Influence of Bi1.5Y0.5O3 Active Layer on the Performance of Nanostructured La0.8Sr0.2MnO3 Cathode
Abstract
:1. Introduction
2. Materials and Methods
Structural, Microstructural, and Electrical Characterization
3. Results and Discussion
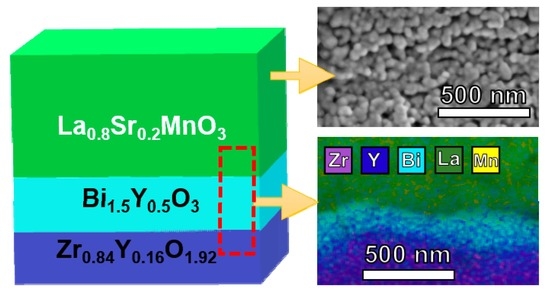
3.1. Structural and Microstructural Analysis
3.2. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kan, W.H.; Samson, A.J.; Thangadurai, V. Trends in electrode development for next generation solid oxide fuel cells. J. Mater. Chem. A 2016, 4, 17913–17932. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Mogni, L.V.; Miller, E.C.; Railsback, J.G.; Barnett, S.A. A perspective on low-temperature solid oxide fuel cells. Energy Environ. Sci. 2016, 9, 1602–1644. [Google Scholar] [CrossRef]
- Adler, S.B. Factors governing oxygen reduction in solid oxide fuel cell cathodes. Chem. Soc. Rev. 2004, 104, 4791–4844. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.P.; Tsai, T.; Barnett, S.A. Oxygen transfer processes in (La,Sr)MnO3/Y2O3-stabilized ZrO2 cathodes: An impedance spectroscopy study. Solid State Ion. 1998, 110, 235–243. [Google Scholar] [CrossRef]
- Minh, Q.; Takahashi, T. Science and Technology of Ceramic Fuel Cell; Elsevier: New York, NY, USA, 1995. [Google Scholar]
- Painter, A.S.; Huang, Y.L.; Wachsman, E.D. Durability of (La0.8Sr0.2)0.95MnO3-δ-(Er0.2Bi0.8)2O3 composite cathodes for low temperature SOFCs. J. Power Sources 2012, 220, 324–330. [Google Scholar] [CrossRef]
- Marrero-López, D.; Dos Santos-Gómez, L.; Canales-Vázquez, J.; Martín, F.; Ramos-Barrado, J.R. Stability and performance of nanostructured La0.8Sr0.2MnO3 cathodes deposited by spray-pyrolysis. Electrochim. Acta 2014, 134, 159–166. [Google Scholar] [CrossRef]
- Pajot, M.; Duffort, V.; Capoen, E.; Mamede, A.-S.; Vannier, R.-N. Influence of the strontium content on the performance of La1-xSrxMnO3/Bi1.5Er0.5O3 composite electrodes for low temperature Solid Oxide Fuel Cells. J. Power Sources 2020, 450, 227649. [Google Scholar] [CrossRef]
- Backhaus-Ricoult, M. Interface chemistry in LSM–YSZ composite SOFC cathodes. Solid State Ion. 2006, 177, 2195–2200. [Google Scholar] [CrossRef]
- Dos Santos-Gómez, L.; Zamudio-García, J.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Highly efficient La0.8Sr0.2MnO3-δ-Ce0.9Gd0.1O1.95 nanocomposite cathodes for solid oxide fuel cells. Ceram. Int. 2018, 44, 4961–4966. [Google Scholar] [CrossRef]
- Dos Santos-Gómez, L.; Losilla, E.R.; Martín, F.; Ramos-Barrado, J.R.; Marrero-López, D. Novel microstructural strategies to enhance the electrochemical performance of La0.8Sr0.2MnO3-δ cathodes. ACS Appl. Mater. Interfaces 2015, 7, 7197–7205. [Google Scholar] [CrossRef] [Green Version]
- Zapata-Ramírez, V.; Dos Santos-Gómez, L.; Mather, G.C.; Marrero-López, D.; Pérez-Coll, D. Enhanced Intermediate-Temperature Electrochemical Performance of Air Electrodes for Solid Oxide Cells with Spray-Pyrolyzed Active Layers. ACS Appl. Mater. Interfaces 2020, 12, 10571–10578. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guan, B.; Ma, L.; Tian, H.; Liu, X. Synergistic Coupling of Proton Conductors BaZr0.1Ce0.7Y0.1Yb0.1O3-δ and La2Ce2O7 to Create Chemical Stable, Interface Active Electrolyte for Steam Electrolysis Cells. ACS Appl. Mater. Interfaces 2019, 11, 18323–18330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xia, C.; Zhao, F.; Chen, F. Thin film ceria–bismuth bilayer electrolytes for intermediate temperature solid oxide fuel cells with La0.85Sr0.15MnO3-δ–Y0.25Bi0.75O1.5 cathodes. Mater. Res. Bull. 2010, 45, 603–608. [Google Scholar] [CrossRef]
