pp. 555–567. [Google Scholar] [CrossRef]
- Alvarez-Filip, L.; Reyes-Bonilla, H.; Calderon-Aguilera, L.E. Community structure of fishes in Cabo Pulmo reef, Gulf of California. Mar. Ecol. 2006, 27, 253–262. [Google Scholar] [CrossRef]
- Fourriére, M.; Reyes-Bonilla, H.; Galván-Villa, C.M.; Ayala Bocos, A.; Rodríguez-Zaragoza, F.A. Reef fish structure assemblages in oceanic islands of the eastern tropical Pacific: Revillagigedo Archipelago and Clipperton atoll. Mar. Ecol. 2019, 40, 12539. [Google Scholar] [CrossRef]
- Clarke, K.R.; Warwick, R.M. A further biodiversity index applicable to species lists: Variation in taxonomic distinctness. Mar. Ecol. Prog. Ser. 2001, 216, 265–278. [Google Scholar] [CrossRef]
- CONANP. Programa de Conservación y Manejo Parque Nacional Islas Marietas; Semarnat, D.F., Ed.; Comisión Nacional de Áreas Naturales Protegidas: Mexico City, Mexico, 2007.
- Munday, P.L.; Donelson, J.M.; Domingos, J.A. Potential for adaptation to climate change in a coral reef fish. Glob. Chang. Biol. 2017, 23, 307–317. [Google Scholar] [CrossRef]
- Attrill, M.J.; Power, M. Climatic influence on a marine fish assemblage. Nature 2002, 417, 275–278. [Google Scholar] [CrossRef]
- Robertson, D.R.; Allen, G.R. Fishes of the Tropical Eastern Pacific; University of Hawaii Press: Honolulu, HI, USA, 2002. [Google Scholar]
- Virgili, A.; Racine, M.; Authier, M.; Monestiez, P.; Ridoux, V. Comparison of habitat models for scarcely detected species. Ecol. Model. 2017, 346, 88–98. [Google Scholar] [CrossRef]
- López-Sandoval, D.C.; Lara-Lara, J.R.; Álvarez-Borrego, S. Phytoplankton production by remote sensing in the region off Cabo Corrientes, Mexico. Hidrobiologica 2009, 19, 185–192. [Google Scholar]
- Blanchard, J.L.; Jennings, S.; Holmes, R.; Harle, J.; Merino, G.; Allen, J.I.; Holt, J.; Dulvy, N.K.; Barange, M. Potential consequences of climate change for primary production and fish production in large marine ecosystems. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2979–2989. [Google Scholar] [CrossRef]
- Páez-Osuna, F.; Sanchez-Cabeza, J.A.; Ruiz-Fernández, A.C.; Alonso-Rodríguez, R.; Piñón-Gimate, A.; Cardoso-Mohedano, J.G.; Flores-Verdugo, F.J.; Carballo, J.L.; Cisneros-Mata, M.A.; Álvarez-Borrego, S. Environmental status of the Gulf of California: A review of responses to climate change and climate variability. Earth-Sci. Rev. 2016, 162, 253–268. [Google Scholar] [CrossRef]
- López-Sandoval, D.C.; Lara-Lara, J.R.; Lavín, M.F.; Álvarez-Borrego, S.; Gaxiola-Castro, G. Primary productivity observations in the eastern tropical Pacific off Cabo Corrientes, Mexico. Cienc. Mar. 2009, 35, 169–182. [Google Scholar] [CrossRef] [Green Version]
- Brander, K. Impacts of climate change on fisheries. J. Mar. Syst. 2010, 79, 389–402. [Google Scholar] [CrossRef]
- Descombes, P.; Wisz, M.S.; Leprieur, F.; Parravicini, V.; Heine, C.; Olsen, S.M.; Swingedouw, D.; Kulbicki, M.; Mouillot, D.; Pellissier, L. Forecasted coral reef decline in marine biodiversity hotspots under climate change. Glob. Change Biol. 2015, 21, 2479–2487. [Google Scholar] [CrossRef] [Green Version]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.M.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. Bioscience 2007, 57, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Bonaldo, R.M.; Hoey, A.S.; Bellwood, D.R. The ecosystem roles of parrotfishes on tropical reefs. Oceanogr. Mar. Biol. Annu. Rev. 2014, 52, 81–132. [Google Scholar] [CrossRef]





