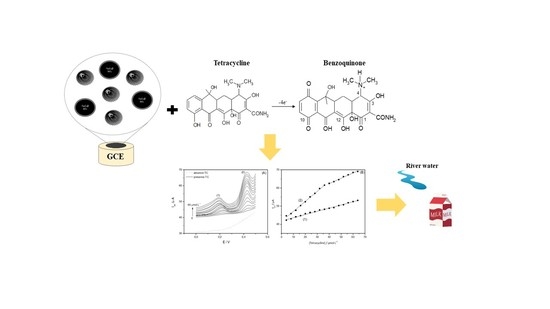
Development of an Electrochemical Sensor Based on Nanocomposite of Fe3O4@SiO2 and Multiwalled Carbon Nanotubes for Determination of Tetracycline in Real Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Synthesis of Nanocomposites of Fe3O4@SiO2
2.3. Characterization of the Modifiers
2.4. Point of Zero Charge of Fe3O4@SiO2 Nanocomposite
2.5. Apparatus
2.6. Preparation of GCE/MWCNT/Fe3O4@SiO2 Modified Electrode
2.7. Evaluation of Experimental Parameters
2.8. Selectivity of the Proposed Sensor
2.9. Application in Real Samples
3. Results and Discussion
3.1. Characterization of the Modifiers
3.2. Study of the Configuration of the Working Electrode
3.3. Electrochemical Behavior of the Sensor
3.4. Optimization of Experimental Parameters for GCE/MWCNT/Fe3O4@SiO2 Sensor
3.5. Influence of Electrochemical Technical
3.6. Analytical Curve
3.7. Selectivity
3.8. Application of the Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, J.; Wang, H.; Qu, J.; Wang, H.; Wang, X. Development and optimization of a naphthoic acid-based ionic liquid as a “non-organic solvent microextraction” for the determination of tetracycline antibiotics in milk and chicken eggs. Food Chem. 2017, 215, 138–148. [Google Scholar] [CrossRef]
- Alawad, A.; Istamboulié, G.; Calas-Blanchard, C.; Noguer, T. A reagentless aptasensor based on intrinsic aptamer redox activity for the detection of tetracycline in water. Sens. Actuators B Chem. 2019, 288, 141–146. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A.; Aydar, S.; Ozyurt, D.; Demirata, B. Determination of Tetracycline on the Surface of a High-Performance Graphene Modified Screen-Printed Carbon Electrode in Milk and Honey Samples. Curr. Nanosci. 2016, 12, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Pailler, J.-Y.; Krein, A.; Pfister, L.; Hoffmann, L.; Guignard, C. Solid phase extraction coupled to liquid chromatography-tandem mass spectrometry analysis of sulfonamides, tetracyclines, analgesics and hormones in surface water and wastewater in Luxembourg. Sci. Total Environ. 2009, 407, 4736–4743. [Google Scholar] [CrossRef]
- Calixto, C.M.F.; Cavalheiro, E.T.G. Determination of Tetracycline in Bovine and Breast Milk Using a Graphite-Polyurethane Composite Electrode. Anal. Lett. 2017, 50, 2323–2334. [Google Scholar] [CrossRef]
- Krepper, G.; Pierini, G.D.; Pistonesi, M.F.; Di Nezio, M.S. “In-situ” antimony film electrode for the determination of tetracyclines in Argentinean honey samples. Sens. Actuators B Chem. 2017, 241, 560–566. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, J.-J.; Xu, Y.; Wu, Z.-Y. Fast and sensitive screening detection of tetracyclines with a paper-based analytical device. Microchem. J. 2019, 145, 703–707. [Google Scholar] [CrossRef]
- Lian, L.; Lv, J.; Wang, X.; Lou, D. Magnetic solid-phase extraction of tetracyclines using ferrous oxidecoated magnetic silica microspheres from water samples. J. Chromatogr. A 2018, 1534. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, P.; Bedi, J.S.; Aulakh, R.S.; Gill, J.P.S.; Kumar, A. Validation of HPLC Multi-residue Method for Determination of Fluoroquinolones, Tetracycline, Sulphonamides and Chloramphenicol Residues in Bovine Milk. Food Anal. Methods 2019, 12, 338–346. [Google Scholar] [CrossRef]
- Ibarra, I.S.; Rodriguez, J.A.; Miranda, J.M.; Veja, M.; Barrado, E. Magnetic solid phase extraction based on phenyl silica adsorbent for the determination of tetracyclines in milk samples by capillary electrophoresis. J. Chromatogr. A 2011, 1218, 2196–2202. [Google Scholar] [CrossRef]