- Lee, K.T.; Jung, D.W.; Camaratta, M.A.; Yoon, H.S.; Ahn, J.S.; Wachsman, E.D. Gd0.1Ce0.9O1.95/Er0.4Bi1.6O3 bilayered electrolytes fabricated by a simple colloidal route using nano-sized Er0.4Bi1.6O3 powders for high performance low temperature solid oxide fuel cells. J. Power Sources 2012, 205, 122–128. [Google Scholar] [CrossRef]
- Leng, Y.J.; Chan, S.H. Anode-supported SOFCs with Y2O3-Doped Bi2O3/Gd2O3-Doped CeO2 composite electrolyte film. Electrochem. Solid State Lett. 2006, 9, A56. [Google Scholar] [CrossRef]
- Joh, D.W.; Park, J.H.; Kim, D.; Wachsman, E.D.; Lee, K.T. Functionally graded bismuth oxide/zirconia bilayer electrolytes for high-performance intermediate-temperature solid oxide fuel cells (IT-SOFCs). ACS Appl. Mater. Interfaces 2017, 9, 8443–8449. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Liu, F.; Gong, Z.; Wu, Y.; Liu, W. Different ceria-based materials Gd0.1Ce0.9O2-δ and Sm0.075Nd0.075Ce0.85O2-δ for ceria–bismuth bilayer electrolyte high performance low temperature solid oxide fuel cells. J. Power Sources 2015, 299, 32–39. [Google Scholar] [CrossRef]
- Hou, J.; Bi, L.; Qian, J.; Zhu, Z.; Zhang, J.; Liu, W. High performance ceria–bismuth bilayer electrolyte low temperature solid oxide fuel cells (LT-SOFCs) fabricated by combining co-pressing with drop-coating. J. Mater. Chem. A 2015, 3, 10219–10224. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.S.; Camaratta, M.A.; Pergolesi, D.; Lee, K.T.; Yoon, H.; Lee, B.W.; Jung, D.W.; Traversa, E.; Wachsman, E.D. Development of High Performance Ceria/Bismuth Oxide Bilayered Electrolyte SOFCs for Lower Temperature Operation. J. Electrochem. Soc. 2010, 157, B376. [Google Scholar] [CrossRef]
- Park, J.Y.; Yoon, H.; Wachsman, E.D. Fabrication and characterization of high-conductivity bilayer electrolytes for intermediate temperature solid oxide fuel cells. J. Am. Ceram. Soc. 2005, 88, 2402–2408. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Zhao, F.; Chen, F.; Xia, C. Sm0.2Ce0.8O1.9/Y0.25Bi0.75O1.5 bilayered electrolytes for low-temperature SOFCs with Ag-Y0.25Bi0.75O1.5 composite cathodes. Solid State Ion. 2011, 192, 557–560. [Google Scholar] [CrossRef]
- Santos-Gómez, L.D.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Improving the efficiency of layered perovskite cathodes by microstructural optimization. J. Mater. Chem. A 2017, 5, 7896–7904. [Google Scholar] [CrossRef]
- Dos Santos-Gómez, L.; Zamudio-García, J.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Highly oriented and fully dense CGO films prepared by spray-pyrolysis and different precursor salts. J. Eur. Ceram. Soc. 2020, 40, 3080–3088. [Google Scholar] [CrossRef]
- X’Pert HighScore Plus; Malvern Panalytical: Almelo, The Netherlands, 2014.
- GSAS Program; Rep. No. LA-UR-86748; Los Alamos, National Lab: Los Alamos, NM, USA, 1994.