| (a) | (b) | (c) | (d) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Is/yr | Nobs | q = 0 | q = 1 | q = 2 | q = 0 Emp | q = 0 Asy | q = 1 Emp | q = 1 Asy | q = 2 Emp | q = 2 Asy | q = 0 | q = 1 | q = 2 | J’ | q = 1 | q = 2 | Rare sp. |
| MI/2010 | 6796 | 0.79 | 0.99 | 0.99 | 68 | 85 | 15.7 | 15.7 | 10.2 | 10.2 | 76.75 | 15.73 | 10.23 | 0.63 | 0.19 | 0.12 | 52.3 |
| MI/2011 | 22,144 | 0.89 | 0.99 | 0.99 | 92 | 102 | 17.4 | 17.4 | 9.8 | 9.7 | 86.27 | 17.34 | 9.77 | 0.64 | 0.19 | 0.1 | 74.6 |
| MI/2012 | 26,452 | 0.89 | 0.99 | 0.99 | 82 | 91 | 14 | 14 | 7.4 | 7.4 | 73.29 | 13.92 | 7.42 | 0.61 | 0.17 | 0.08 | 68.8 |
| MI/2013 | 57,546 | 0.94 | 0.99 | 0.99 | 88 | 92 | 15.9 | 16 | 9.2 | 9.2 | 74.25 | 15.93 | 9.21 | 0.64 | 0.2 | 0.11 | 72.1 |
| MI/2014 | 50,451 | 0.94 | 0.99 | 0.99 | 89 | 93 | 14.2 | 14.2 | 7.5 | 7.5 | 76.26 | 14.23 | 7.46 | 0.61 | 0.17 | 0.08 | 74.8 |
| MI/2015 | 45,233 | 0.95 | 0.99 | 0.99 | 82 | 85 | 13.2 | 13.2 | 6.9 | 6.8 | 69.19 | 13.16 | 6.87 | 0.60 | 0.17 | 0.08 | 68.8 |
| MI/2016 | 45,280 | 0.94 | 0.99 | 0.99 | 90 | 94 | 13.9 | 13.9 | 7.4 | 7.3 | 77.05 | 13.88 | 7.38 | 0.60 | 0.16 | 0.08 | 76.1 |
| MI/2017 | 41,388 | 0.87 | 0.99 | 0.99 | 88 | 100 | 13.7 | 13.7 | 7.3 | 7.3 | 75.88 | 13.69 | 7.38 | 0.60 | 0.16 | 0.08 | 74.3 |
| II/2010 | 10,770 | 0.87 | 0.99 | 0.99 | 61 | 61 | 14.3 | 14.4 | 8.3 | 8.2 | 56.56 | 14.29 | 8.25 | 0.65 | 0.23 | 0.13 | 46.7 |
| II/2011 | 8653 | 0.86 | 0.99 | 0.99 | 68 | 78 | 19.6 | 19.7 | 14 | 14 | 66.95 | 19.64 | 14.01 | 0.70 | 0.28 | 0.19 | 48.4 |
| II/2012 | 7718 | 0.89 | 0.99 | 0.99 | 60 | 66 | 9.8 | 9.9 | 4.4 | 4.4 | 56.49 | 9.84 | 4.37 | 0.56 | 0.15 | 0.06 | 50.2 |
| II/2013 | 14,515 | 0.90 | 0.99 | 0.99 | 77 | 84 | 17.2 | 17.2 | 11.6 | 11.6 | 69.54 | 17.22 | 11.26 | 0.67 | 0.23 | 0.15 | 59.8 |
| II/2014 | 1098 | 0.97 | 0.99 | 0.99 | 40 | 40 | 15.5 | 15.5 | 9.8 | 9.8 | 40.06 | 15.27 | 9.81 | 0.73 | 0.36 | 0.22 | 24.5 |
| II/2015 | 6660 | 0.95 | 0.99 | 0.99 | 58 | 60 | 13.1 | 13.1 | 8.2 | 8.2 | 55.71 | 13.1 | 8.2 | 0.64 | 0.22 | 0.13 | 44.9 |
| II/2017 | 1927 | 0.86 | 0.99 | 0.99 | 51 | 58 | 15.8 | 16 | 10.1 | 10.1 | 56.05 | 15.97 | 10.17 | 0.68 | 0.27 | 0.16 | 35.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez de-Silva, C.V.; Cupul-Magaña, A.L.; Rodríguez-Troncoso, A.P.; Rodríguez-Zaragoza, F.A. Reef Fish Assemblage in Two Insular Zones within the Mexican Central Pacific. Oceans 2022, 3, 204-217. https://0-doi-org.brum.beds.ac.uk/10.3390/oceans3020015
Pérez de-Silva CV, Cupul-Magaña AL, Rodríguez-Troncoso AP, Rodríguez-Zaragoza FA. Reef Fish Assemblage in Two Insular Zones within the Mexican Central Pacific. Oceans. 2022; 3(2):204-217. https://0-doi-org.brum.beds.ac.uk/10.3390/oceans3020015
Chicago/Turabian StylePérez de-Silva, Carlos Vladimir, Amílcar Leví Cupul-Magaña, Alma Paola Rodríguez-Troncoso, and Fabián Alejandro Rodríguez-Zaragoza. 2022. "Reef Fish Assemblage in Two Insular Zones within the Mexican Central Pacific" Oceans 3, no. 2: 204-217. https://0-doi-org.brum.beds.ac.uk/10.3390/oceans3020015