- Kowalski, P. Capillary electrophoretic method for the simultaneous determination of tetracycline residues in fish samples. J. Pharm. Biomed. Anal. 2008, 47, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Traviesa-Alvarez, J.M.; Costa-Fernández, J.M.; Pereiro, R.; Sanz-Medel, A. Direct screening of tetracyclines in water and bovine milk using room temperature phosphorescence detection. Anal. Chim. Acta 2007, 589, 51–58. [Google Scholar] [CrossRef]
- Wang, J. Analytical Electrochemistry, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Wong, A.; Scontri, M.; Materon, E.M.; Lanza, M.R.V.; Sotomayor, M.D.P.T. Development and application of an electrochemical sensor modified with multi-walled carbon nanotubes and graphene oxide for the sensitive and selective detection of tetracycline. J. Electroanal. Chem. 2015, 757, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Bianco, A.; Chen, Y.; Frackowiak, E.; Holzinger, M.; Koratkar, N.; Meunier, V.; Mikhailovsky, S.; Strano, M.; Tascon, J.M.D.; Terrones, M. Carbon science perspective in 2020: Current research and future challenges. Carbon 2020, 161, 373–391. [Google Scholar] [CrossRef]
- Zhou, L.; Pan, S.; Chen, X.; Zhao, Y.; Zou, B.; Jin, M. Kinetics and thermodynamics studies of pentachlorophenol adsorption on covalently functionalized Fe3O4@SiO2-MWCNTs core-shell magnetic microspheres. Chem. Eng. J. 2014, 257, 10–19. [Google Scholar] [CrossRef]
- Rocha-Santos, T.A.P. Sensors and biosensors based on magnetic nanoparticles. Trends Anal. Chem. 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Irandoust, M.; Haghighi, M.; Taherpour, A.A.; Jafarzadeh, M. Electrochemical sensing of trifluralin in water by fluconazole-immobilized Fe3O4@SiO2 nanomagnetic core-shell linked to carbon nanotube modified glassy carbon electrode; an experimental and theoretical modeling. J. Iran. Chem. Soc. 2017, 15, 719–732. [Google Scholar] [CrossRef]
- Ríos, A.; Zougagh, M. Recent advances in magnetic nanomaterials for improving analytical processes. Trends Anal. Chem. 2016, 84, 72–83. [Google Scholar] [CrossRef]
- Zhou, L.; Li, D.-J.; Gai, L.; Wang, J.-P.; Li, Y.-B. Electrochemical aptasensor for the detection of tetracycline with multi-walled carbon nanotubes amplification. Sens. Actuators B Chem. 2012, 162, 201–208. [Google Scholar] [CrossRef]
- Kogularasu, S.; Akilarasan, M.; Chen, S.-M.; Elaiyappillai, E.; Johnson, P.M.; Chen, T.-W.; Al-Hemaid, F.M.A.; Ali, M.A.; Elshikh, M.S. A comparative study on conventionally prepared MnFe2O4 nanospheres and template-synthesized novel MnFe2O4 nano-agglomerates as the electrodes for biosensing of mercury contaminations and supercapacitor applications. Electrochim. Acta 2018, 290, 533–543. [Google Scholar] [CrossRef]
- Kogularasu, S.; Govindasamy, M.; Chen, S.-M.; Lin, S.-H.; Akilarasan, M.; Mani, V. A novel synthesis of non-aggregated spinel nickel ferrite nanosheets for developing non-enzymatic reactive oxygen species sensor in biological samples. J. Electroanal. Chem. 2018, 820, 161–167. [Google Scholar]
- Akilarasan, M.; Kogularasu, S.; Chen, S.-M.; Chen, T.-W.; Lou, B.-S. A novel approach to iron oxide separation from e-waste and bisphenol A detection in thermal paper receipts using recovered nanocomposites. RSC Adv. 2018, 8, 39870–39878. [Google Scholar] [CrossRef] [Green Version]
