Effect of Ag Nanoparticle Size on Ion Formation in Nanoparticle Assisted LDI MS
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Nanoparticle Characterization
3.2. Riboflavin Fragmentation
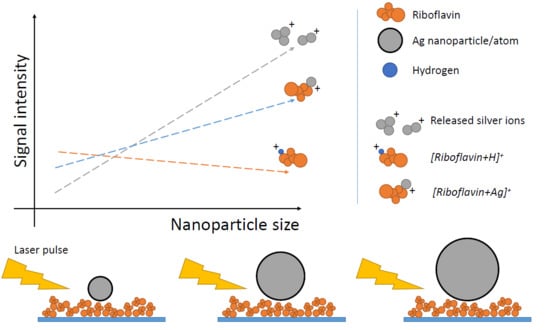
3.3. Effect of Laser Fluence and NP Size on Mass Spectra of Riboflavin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lei, C.; Qian, K.; Noonan, O.; Nouwens, A.; Yu, C. Applications of nanomaterials in mass spectrometry analysis. Nanoscale 2013, 5, 12033–12042. [Google Scholar] [CrossRef]
- Keshavarz, M.; Tan, B.; Venkatakrishnan, K. Multiplex photoluminescent silicon nanoprobe for diagnostic bioimaging and intracellular analysis. Adv. Sci. 2018, 5, 1700548. [Google Scholar] [CrossRef] [Green Version]
- Camden, J.P.; Dieringer, J.A.; Wang, Y.; Masiello, D.J.; Marks, L.D.; Schatz, G.C.; van Duyne, R.P. Probing the structure of single-molecule surface-enhanced Raman scattering hot spots. J. Am. Chem. Soc. 2008, 130, 12616–12617. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Kassanos, P.; Tan, B.; Venkatakrishnan, K. Metal-oxide surface-enhanced Raman biosensor template towards point-of-care EGFR detection and cancer diagnostics. Nanoscale Horiz. 2020, 130, 294–307. [Google Scholar] [CrossRef]
- Keshavarz, M.; Tan, B.; Venkatakrishnan, K. Label-free sers quantum semiconductor probe for molecular-level and in vitro cellular detection: A noble-metal-free methodology. ACS Appl. Mater. Interfaces 2018, 10, 34886–34904. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Baillargeat, D.; Ho, H.P.; Yong, K.T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef]
- Tsuzuki, T. Commercial scale production of inorganic nanoparticles. Int. J. Nanotechnol. 2009, 6, 567–588. [Google Scholar] [CrossRef]
- Liz-Marzán, L.M. Tailoring surface plasmons through the morphology and assembly of metal nanoparticles. Langmuir 2006, 22, 32–41. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. Nanoparticle assisted laser desorption/ionization mass spectrometry for small molecule analytes. Microchim. Acta 2018, 185, 200. [Google Scholar] [CrossRef]
- Gemperline, E.; Rawson, S.; Li, L. Optimization and comparison of multiple MALDI matrix application methods for small molecule mass spectrometric imaging. Anal. Chem. 2014, 86, 10030–10035. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Waki, H.; Ido, Y.; Akita, S.; Yoshida, Y.; Yoshida, T. Protein and polymer analyses up to m/z 100 000 by laser ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 1988, 2, 151–153. [Google Scholar] [CrossRef]
- Taira, S.; Sugiura, Y.; Moritake, S.; Shimma, S.; Ichiyanagi, Y.; Setou, M. Nanoparticle-assisted laser desorption/ionization based mass imaging with cellular resolution. Anal. Chem. 2008, 80, 4761–4766. [Google Scholar] [CrossRef] [PubMed]
- Boerigter, C.; Aslam, U.; Linic, S. Mechanism of Charge Transfer from Plasmonic Nanostructures to Chemically Attached Materials. ACS Nano 2016, 10, 6108–6115. [Google Scholar] [CrossRef] [PubMed]
- Kailasa, S.K.; Wu, H.F. One-pot synthesis of dopamine dithiocarbamate functionalized gold nanoparticles for quantitative analysis of small molecules and phosphopeptides in SALDI- and MALDI-MS. Analyst 2012, 137, 1629–1638. [Google Scholar] [CrossRef]