- Johnson, D. ZView: A Software Program for IES Analysis; Version 2.9; Scribner Associates, Inc.: Southern Pines, NC, USA, 2005. [Google Scholar]
- Wan, T.H.; Saccoccio, M.; Chen, C.; Ciucci, F. Influence of the Discretization Methods on the Distribution of Relaxation Times Deconvolution: Implementing Radial Basis Functions with DRTtools. Electrochim. Acta 2015, 184, 483–499. [Google Scholar] [CrossRef]
- Battle, P.D.; Catlow, C.R.A.; Heap, J.W.; Moroney, L.M. Structural and Dynamical Studies of δ-Bi2O3 Oxide Ion Conductors: I. The Structure of (Bi2O3)1−x(Y2O3)x as a Function of x and Temperature. J. Solid State Chem. 1986, 63, 8–15. [Google Scholar] [CrossRef]
- Gao, Z.; Zenou, V.Y.; Kennouche, D.; Marks, L.; Barnett, S.A. Solid oxide cells with zirconia/ceria Bi Layer electrolytes fabricated by reduced temperature firing. J. Mater. Chem. A 2015, 3, 9955–9964. [Google Scholar] [CrossRef]
- Molin, S.; Chrzan, A.; Karczewki, J.; Szymczewska, D.; Jasinki, P. The role of thin functional layers in solid oxide fuel cells, Electrochim. Acta 2016, 204, 136–145. [Google Scholar] [CrossRef]
- Pan, K.-J.; Hussain, A.M.; Huang, Y.-L.; Gong, Y.; Cohn, G.; Ding, D.; Wachsman, E.D. Evolution of Solid Oxide Fuel Cells via Fast Interfacial Oxygen Crossover. ACS Appl. Energy Mater. 2019, 2, 4069–4074. [Google Scholar] [CrossRef]
- Fleig, J. Solid oxide fuel cell cathodes: Polarization mechanisms and modeling of the electrochemical performance. Annu. Rev. Mater. Res. 2003, 33, 361–382. [Google Scholar] [CrossRef]
- Ghamarinia, M.; Babaei, A.; Zamani, C. Electrochemical characterization of La2NiO4-infiltrated La0.6Sr0.4Co0.2Fe0.8O3-d by analysis of distribution of relaxation times. Electrochim. Acta 2020, 353, 136520. [Google Scholar] [CrossRef]
- Kromp, A.; Leonide, A.; Weber, A.; Ivers-Tiffée, E. Electrochemical Analysis of Reformate-Fuelled Anode Supported SOFC. J. Electrochem. Soc. 2011, 158, B980. [Google Scholar] [CrossRef]
- Chen, Y.; Choi, Y.M.; Yoo, S.; Ding, Y.; Yan, R.; Pei, K.; Qu, C.; Zhang, L.; Chang, I.; Zhao, B.; et al. A highly efficient multi-phase catalyst dramatically enhances the rate of oxygen reduction. Joule 2018, 2, 938–949. [Google Scholar] [CrossRef]
- Lee, J.G.; Park, M.G.; Yoon, H.H.; Soul, Y.G. Application of GDC-YDB Bilayer and LSMYDB Cathode for Intermediate Temperature Solid Oxide Fuel Cells. J. Electroceram. 2013, 31, 231–237. [Google Scholar] [CrossRef]
- He, Z.; Ai, N.; He, S.; Jiang, S.P.; Zhang, L.; Rickard, W.D.A.; Tang, D.; Chen, K. Positive Effect of Incorporating Er0.4Bi1.6O3 on the Performance and Stability of La2NiO4+δ Cathode. J. Electrochem. Soc. 2019, 166, F796–F804. [Google Scholar] [CrossRef]
- Lenser, C.; Menzler, N.H. Impedance characterization of supported oxygen ion conducting electrolytes. Solid State Ion. 2019, 334, 70–81. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamudio-García, J.; Albarrán-Aroca, N.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Influence of Bi1.5Y0.5O3 Active Layer on the Performance of Nanostructured La0.8Sr0.2MnO3 Cathode. Appl. Nano 2020, 1, 14-24. https://0-doi-org.brum.beds.ac.uk/10.3390/applnano1010003
Zamudio-García J, Albarrán-Aroca N, Porras-Vázquez JM, Losilla ER, Marrero-López D. Influence of Bi1.5Y0.5O3 Active Layer on the Performance of Nanostructured La0.8Sr0.2MnO3 Cathode. Applied Nano. 2020; 1(1):14-24. https://0-doi-org.brum.beds.ac.uk/10.3390/applnano1010003
Chicago/Turabian StyleZamudio-García, Javier, Nerea Albarrán-Aroca, José M. Porras-Vázquez, Enrique R. Losilla, and David Marrero-López. 2020. "Influence of Bi1.5Y0.5O3 Active Layer on the Performance of Nanostructured La0.8Sr0.2MnO3 Cathode" Applied Nano 1, no. 1: 14-24. https://0-doi-org.brum.beds.ac.uk/10.3390/applnano1010003