- Beveridge, J.S.; Stephens, J.R.; Williams, M.E. The Use Magnetic Nanoparticles in Analytical Chemistry. Annu. Rev. Anal. Chem. 2011, 4, 251–273. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, A.; Shirzadmehr, A.; Madrakian, T.; Bagheri, H. Improvement in the performance of a Pb2+ selective potentiometric sensor using modified core/shell SiO2/Fe3O4 nano-structure. J. Mol. Liq. 2014, 199, 108–114. [Google Scholar] [CrossRef]
- Silva, M.C.; Torres, J.A.; Nogueira, F.G.E.; Tavares, T.S.; Corrêa, A.D.; Oliveira, L.; Ramalho, T.C. Immobilization of soybean peroxidase on silica-coated magnetic particles: A magnetically recoverable biocatalyst for pollutants removal. RSC Adv. 2016, 6, 83856–83863. [Google Scholar] [CrossRef]
- Agência Nacional de Águas. Guia Nacional de Coleta e Preservação de Amostras: Água, Sedimento, Comunidades Aquáticas e Efluentes Líquidos/Companhia Ambiental do Estado de São Paulo; Organizadores, Carlos Jesus Jesus Brandão: São Paulo, Brazil; CETESB: Brasília, Brazil; ANA: Brasília, Brazil, 2011. [Google Scholar]
- Yao, C.-Y.; Yang, J.-Y.; Xu, Z.-L.; Wang, H.; Lei, H.-T.; Sun, Y.-M.; Tian, Y.-X.; Shen, Y.-D. Indirect Competitive Enzyme-Linked Immunosorbent Assay for Detection of Tylosin in Milk and Water Samples. Chin. J. Anal. Chem. 2018, 46, 1275–1281. [Google Scholar] [CrossRef]
- Baby, T.T.; Ramaprabhu, S. SiO2 coated Fe3O4 magnetic nanoparticle dispersed multiwalled carbon nanotubes based amperometric glucose biosensor. Talanta 2010, 80, 2016–2022. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, Z.; Hu, Y.; Li, J.; Fan, X. Multiple functionalization of multi-walled carbon nanotubes with carboxyl and amino groups. Appl. Surf. Sci. 2013, 276, 476–481. [Google Scholar] [CrossRef]
- Yamaura, M.; Camilo, R.; Sampaio, L.; Macêdo, M.; Nakamura, M.; Toma, H. Preparation and characterization of (3-aminopropyl)triethoxysilane-coated magnetite nanoparticles. J. Magn. Magn. Mater. 2004, 279, 210–217. [Google Scholar] [CrossRef]
- Bini, R.A.; Marques, R.F.C.; Santos, F.J.; Chaker, J.A.; Jafelicci, M. Synthesis and functionalization of magnetite nanoparticles with different amino-functional alkoxysilanes. J. Magn. Magn. Mater. 2012, 324, 534–539. [Google Scholar] [CrossRef] [Green Version]
- Hui, C.; Shen, C.; Tian, J.; Bao, L.; Ding, H.; Li, C.; Tian, Y.; Shi, X.; Gao, H.-J. Core-shell Fe3O4@SiO2 nanoparticles synthesized with well-dispersed hydrophilic Fe3O4 seeds. Nanoscale 2011, 3, 701–705. [Google Scholar] [CrossRef] [Green Version]
- Silva, F.D.; Goulart, A.T.; Couceiro, P.R.C.; Fabris, J.D. Mecanismos químicos e mineralógicos de transformação da magnesioferrita de solo derivado de tufito, da região do Alto Paranaíba, MG. Quím. Nova 2009, 32, 1850–1855. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; Germania: Heppenheim, Germany, 2003. [Google Scholar]
- Fan, X.; Li, X. Preparation and magnetic property of multiwalled carbon nanotubes decorated by Fe3O4 nanoparticles. New Carbon Mater. 2012, 27, 111–116. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.; Zhang, Z.; Zhang, H.; Luo, L.; Yao, S. Imprinted sol-gel electrochemical sensor for the determination of benzylpenicillin based on Fe3O4@SiO2/multi-walled carbon nanotubes-chitosans nanocomposite film modified carbon electrode. Anal. Chim. Acta 2011, 698, 61–68. [Google Scholar] [CrossRef]
- Duan, H.; Wang, X.; Wang, Y.; Li, J.; Luo, C. Bioreceptor multi-walled carbon nanotubes@Fe3O4@SiO2-surface molecular imprinted polymer in an ultrasensitive chemiluminescent biosensor for bovine hemoglobin. RSC Adv. 2015, 5, 88492–88499. [Google Scholar] [CrossRef]
- Shriver, D.F.; Atkins, P.W. Química Inorgânica, 3rd ed.; Bookman: Porto Alegre, Brazil, 1999. [Google Scholar]