- Iwaki, Y.; Kawasaki, H.; Arakawa, R. Human serum albumin-modified Fe3O4 magnetic nanoparticles for affinity-SALDI-MS of small-molecule drugs in biological liquids. Anal. Sci. 2012, 28, 893–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zenobi, R.; Knochenmuss, R. Ion formation in MALDI mass spectrometry. Mass Spectrom. Rev. 2002, 17, 337–366. [Google Scholar] [CrossRef]
- Lai, E.P.C.; Owega, S.; Kulczycki, R. Time-of-flight mass spectrometry of bioorganic molecules by laser ablation of silver thin film substrates and particles. J. Mass Spectrom. 1998, 33, 554–564. [Google Scholar] [CrossRef]
- Teng, C.H.; Ho, K.C.; Lin, Y.S.; Chen, Y.C. Gold nonoparticles as selective and concentrating probes for samples in MALDI MS analysis. Anal. Chem. 2004, 76, 4337–4342. [Google Scholar] [CrossRef]
- Dong, X.; Cheng, J.; Li, J.; Wang, Y. Graphene as a novel matrix for the analysis of small molecules by MALDI-TOF MS. Anal. Chem. 2010, 82, 6208–6214. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Song, N.; Li, X.; Ma, J.; Jia, Q. Highly selective enrichment of phosphopeptides by on-chip indium oxide functionalized magnetic nanoparticles coupled with MALDI-TOF MS. Proteomics 2017, 17, 17–18. [Google Scholar] [CrossRef]
- Lu, M.; Yang, X.; Yang, Y.; Qin, P.; Wu, X.; Cai, Z. Nanomaterials as Assisted Matrix of Laser Desorption/Ionization Time-of-Flight Mass Spectrometry for the Analysis of Small Molecules. Nanomaterials 2017, 7, 87. [Google Scholar] [CrossRef]
- Jackson, S.N.; Baldwin, K.; Muller, L.; Womack, V.M.; Schultz, J.A.; Balaban, C.; Woods, A.S. Imaging of lipids in rat heart by MALDI-MS with silver nanoparticles. Anal. Bioanal. Chem. 2014, 406, 1377–1386. [Google Scholar] [CrossRef] [Green Version]
- Goolsby, B.J.; Brodbelt, J.S.; Adou, E.; Blanda, M. Determination of alkali metal ion binding selectivities of calixarenes by matrix-assisted laser desorption ionization and electrospray ionization in a quadrupole ion trap. Int. J. Mass Spectrom. 1999, 193, 197–204. [Google Scholar] [CrossRef]
- Hortal, A.R.; Hurtado, P.; Martínez-Haya, B.A.; Arregui, L. Bañares, Solvent-free MALDI investigation of the cationization of linear polyethers with alkali metals. J. Phys. Chem. B 2008, 112, 8530–8535. [Google Scholar] [CrossRef]
- Yang, S.; Shi, Y.; Ma, Y.; Mu, L.; Kong, X. Competition between metal cationization and protonation/reduction in MALDI process: An example of riboflavin. Int. J. Mass Spectrom. 2018, 434, 209–214. [Google Scholar] [CrossRef]
- Knochenmuss, R.; Dubois, F.; Dale, M.J.; Zenobi, R. The matrix suppression effect and ionization mechanisms in matrix-assisted laser desorption/ionization. Rapid Commun. Mass Spectrom. 1996, 10, 871–877. [Google Scholar] [CrossRef]
- Wong, C.K.L.; Dominic Chan, T.-W. Cationization processes in matrix-assisted laser desorption/ionization mass spectrometry: Attachment of divalent and trivalent metal ions. Rapid Commun. Mass Spectrom. 1997, 11, 513–519. [Google Scholar] [CrossRef]
- Montaudo, G.; Montaudo, M.S.; Puglisi, C.; Samperi, F.; Sepulchre, M. End-group characterization of poly(methylphenylsilane) by alkali metal salts doped MALDI-TOF mass spectra. Macromol. Chem. Phys. 1996, 197, 2615–2625. [Google Scholar] [CrossRef]
- Wu, S.; Qian, L.; Huang, L.; Sun, X.; Su, H.; Gurav, D.D.; Jiang, M.; Cai, W.; Qian, K. A Plasmonic mass spectrometry approach for detection of small nutrients and toxins. Nano-Micro Lett. 2018, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Prysiazhnyi, V.; Dycka, F.; Kratochvil, J.; Sterba, J.; Stranak, V. Gas-aggregated Ag nanoparticles for detection of small molecules using LDI MS. Anal. Bioanal. Chem. 2020, 412, 1037–1047. [Google Scholar] [CrossRef]
- Sekuła, J.; Nizioł, J.; Rode, W.; Ruman, T. Silver nanostructures in laser desorption/ionization mass spectrometry and mass spectrometry imaging. Analyst 2015, 140, 6195–6209. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Baldwin, K.; Barbacci, D.C.; Jackson, S.N.; Roux, A.; Balaban, C.D.; Brinson, B.E.; McCully, M.I.; Lewis, E.K.; Schultz, J.A.; et al. Laser Desorption/Ionization Mass Spectrometric Imaging of Endogenous Lipids from Rat Brain Tissue Implanted with Silver Nanoparticles. J. Am. Soc. Mass Spectrom. 2017, 28, 1716–1728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, S.; Liu, J.; Liu, L.; Yang, H.; Zhang, Y.; Wang, T.; Zhao, Z. Silver nanoparticles as matrix for MALDI FTICR MS profiling and imaging of diverse lipids in brain. Talanta 2018, 179, 624–631. [Google Scholar] [CrossRef]
- Beztsinna, N.; Tsvetkova, Y.; Jose, J.; Rhourri-Frih, B.; al Rawashdeh, W.; Lammers, T.; Kiessling, F.; Bestel, I. Photoacoustic imaging of tumor targeting with riboflavin-functionalized theranostic nanocarriers. Int. J. Nanomed. 2017, 12, 3813–3825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.B.; Chen, Y.C.; Urban, P.L. Coffee-ring effects in laser desorption/ionization mass spectrometry. Anal. Chim. Acta. 2013, 766, 77–82. [Google Scholar] [CrossRef]
- Hanif, M.; Popok, V.N. Magnetron Sputtering Cluster Apparatus for Formation and Deposition of Size-Selected Metal Nanoparticles. In Physics, Chemistry and Applications of Nanostructures; Borisenko, V.E., Gaponenko, S.V., Gurin, V.S., Kam, C.H., Eds.; World Sci. Publ.: Singapore, 2015; pp. 416–419. [Google Scholar]
- Hanif, M.; Juluri, R.R.; Chirumamilla, M.; Popok, V.N. Poly(methyl methacrylate) composites with size-selected silver nanoparticles fabricated using cluster beam technique. J. Polym. Sci. Part. B Polym. Phys. 2016, 54, 1152–1159. [Google Scholar] [CrossRef]
- Popok, V. Energetic Cluster-Surface Collisions. In Handbook of Nanophysics: Clusters and Fullerenes; Sattler, K.D., Ed.; CRC Press: Boca Raton, CA, USA, 2010; pp. 19-1–19-19. [Google Scholar]
- Popok, V.N.; Gurevich, L. Charge states of size-selected silver nanoparticles produced by magnetron sputtering. J. Nanoparticle Res. 2019, 21, 171. [Google Scholar] [CrossRef]
- Novikov, S.M.; Popok, V.N.; Evlyukhin, A.B.; Hanif, M.; Morgen, P.; Fiutowski, J.; Beermann, J.; Rubahn, H.-G.; Bozhevolnyi, S.I. Highly-stable monocrystalline silver clusters for plasmonic applications. Langmuir 2017, 33, 6062–6070. [Google Scholar] [CrossRef]