- Sidhu, P.S.; Gilkes, R.J.; Posner, A.M. Mechanism of the low temperature oxidation of synthetic magnetites. J. Inorg. Nucl. Chem. 1977, 39, 1953–1958. [Google Scholar] [CrossRef]
- Kushikawa, R.T.; Silva, M.R.; Angelo, A.C.D.; Teixeira, M.F.S. Construction of an electrochemical sensing platform based on platinum nanoparticles supported on carbon for tetracycline determination. Sens. Actuators B Chem. 2016, 228, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Skerjanc, J.; Kogej, K.; Cerar, J. Equilibrium and Transport Properties of Alkylpyridinium Bromides. Langmuir 1999, 15, 5023–5028. [Google Scholar] [CrossRef]
- Reddy, Y.V.M.; Sravani, B.; Agarwal, S.; Guptha, V.K.; Madhavi, G. Electrochemical sensor for detection of uric acid in the presence of ascorbic acid and dopamine using the poly(DPA)/SiO2 @Fe3O4 modified carbon paste electrode. J. Electroanal. Chem. 2018, 820, 168–175. [Google Scholar] [CrossRef]
- Dang, X.; Hu, C.; Wei, Y.; Chen, W.; Hu, S. Sensitivity Improvement of the Oxidation of Tetracycline at Acetylene Black Electrode in the Presence of Sodium Dodecyl Sulfate. Electroanalysis 2004, 16, 1949–1955. [Google Scholar] [CrossRef]
- Devkota, L.; Nguyen, L.T.; Vu, T.T.; Piro, B. Electrochemical determination of tetracycline using AuNP-coated molecularly imprinted overoxidized polypyrrole sensing interface. Electrochim. Acta 2018, 270, 535–542. [Google Scholar] [CrossRef]
- Delgado, K.P.; Raymundo-Pereira, P.A.; Campos, A.M.; Oliveira, O.N.; Janegitz, B.C. Ultralow Cost Electrochemical Sensor made of Potato Starch and Carbon Black Nanoballs to Detect Tetracycline in Waters and Milk. Electroanalysis 2018, 30, 2153–2159. [Google Scholar] [CrossRef]










| Technical | Sensitivity/μAmol L−1 |
|---|---|
| AdSDPV | 0.51 |
| DPV | 0.14 |
| SWV | 0.0037 |
| Electrode | Linear Range (µmol L−1) | LOD (µmol L−1) | Reference |
|---|---|---|---|
| GR-Pol [a] | 3.0–95.0 | 2.6 | [5] |
| CB-FB/GCE [b] | 5.0–120 | 1.15 | [46] |
| MIOPPy-AuNP/SPCE [c] | 1.0–20 | 0.65 | [45] |
| PtNPs/C/GCE [d] | 9.99–44.01 | 4.28 | [41] |
| GCE/MWCNT/Fe3O4@SiO2 | 4.0–36 | 1.67 | This work |
| River Water Sample | Tetracycline/µmol L−1 | Recovery (%) | |
|---|---|---|---|
| Added | Detected * | ||
| No. 1 | 5.2 | (6.1 ± 0.8) | 117.9 |
| No. 2 | 9.2 | (8.7 ± 0.4) | 94.9 |
| Milk Sample | Tetracycline/µmol L−1 | Recovery (%) | |
|---|---|---|---|
| Added | Detected * | ||
| No. 1 | 10 | (10 ± 0.2) | 102.0 |
| 20 | (20.1 ± 1.2) | 100.3 | |
| No. 2 | 10 | (9.1 ± 0.1) | 91.0 |
| 20 | (21.8 ± 1.8) | 108.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, E.F.; da Silva, D.N.; Silva, M.C.; Pereira, A.C. Development of an Electrochemical Sensor Based on Nanocomposite of Fe3O4@SiO2 and Multiwalled Carbon Nanotubes for Determination of Tetracycline in Real Samples. Electrochem 2021, 2, 251-263. https://0-doi-org.brum.beds.ac.uk/10.3390/electrochem2020018
Amaral EF, da Silva DN, Silva MC, Pereira AC. Development of an Electrochemical Sensor Based on Nanocomposite of Fe3O4@SiO2 and Multiwalled Carbon Nanotubes for Determination of Tetracycline in Real Samples. Electrochem. 2021; 2(2):251-263. https://0-doi-org.brum.beds.ac.uk/10.3390/electrochem2020018
Chicago/Turabian StyleAmaral, Edna Ferreira, Daniela Nunes da Silva, Maria Cristina Silva, and Arnaldo César Pereira. 2021. "Development of an Electrochemical Sensor Based on Nanocomposite of Fe3O4@SiO2 and Multiwalled Carbon Nanotubes for Determination of Tetracycline in Real Samples" Electrochem 2, no. 2: 251-263. https://0-doi-org.brum.beds.ac.uk/10.3390/electrochem2020018