- McMahon, J.M. The analyses of six common vitamins by laser desorption mass spectroscopy. Anal. Biochem. 1985, 147, 535–545. [Google Scholar] [CrossRef]
- Spengler, B.; Bahr, U.; Karas, M.; Hillenkamp, F. Postionization of laser-desorbed organic and inorganic compounds in a time of flight mass spectrometer. Instrum. Sci. Technol. 1988, 17, 173–193. [Google Scholar] [CrossRef]
- Ingendoh, A.; Karas, M.; Hillenkamp, F.; Giessmann, U. Factors affecting the resolution in matrix-assisted laser desorption-ionization mass spectrometry. Int. J. Mass Spectrom. Ion Process. 1994, 131, 345–354. [Google Scholar] [CrossRef]
- Govorov, A.O.; Richardson, H.H. Generating heat with metal nanoparticles. Nano Today 2007, 2, 30–38. [Google Scholar] [CrossRef]
- González, A.L.; Noguez, C.; Beránek, J.; Barnard, A.S. Size, shape, stability, and color of plasmonic silver nanoparticles. J. Phys. Chem. C 2014, 118, 9128–9136. [Google Scholar] [CrossRef]
- Hlaing, M.; Gebear-Eigzabher, B.; Roa, A.; Marcano, A.; Radu, D.; Lai, C.Y. Absorption and scattering cross-section extinction values of silver nanoparticles. Opt. Mater. 2016, 58, 439–444. [Google Scholar] [CrossRef] [Green Version]
- Chandler, J.; Haslam, C.; Hardy, N.; Leveridge, M.; Marshall, P. A Systematic Investigation of the Best Buffers for Use in Screening by MALDI–Mass Spectrometry. SLAS Discov. 2017, 22, 1262–1269. [Google Scholar] [CrossRef] [Green Version]







| Filtering Voltage, V | 200 | 500 | 700 | 1100 | 1600 |
|---|---|---|---|---|---|
| NP height, nm | 10.2 ± 1.0 | 14.6 ± 1.4 | 17.1 ± 1.6 | 19.5 ± 2.0 | 23.3 ± 2.4 |
| Attributed Structure | Experimental m/z | Theoretical m/z | Formula |
|---|---|---|---|
| R2 + (CH2) + Na | 172.07 | 172.07 | C6H13O4Na |
| R2 + (CH2)2 + H | 186.06 | 186.08 | C7H15O4Na |
| Ag2 + H | 216.80 | 216.81 | Ag2H |
| R2 + Ag | 241.81 | 241.97 | C5H11O4Ag |
| R1 + H | 243.07 | 243.08 | C12H11N4O2 |
| R2 + CH2-H + Ag | 254.91 | 254.97 | C6H12O4Ag |
| R1 + CH2 + H | 257.09 | 257.10 | C13H13N4O2 |
| R1 + Ag | 348.96 | 348.98 | C12H10N4O2Ag |
| R1 + CH2 + Ag | 362.97 | 363.00 | C13H12N4O2Ag |
| M + H | 377.07 | 377.15 | C17H21N4O6 |
| M + Na | 399.07 | 399.12 | C17H20N4O6Na |
| R1 + C4H10O2 + Ag | 439.00 | 439.05 | C16H20N4O4Ag |
| R1 + Ag2 | 457.83 | 457.89 | C12H10N4O2Ag2 |
| M + Ag | 482.94 | 483.04 | C17H20N4O6Ag |
| M + CH2-H + Ag | 497.03 | 497.05 | C18H22N4O6Ag |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prysiazhnyi, V.; Dycka, F.; Kratochvil, J.; Stranak, V.; Popok, V.N. Effect of Ag Nanoparticle Size on Ion Formation in Nanoparticle Assisted LDI MS. Appl. Nano 2020, 1, 3-13. https://0-doi-org.brum.beds.ac.uk/10.3390/applnano1010002
Prysiazhnyi V, Dycka F, Kratochvil J, Stranak V, Popok VN. Effect of Ag Nanoparticle Size on Ion Formation in Nanoparticle Assisted LDI MS. Applied Nano. 2020; 1(1):3-13. https://0-doi-org.brum.beds.ac.uk/10.3390/applnano1010002
Chicago/Turabian StylePrysiazhnyi, Vadym, Filip Dycka, Jiri Kratochvil, Vitezslav Stranak, and Vladimir N. Popok. 2020. "Effect of Ag Nanoparticle Size on Ion Formation in Nanoparticle Assisted LDI MS" Applied Nano 1, no. 1: 3-13. https://0-doi-org.brum.beds.ac.uk/10.3390/applnano1010002